Bob
Have a question related to this hub?
Alice
Got something to say related to this hub?
Share it here.
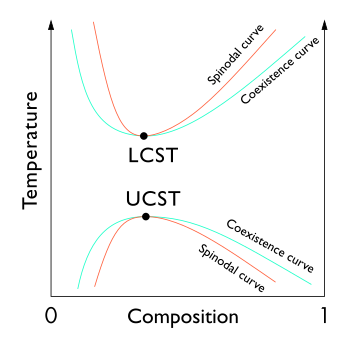
In thermodynamics, the binodal, also known as the coexistence curve or binodal curve, denotes the state of a multi-component system at which the system's distinct phases straddle between coexistence or miscibility. Equivalently, it is the boundary on which thermodynamics favors the system components to dissolve or separate into two phases.[1] In general, the binodal is defined by the condition at which the chemical potential of all solution components is equal in each phase. The extremum of a binodal curve in temperature coincides with the extremum of the spinodal curve, and is known as a critical point.
In binary (two component) mixtures, the binodal can be determined at a given temperature by drawing a tangent line to the free energy.[1]
Redhi, Gyanisavan Govindsamy (2003). "5.7 Fitting Mathematical Equations to the Binodal Curve Data". THERMODYNAMICS OF LIQUID MIXTURES CONTAINING CARBOXYLIC ACIDS (PDF) (Ph.D.). University of Natal, Durban, South Africa. p. 123. Retrieved 22 July 2018.