Recent from talks
Nothing was collected or created yet.
BAPTA
View on Wikipedia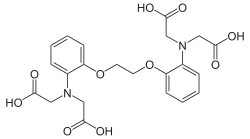 | |
 | |
| Names | |
|---|---|
| Preferred IUPAC name
2,2′,2′′,2′′′-[Ethane-1,2-diylbis(oxy-2,1-phenylenenitrilo)]tetraacetic acid | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.157.377 |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C22H24N2O10 | |
| Molar mass | 476.433 |
| Density | 1.494 g/cm3 |
| Melting point | 177 to 179 °C (351 to 354 °F; 450 to 452 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
BAPTA (1,2-bis(o-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid) is an aminopolycarboxylic acid with a high affinity for calcium. It is a white solid. It is used in research to chelate Ca2+, as it behaves similarly to EGTA and EDTA.
Complexation
[edit]BAPTA, as its conjugate base, binds calcium ions as a decadentate ligand:
- [CH2OC6H4N(CH2CO2H)2]2 + Ca2+ → Ca[CH2OC6H4N(CH2CO2)2]2−2 + 4 H+
According to X-ray crystallography. the four carboxylates, two amines, and two ether oxygens bind to Ca2+.[1]
There is a range of reported values for the dissociation constant of BAPTA, though 0.2 μM appears consistently.[2] The rate constant for calcium binding is 500 μM−1 s−1.[2] The complexation process of calcium ion to BAPTA can be deconvoluted into three main processes: conformational changes of the glicol linker, nitrogen conjugation and electronic effects changes of the benzene rings.[3]
BAPTA is a component of some fluorescent calcium ion indicators such as Calcium Green and Oregon Green 488 BAPTA-1 and -2 (OGB-1, OGB2). These indicators change their fluorescence intensity and fluorescence lifetime depending on the calcium ion concentration.[4]
See also
[edit]References
[edit]- ^ Gerig, John T.; Singh, Phirtu; Levy, Louis A.; London, Robert E. (1987). "Calcium complexation with a highly calcium selective chelator: Crystal structure of ca(CaFBAPTA) ·5H2O". Journal of Inorganic Biochemistry. 31 (2): 113–121. doi:10.1016/0162-0134(87)80056-9.
- ^ a b Ricci AJ, Wu YC, Fettiplace R (15 October 1998). "The endogenous calcium buffer and the time course of transducer adaptation in auditory hair cells". The Journal of Neuroscience. 18 (20): 8261–77. doi:10.1523/JNEUROSCI.18-20-08261.1998. PMC 6792854. PMID 9763471.
- ^ Csomós, Attila; Kontra, Bence; Jancsó, Attila; Galbács, Gábor; Deme, Ruth; Kele, Zoltán; Rózsa, Balázs; Kovács, Ervin; Mucsi, Zoltán (Sep 2021). "A Comprehensive Study of the Ca2+ Ion Binding of Fluorescently Labelled BAPTA Analogues". European Journal of Organic Chemistry. 2021 (37): 5248–5261. doi:10.1002/ejoc.202100948.
- ^ "Fluorescent Ca2+ Indicators Excited with Visible Light—Section 19.3". The Molecular Probes Handbook. Retrieved 2024-01-22.

