Recent from talks
All channels
Be the first to start a discussion here.
Be the first to start a discussion here.
Be the first to start a discussion here.
Be the first to start a discussion here.
Welcome to the community hub built to collect knowledge and have discussions related to Rubidium triiodide.
Nothing was collected or created yet.
Rubidium triiodide
View on Wikipediafrom Wikipedia
 | |
| Identifiers | |
|---|---|
3D model (JSmol)
|
|
PubChem CID
|
|
| |
| |
| Properties | |
| RbI3 | |
| Appearance | black crystals |
| Related compounds | |
Other cations
|
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Rubidium triiodide is an inorganic compound with the chemical formula RbI3. It is composed of Rb+ and I−
3.
Preparation
[edit]Rubidium triiodide can be obtained by heating rubidium iodide and iodine in aqueous solution:[1]
- RbI + I2 → RbI3
Properties
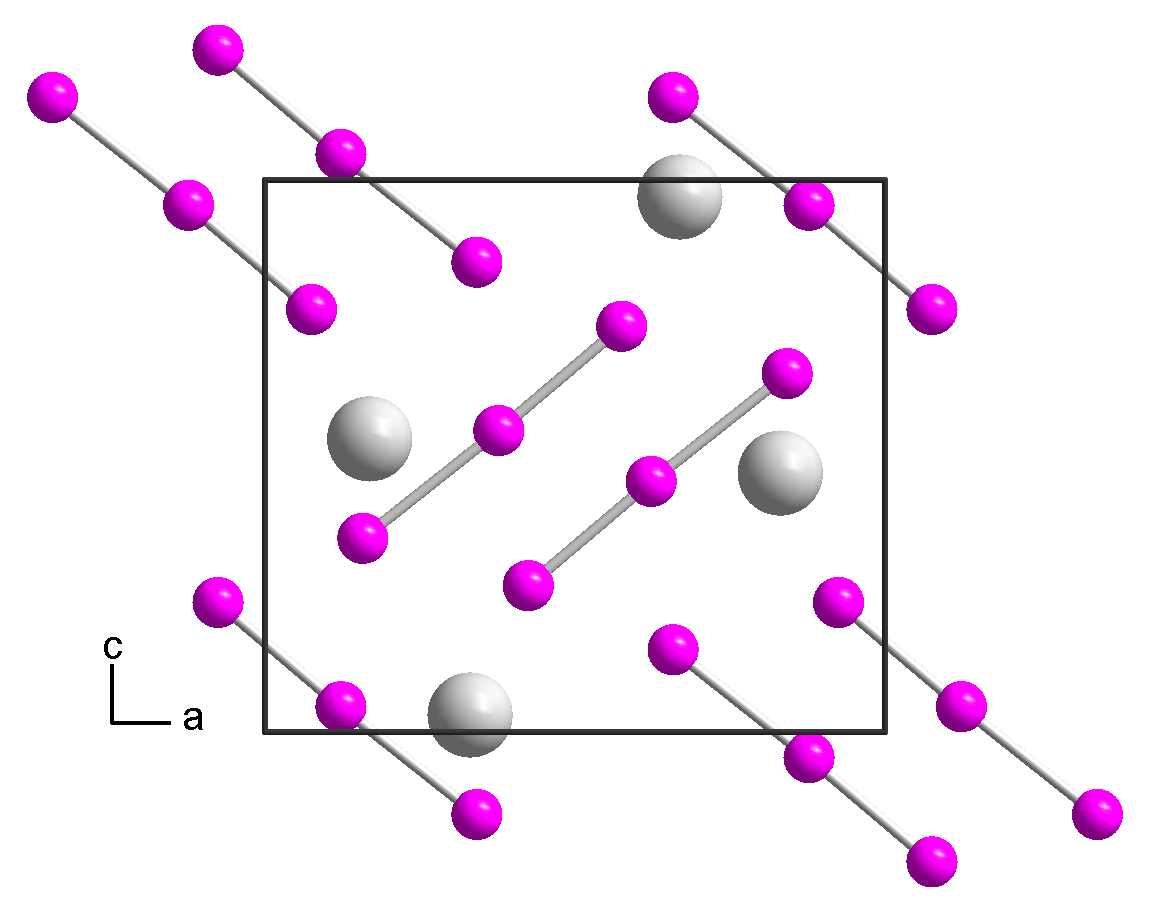
[edit]Rubidium triiodide is an orthorhombic black crystal, isomorphic to caesium triiodide, with space group Pnma, unit cell parameters a = 1090.8 pm, b = 665.5 pm, c = 971.1 pm.[2] Upon heating to 270 °C, the rubidium triiodide decomposes into rubidium iodide and elemental iodine.[1] It is soluble in ethanol and decomposes in ether.[1]
Reactions
[edit]It has long been believed that rubidium triiodide reacts further with iodine to form RbI7 and RbI9,[3] but this has been refuted by recent studies.[4][2]
References
[edit]- ^ a b c Wells, H. L.; Wheeler, H. L.; Penfield, S. L. (1892). "Über Trihalogenverbindungen des Rubidiums und Kaliums. nebst ihrer Krystallographie". Zeitschrift für anorganische Chemie. 1 (1): 442–455. doi:10.1002/zaac.18920010140.
- ^ a b Tebbe, K. F.; Georgy, U. (1986-12-15). "Die Kristallstruckturen von Rubidiumtriiodid und Thalliumtriiodid". Acta Crystallographica Section C Crystal Structure Communications. 42 (12). International Union of Crystallography (IUCr): 1675–1678. Bibcode:1986AcCrC..42.1675T. doi:10.1107/s0108270186090972. ISSN 0108-2701.
- ^ Abegg, R. (1906-09-21). "Über die festen Polyjodide der Alkalien, ihre Stabilität und Existenzbedingungen bei 25°". Zeitschrift für anorganische Chemie. 50 (1). Wiley: 403–438. doi:10.1002/zaac.19060500136. ISSN 0863-1778.
- ^ Briggs, T. R.; Patterson, E. S. (1932-10-01). "The Polyiodides of Rubidium. I". The Journal of Physical Chemistry. 36 (10). American Chemical Society (ACS): 2621–2624. doi:10.1021/j150340a011. ISSN 0092-7325.