Recent from talks
Nothing was collected or created yet.
Gonad
View on WikipediaThis article may require cleanup to meet Wikipedia's quality standards. The specific problem is: Writing does not cover the subject in a clear and concise manner. (May 2012) |
| Gonad | |
|---|---|
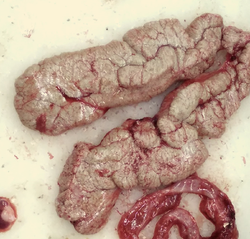
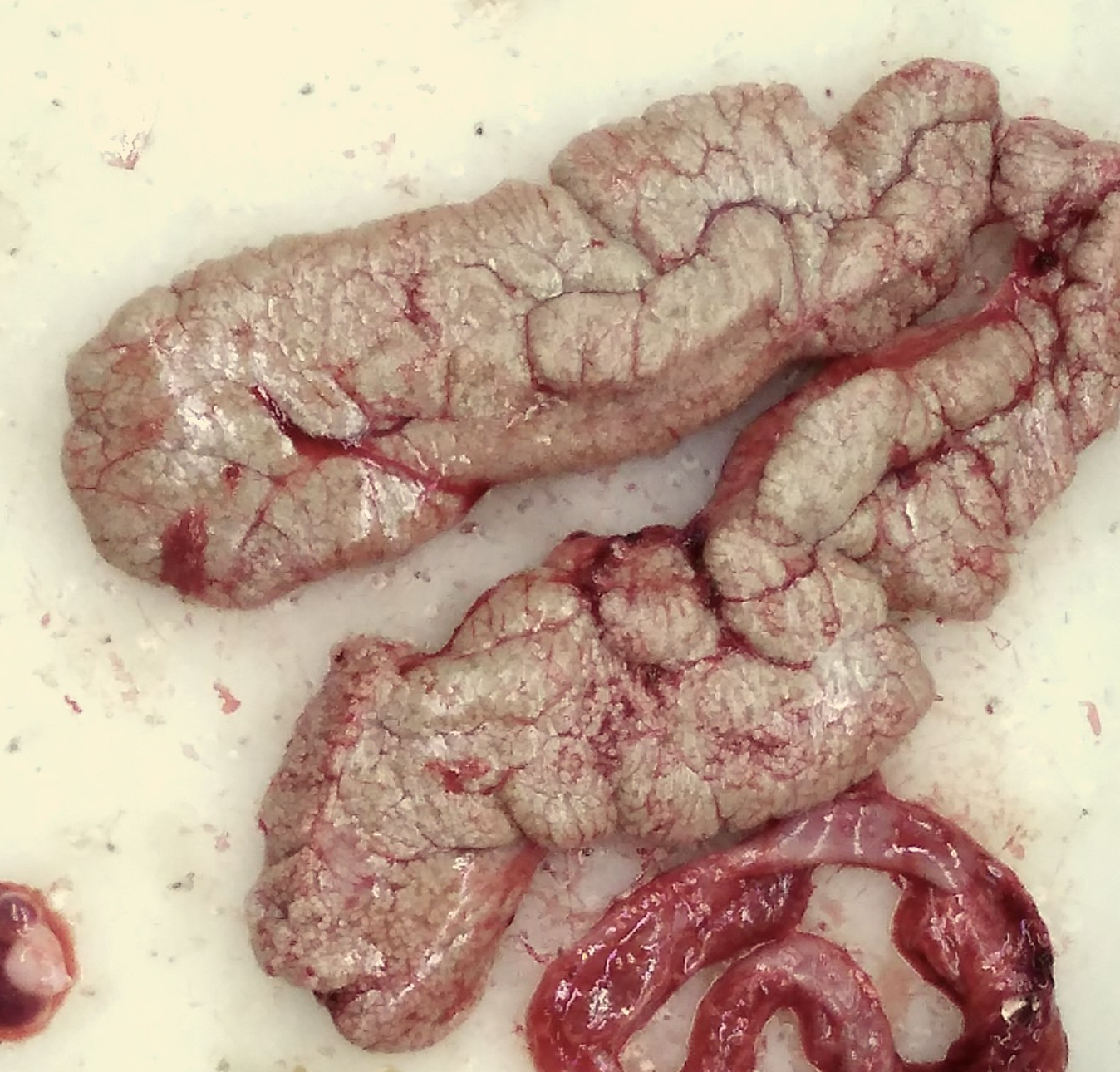
 A pair of ovaries of Cyprinus carpio (common carp) placed in dissecting dish | |
| Identifiers | |
| MeSH | D006066 |
| FMA | 18250 |
| Anatomical terminology | |
A gonad, sex gland, or reproductive gland[1] is a mixed gland and sex organ that produces the gametes and sex hormones of an organism. Female reproductive cells are egg cells, and male reproductive cells are sperm.[2] The male gonad, the testicle, produces sperm in the form of spermatozoa. The female gonad, the ovary, produces egg cells. Both of these gametes are haploid cells. Some hermaphroditic animals (and some humans— see Ovotesticular syndrome) have a type of gonad called an ovotestis.
Evolution
[edit]It is hard to find a common origin for gonads, but gonads most likely evolved independently several times.[3]
Regulation
[edit]The gonads are controlled by luteinizing hormone (LH) and follicle-stimulating hormone (FSH), produced and secreted by gonadotropes or gonadotrophins in the anterior pituitary gland.[4] This secretion is regulated by gonadotropin-releasing hormone (GnRH) produced in the hypothalamus.[5][6]
Development
[edit]The gonads develop from three sources; the mesothelium, underlying mesenchyme and the primordial germ cells. Gonads start developing as a common primordium (an organ in the earliest stage of development), in the form of genital ridges,[7] at the sixth week, which are only later differentiated to male or female sex organs (except when they are not differentiated). The presence of the SRY gene,[8] located on the short arm of the Y chromosome and encoding the testis determining factor, usually determines male sexual differentiation. In the absence of the SRY gene from the Y chromosome, usually the female sex (ovaries instead of testes) will develop. The development of the gonads is a part of the development of the urinary and reproductive organs.[citation needed]
Disease
[edit]This section needs expansion. You can help by adding to it. (September 2022) |
The gonads are subject to many diseases, such as hypergonadism, hypogonadism, agonadism, tumors, and cancer, among others.[citation needed]
Aging
[edit]Ovarian aging
[edit]A delay in having children is common in the developed world and this delay is often associated with ovarian female infertility and subfertility. Ovarian aging is characterized by progressive decline of the quality and number of oocytes.[9] This decline is likely due, in part, to reduced expression of genes that encode proteins necessary for DNA repair and meiosis.[10][11] Such reduced expression can lead to increased DNA damage and errors in meiotic recombination.[9]
Testicular aging
[edit]The testes of older men often have sperm abnormalities that can ultimately lead to male infertility.[12] These abnormalities include accumulation of DNA damage and decreased DNA repair ability.[12] During spermatogenesis in the testis, spontaneous new mutations arise and tend to accumulate with age.[13]
See also
[edit]References
[edit]- ^ "the definition of sex gland". Dictionary.com. Archived from the original on 22 July 2015. Retrieved 8 May 2018.
- ^ "gonad (noun) American English definition and synonyms - Macmillan Dictionary". www.macmillandictionary.com. Archived from the original on 8 May 2018. Retrieved 8 May 2018.
- ^ Schmidt-Rhaesa, Andreas (2007-08-30). "13. Reproductive organs". The Evolution of Organ Systems. Oxford University Press. p. 252. ISBN 978-0-19-856668-7. OCLC 190852859.
The diversity of modes in which gonads are formed makes it hard to substantiate a common origin of gonads. It appears to be more likely that gonads evolved independently several times.
- ^ "gonadotropin". The Free Dictionary. Mosby's Medical Dictionary, 8th edition. Elsevier. 2009. Retrieved 4 June 2012.
- ^ Kimball, John W. (12 February 2011). "Hormones of the Hypothalamus: Gonadotropin-releasing hormone (GnRH)". Kimball's Biology Pages. John W. Kimball (The Saylor Foundation). Archived from the original on 27 June 2012. Retrieved 4 June 2012.
- ^ Marieb, Elaine (2013). Anatomy & physiology. Benjamin-Cummings. p. 915. ISBN 978-0-321-88760-3. OCLC 43903780.
- ^ Schoenwolf, Gary C. (2015). Larsen's human embryology (5th ed.). Elsevier. p. 16. ISBN 978-1-4557-0684-6. OCLC 862800082.
- ^ "Human Developmental Genetics". Institut Pasteur. Archived from the original on 5 May 2012. Retrieved 4 June 2012.
- ^ a b Park SU, Walsh L, Berkowitz KM (July 2021). "Mechanisms of ovarian aging". Reproduction. 162 (2): R19 – R33. doi:10.1530/REP-21-0022. PMC 9354567. PMID 33999842.
- ^ Yang Q, Mumusoglu S, Qin Y, Sun Y, Hsueh AJ (August 2021). "A kaleidoscopic view of ovarian genes associated with premature ovarian insufficiency and senescence". FASEB J. 35 (8): e21753. doi:10.1096/fj.202100756R. PMID 34233068.
- ^ Turan V, Oktay K (January 2020). "BRCA-related ATM-mediated DNA double-strand break repair and ovarian aging". Hum Reprod Update. 26 (1): 43–57. doi:10.1093/humupd/dmz043. PMC 6935693. PMID 31822904.
- ^ a b Dong S, Chen C, Zhang J, Gao Y, Zeng X, Zhang X (2022). "Testicular aging, male fertility and beyond". Front Endocrinol (Lausanne). 13 1012119. doi:10.3389/fendo.2022.1012119. PMC 9606211. PMID 36313743.
- ^ Cioppi F, Casamonti E, Krausz C (2019). "Age-Dependent De Novo Mutations During Spermatogenesis and Their Consequences". Genetic Damage in Human Spermatozoa. Adv Exp Med Biol. Vol. 1166. pp. 29–46. doi:10.1007/978-3-030-21664-1_2. ISBN 978-3-030-21663-4. PMID 31301044.