Recent from talks
Contribute something
Nothing was collected or created yet.
Kidney
View on WikipediaThis article may incorporate text from a large language model. (October 2025) |
| Kidneys | |
|---|---|
 The kidneys lie in the retroperitoneal space behind the abdomen, and act to filter blood to create urine | |
 Location of kidneys with associated organs (adrenal glands and bladder) | |
| Details | |
| System | Urinary system and endocrine system |
| Artery | Renal artery |
| Vein | Renal vein |
| Nerve | Renal plexus |
| Identifiers | |
| Latin | ren |
| Greek | νεφρός (nephros) |
| MeSH | D007668 |
| TA98 | A08.1.01.001 |
| TA2 | 3358 |
| FMA | 7203 |
| Anatomical terminology | |
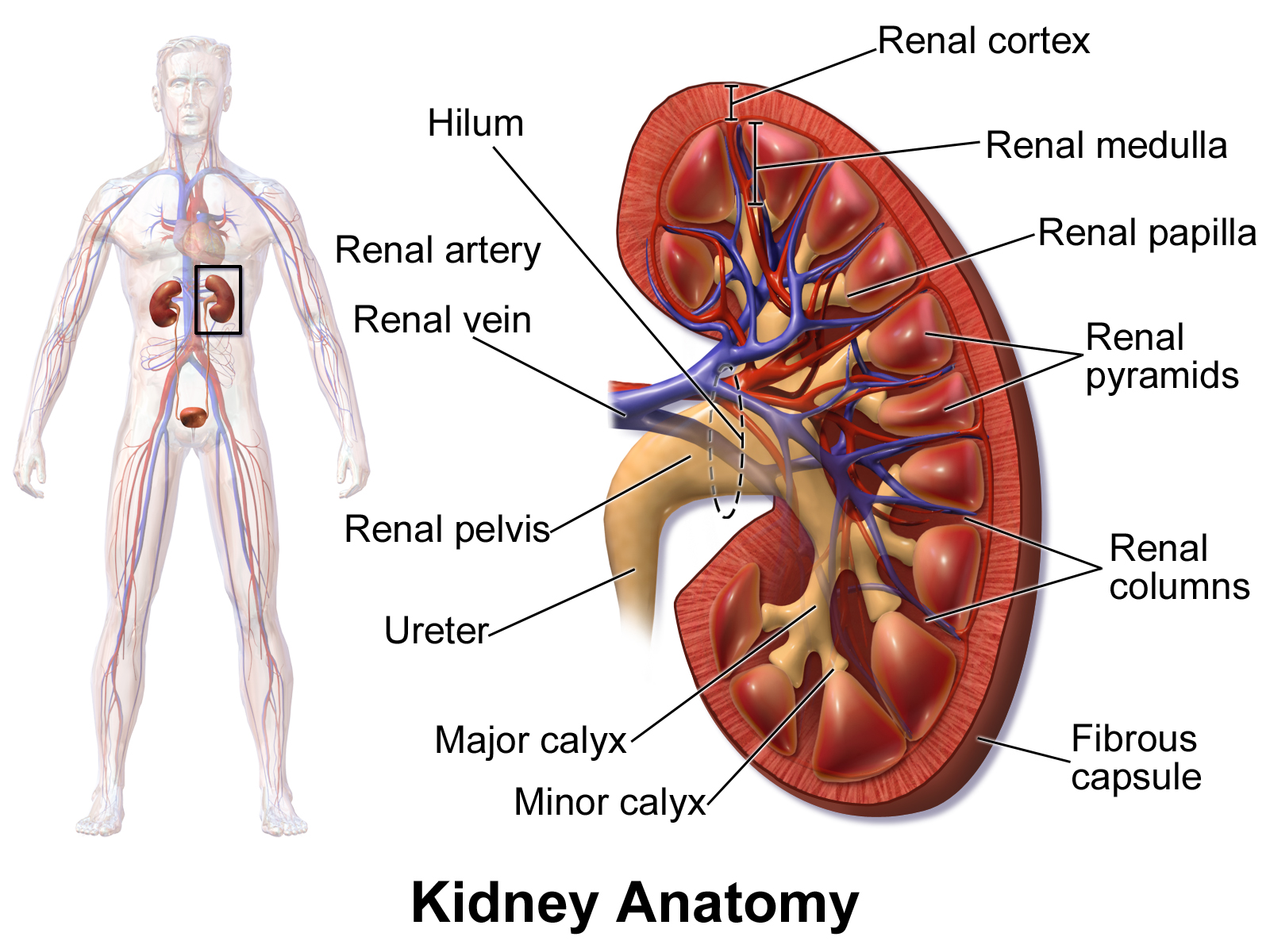
In humans, the kidneys are two reddish-brown bean-shaped blood-filtering organs[1] that are a multilobar, multipapillary form of mammalian kidneys, usually without signs of external lobulation.[2][3] They are located on the left and right in the retroperitoneal space, and in adult humans are about 12 centimetres (4+1⁄2 inches) in length.[4][5] They receive blood from the paired renal arteries; blood exits into the paired renal veins. Each kidney is attached to a ureter, a tube that carries excreted urine to the bladder.
The kidney participates in the control of the volume of various body fluids, fluid osmolality, acid-base balance, various electrolyte concentrations, and removal of toxins. Filtration occurs in the glomerulus: one-fifth of the blood volume that enters the kidneys is filtered. Examples of substances reabsorbed are solute-free water, sodium, bicarbonate, glucose, and amino acids. Examples of substances secreted are hydrogen, ammonium, potassium and uric acid. The nephron is the structural and functional unit of the kidney. Each adult human kidney contains around 1 million nephrons, while a mouse kidney contains only about 12,500 nephrons. The kidneys also carry out functions independent of the nephrons. For example, they convert a precursor of vitamin D to its active form, calcitriol; and synthesize the hormones erythropoietin and renin.
Chronic kidney disease (CKD) has been recognized as a leading public health problem worldwide. The global estimated prevalence of CKD is 13.4%, and patients with kidney failure needing renal replacement therapy are estimated between 5 and 7 million.[6] Procedures used in the management of kidney disease include chemical and microscopic examination of the urine (urinalysis), measurement of kidney function by calculating the estimated glomerular filtration rate (eGFR) using the serum creatinine; and kidney biopsy and CT scan to evaluate for abnormal anatomy. Dialysis and kidney transplantation are used to treat kidney failure; one (or both sequentially) of these are almost always used when renal function drops below 15%. Nephrectomy is frequently used to cure renal cell carcinoma.
Renal physiology is the study of kidney function. Nephrology is the medical specialty which addresses diseases of kidney function: these include CKD, nephritic and nephrotic syndromes, acute kidney injury, and pyelonephritis. Urology addresses diseases of kidney (and urinary tract) anatomy: these include cancer, renal cysts, kidney stones and ureteral stones, and urinary tract obstruction.[7]
The word "renal" is an adjective meaning "relating to the kidneys", and its roots are French or late Latin. Whereas according to some opinions, "renal" should be replaced with "kidney" in scientific writings such as "kidney artery", other experts have advocated preserving the use of "renal" as appropriate including in "renal artery".[8]
Structure
[edit]
In humans, the kidneys are located high in the abdominal cavity, one on each side of the spine, and lie in a retroperitoneal position at a slightly oblique angle.[9] The asymmetry within the abdominal cavity, caused by the position of the liver, typically results in the right kidney being slightly lower and smaller than the left, and being placed slightly more to the middle than the left kidney.[10][11][12] The left kidney is approximately at the vertebral level T12 to L3,[13] and the right is slightly lower. The right kidney sits just below the diaphragm and posterior to the liver. The left kidney sits below the diaphragm and posterior to the spleen. On top of each kidney is an adrenal gland. The upper parts of the kidneys are partially protected by the 11th and 12th ribs. Each kidney, with its adrenal gland is surrounded by two layers of fat: the perirenal fat present between renal fascia and renal capsule and pararenal fat superior to the renal fascia.
The human kidney is a bean-shaped structure with a convex and a concave border.[14] A recessed area on the concave border is the renal hilum, where the renal artery enters the kidney and the renal vein and ureter leave. The kidney is surrounded by tough fibrous tissue, the renal capsule, which is itself surrounded by perirenal fat, renal fascia, and pararenal fat. The anterior (front) surface of these tissues is the peritoneum, while the posterior (rear) surface is the transversalis fascia.
The superior pole of the right kidney is adjacent to the liver. For the left kidney, it is next to the spleen. Both, therefore, move down upon inhalation.
| Sex | Weight, standard reference range | |
| Right kidney | Left kidney | |
| Male[15] | 80–160 g (2+3⁄4–5+3⁄4 oz) | 80–175 g (2+3⁄4–6+1⁄4 oz) |
| Female[16] | 40–175 g (1+1⁄2–6+1⁄4 oz) | 35–190 g (1+1⁄4–6+3⁄4 oz) |
A Danish study measured the median renal length to be 11.2 cm (4+7⁄16 in) on the left side and 10.9 cm (4+5⁄16 in) on the right side in adults. Median renal volumes were 146 cm3 (8+15⁄16 cu in) on the left and 134 cm3 (8+3⁄16 cu in) on the right.[17]
Gross anatomy
[edit]
The functional substance, or parenchyma, of the human kidney is divided into two major structures: the outer renal cortex and the inner renal medulla. Grossly, these structures take the shape of eight to 18 cone-shaped renal lobes, each containing renal cortex surrounding a portion of medulla called a renal pyramid.[18] Between the renal pyramids are projections of cortex called renal columns.
The tip, or papilla, of each pyramid empties urine into a minor calyx; minor calyces empty into major calyces, and major calyces empty into the renal pelvis. This becomes the ureter. At the hilum, the ureter and renal vein exit the kidney and the renal artery enters. Hilar fat and lymphatic tissue with lymph nodes surround these structures. The hilar fat is contiguous with a fat-filled cavity called the renal sinus. The renal sinus collectively contains the renal pelvis and calyces and separates these structures from the renal medullary tissue.[19]
The kidneys possess no overtly moving structures.
-
Normal adult right kidney as seen on abdominal ultrasound with a pole to pole measurement of 9.34 cm
-
Image showing the structures that the kidney lies near
-
Cross-section through a cadaveric specimen showing the position of the kidneys
Blood supply
[edit]The kidneys receive blood from the renal arteries, left and right, which branch directly from the abdominal aorta. The kidneys receive approximately 20–25% of cardiac output in adult human.[18][20][21] Each renal artery branches into segmental arteries, dividing further into interlobar arteries, which penetrate the renal capsule and extend through the renal columns between the renal pyramids. The interlobar arteries then supply blood to the arcuate arteries that run through the boundary of the cortex and the medulla. Each arcuate artery supplies several interlobular arteries that feed into the afferent arterioles that supply the glomeruli.
Blood drains from the kidneys, ultimately into the inferior vena cava. After filtration occurs, the blood moves through a small network of small veins (venules) that converge into interlobular veins. As with the arteriole distribution, the veins follow the same pattern: the interlobular provide blood to the arcuate veins then back to the interlobar veins, which come to form the renal veins which exit the kidney.
Nerve supply
[edit]The kidney and nervous system communicate via the renal plexus, whose fibers course along the renal arteries to reach each kidney.[22] Input from the sympathetic nervous system triggers vasoconstriction in the kidney, thereby reducing renal blood flow.[22] The kidney also receives input from the parasympathetic nervous system,[23] by way of the renal branches of the vagus nerve; the function of this is yet unclear.[22][24] Sensory input from the kidney travels to the T10–11 levels of the spinal cord and is sensed in the corresponding dermatome.[22] Thus, pain in the flank region may be referred from corresponding kidney.[22]
Microanatomy
[edit]Nephrons, the urine-producing functional structures of the kidney, span the cortex and medulla. The initial filtering portion of a nephron is the renal corpuscle, which is located in the cortex. This is followed by a renal tubule that passes from the cortex deep into the medullary pyramids. Part of the renal cortex, a medullary ray is a collection of renal tubules that drain into a single collecting duct.[citation needed]
Renal histology is the study of the microscopic structure of the kidney. The adult human kidney contains at least 26 distinct cell types,[25] including epithelial, endothelial, stromal and smooth muscle cells. Distinct cell types include:
- Kidney glomerulus parietal cell
- Kidney glomerulus podocyte
- Intraglomerular mesangial cell
- Extraglomerular mesangial cell
- Juxtaglomerular cell
- Kidney proximal tubule brush border cell
- Loop of Henle thin segment cell
- Thick ascending limb cell
- Kidney distal tubule cell
- Collecting duct principal cell
- Collecting duct intercalated cell
- Interstitial kidney cells
Gene and protein expression
[edit]In humans, about 20,000 protein coding genes are expressed in human cells and almost 70% of these genes are expressed in normal, adult kidneys.[26][27] Just over 300 genes are more specifically expressed in the kidney, with only some 50 genes being highly specific for the kidney. Many of the corresponding kidney specific proteins are expressed in the cell membrane and function as transporter proteins. The highest expressed kidney specific protein is uromodulin, the most abundant protein in urine with functions that prevent calcification and growth of bacteria. Specific proteins are expressed in the different compartments of the kidney with podocin and nephrin expressed in glomeruli, Solute carrier family protein SLC22A8 expressed in proximal tubules, calbindin expressed in distal tubules and aquaporin 2 expressed in the collecting duct cells.[28]
Development
[edit]The mammalian kidney develops from intermediate mesoderm. Kidney development, also called nephrogenesis, proceeds through a series of three successive developmental phases: the pronephros, mesonephros, and metanephros. The metanephros are primordia of the permanent kidney.[29]
Function
[edit]
The kidneys excrete a variety of waste products produced by metabolism into the urine. The microscopic structural and functional unit of the kidney is the nephron. It processes the blood supplied to it via filtration, reabsorption, secretion and excretion; the consequence of those processes is the production of urine. These include the nitrogenous wastes urea, from protein catabolism, and uric acid, from nucleic acid metabolism. The ability of mammals and some birds to concentrate wastes into a volume of urine much smaller than the volume of blood from which the wastes were extracted is dependent on an elaborate countercurrent multiplication mechanism. This requires several independent nephron characteristics to operate: a tight hairpin configuration of the tubules, water and ion permeability in the descending limb of the loop, water impermeability in the ascending loop, and active ion transport out of most of the ascending limb. In addition, passive countercurrent exchange by the vessels carrying the blood supply to the nephron is essential for enabling this function.
The kidney participates in whole-body homeostasis, regulating acid–base balance, electrolyte concentrations, extracellular fluid volume, and blood pressure. The kidney accomplishes these homeostatic functions both independently and in concert with other organs, particularly those of the endocrine system. Various endocrine hormones coordinate these endocrine functions; these include renin, angiotensin II, aldosterone, antidiuretic hormone, and atrial natriuretic peptide, among others.
Formation of urine
[edit]
Filtration
[edit]Filtration, which takes place at the renal corpuscle, is the process by which cells and large proteins are retained while materials of smaller molecular weights are[30] filtered from the blood to make an ultrafiltrate that eventually becomes urine. The adult human kidney generates approximately 180 liters of filtrate a day, most of which is reabsorbed.[31] The normal range for a twenty four hour urine volume collection is 800 to 2,000 milliliters per day.[32] The process is also known as hydrostatic filtration due to the hydrostatic pressure exerted on the capillary walls.
Reabsorption
[edit]
Reabsorption is the transport of molecules from this ultrafiltrate and into the peritubular capillary network that surrounds the nephron tubules.[33] It is accomplished via selective receptors on the luminal cell membrane. Water is 55% reabsorbed in the proximal tubule. Glucose at normal plasma levels is completely reabsorbed in the proximal tubule. The mechanism for this is the Na+/glucose cotransporter. A plasma level of 350 mg/dL will fully saturate the transporters and glucose will be lost in the urine. A plasma glucose level of approximately 160 is sufficient to allow glucosuria, which is an important clinical clue to diabetes mellitus.
Amino acids are reabsorbed by sodium dependent transporters in the proximal tubule. Hartnup disease is a deficiency of the tryptophan amino acid transporter, which results in pellagra.[34]
| Location of reabsorption | Reabsorbed nutrient | Notes |
|---|---|---|
| Early proximal tubule | Glucose (100%), amino acids (100%), bicarbonate (90%), Na+ (65%), Cl− (65%), phosphate (65%) and H2O (65%) | |
| Thin descending loop of Henle | H2O |
|
| Thick ascending loop of Henle | Na+ (10–20%), K+, Cl−; indirectly induces para cellular reabsorption of Mg2+, Ca2+ |
|
| Early distal convoluted tubule | Na+, Cl− |
|
| Collecting tubules | Na+(3–5%), H2O |
|
Secretion
[edit]Secretion is the reverse of reabsorption: molecules are transported from the peritubular capillary through the interstitial fluid, then through the renal tubular cell and into the ultrafiltrate.
Excretion
[edit]The last step in the processing of the ultrafiltrate is excretion: the ultrafiltrate passes out of the nephron and travels through a tube called the collecting duct, which is part of the collecting duct system, and then to the ureters where it is renamed urine. In addition to transporting the ultrafiltrate, the collecting duct also takes part in reabsorption.
Hormone secretion
[edit]The kidneys secrete a variety of hormones, including erythropoietin, calcitriol, and renin. Erythropoietin (EPO) is released in response to hypoxia (low levels of oxygen at tissue level) in the renal circulation. It stimulates erythropoiesis (production of red blood cells) in the bone marrow. Calcitriol, the activated form of vitamin D, promotes intestinal absorption of calcium and the renal reabsorption of phosphate. Renin is an enzyme which regulates angiotensin and aldosterone levels.
Blood pressure regulation
[edit]Although the kidney cannot directly sense blood, long-term regulation of blood pressure predominantly depends upon the kidney. This primarily occurs through maintenance of the extracellular fluid compartment, the size of which depends on the plasma sodium concentration. Renin is the first in a series of important chemical messengers that make up the renin–angiotensin system. Changes in renin ultimately alter the output of this system, principally the hormones angiotensin II and aldosterone. Each hormone acts via multiple mechanisms, but both increase the kidney's absorption of sodium chloride, thereby expanding the extracellular fluid compartment and raising blood pressure. When renin levels are elevated, the concentrations of angiotensin II and aldosterone increase, leading to increased sodium chloride reabsorption, expansion of the extracellular fluid compartment, and an increase in blood pressure. Conversely, when renin levels are low, angiotensin II and aldosterone levels decrease, contracting the extracellular fluid compartment, and decreasing blood pressure.
Acid–base balance
[edit]The two organ systems that help regulate the body's acid–base balance are the kidneys and lungs. Acid–base homeostasis is the maintenance of pH around a value of 7.4. The lungs are the part of respiratory system which helps to maintain acid–base homeostasis by regulating carbon dioxide (CO2) concentration in the blood. The respiratory system is the first line of defense when the body experiences and acid–base problem. It attempts to return the body pH to a value of 7.4 by controlling the respiratory rate. When the body is experiencing acidic conditions, it will increase the respiratory rate which in turn drives off CO2 and decreases the H+ concentration, therefore increasing the pH. In basic conditions, the respiratory rate will slow down so that the body holds onto more CO2 and increases the H+ concentration and decreases the pH.[35]
The kidneys have two cells that help to maintain acid-base homeostasis: intercalated A and B cells. The intercalated A cells are stimulated when the body is experiencing acidic conditions. Under acidic conditions, the high concentration of CO2 in the blood creates a gradient for CO2 to move into the cell and push the reaction HCO3 + H ↔ H2CO3 ↔ CO2 + H2O to the left. On the luminal side of the cell there is a H+ pump and a H/K exchanger. These pumps move H+ against their gradient and therefore require ATP. These cells will remove H+ from the blood and move it to the filtrate which helps to increase the pH of the blood. On the basal side of the cell there is a HCO3/Cl exchanger and a Cl/K co-transporter (facilitated diffusion). When the reaction is pushed to the left it also increases the HCO3 concentration in the cell and HCO3 is then able to move out into the blood which additionally raises the pH. The intercalated B cell responds very similarly, however, the membrane proteins are flipped from the intercalated A cells: the proton pumps are on the basal side and the HCO3/Cl exchanger and K/Cl co-transporter are on the luminal side. They function the same, but now release protons into the blood to decrease the pH.[36]
Regulation of osmolality
[edit]The kidneys help maintain the water and salt level of the body. Any significant rise in plasma osmolality is detected by the hypothalamus, which communicates directly with the posterior pituitary gland. An increase in osmolality causes the gland to secrete antidiuretic hormone (ADH), resulting in water reabsorption by the kidney and an increase in urine concentration. The two factors work together to return the plasma osmolality to its normal levels.
Measuring function
[edit]Various calculations and methods are used to try to measure kidney function. Renal clearance is the volume of plasma from which the substance is completely cleared from the blood per unit time. The filtration fraction is the amount of plasma that is actually filtered through the kidney. This can be defined using the equation. The kidney is a very complex organ and mathematical modelling has been used to better understand kidney function at several scales, including fluid uptake and secretion.[37][38]
Clinical significance
[edit]Nephrology is the subspeciality under Internal Medicine that deals with kidney function and disease states related to renal malfunction and their management including dialysis and kidney transplantation. Urology is the specialty under Surgery that deals with kidney structure abnormalities such as kidney cancer and cysts and problems with urinary tract. Nephrologists are internists, and urologists are surgeons, whereas both are often called "kidney doctors". There are overlapping areas that both nephrologists and urologists can provide care such as kidney stones and kidney related infections.
There are many causes of kidney disease. Some causes are acquired over the course of life, such as diabetic nephropathy whereas others are congenital, such as polycystic kidney disease.
Medical terms related to the kidneys commonly use terms such as renal and the prefix nephro-. The adjective renal, meaning related to the kidney, is from the Latin rēnēs, meaning kidneys; the prefix nephro- is from the Ancient Greek word for kidney, nephros (νεφρός).[39] For example, surgical removal of the kidney is a nephrectomy, while a reduction in kidney function is called renal dysfunction.
Acquired disease
[edit]- Diabetic nephropathy
- Glomerulonephritis
- Hydronephrosis is the enlargement of one or both of the kidneys caused by obstruction of the flow of urine.
- Interstitial nephritis is inflammation of the area of the kidney known as the renal interstitium.
- Kidney stones (nephrolithiasis) are a relatively common and particularly painful disorder. A chronic condition can result in scars to the kidneys. The removal of kidney stones involves ultrasound treatment to break up the stones into smaller pieces, which are then passed through the urinary tract. One common symptom of kidney stones is a sharp to disabling pain in the middle and sides of the lower back or groin.
- Kidney tumour
- Lupus nephritis
- Minimal change disease
- In nephrotic syndrome, the glomerulus has been damaged so that a large amount of protein in the blood enters the urine. Other frequent features of the nephrotic syndrome include swelling, low serum albumin, and high cholesterol.
- Pyelonephritis is infection of the kidneys and is frequently caused by complication of a urinary tract infection.
- Kidney failure
- Renal artery stenosis
- Renovascular hypertension
Kidney injury and failure
[edit]Generally, humans can live normally with just one kidney, as one has more functioning renal tissue than is needed to survive. Only when the amount of functioning kidney tissue is greatly diminished does one develop chronic kidney disease. Renal replacement therapy, in the form of dialysis or kidney transplantation, is indicated when the glomerular filtration rate has fallen very low or if the renal dysfunction leads to severe symptoms.[40]
Dialysis
[edit]
Dialysis is a treatment that substitutes for the function of normal kidneys. Dialysis may be instituted when approximately 85%–90% of kidney function is lost, as indicated by a glomerular filtration rate (GFR) of less than 15. Dialysis removes metabolic waste products as well as excess water and sodium (thereby contributing to regulating blood pressure); and maintains many chemical levels within the body. Life expectancy is 5–10 years for those on dialysis; some live up to 30 years. Dialysis can occur via the blood (through a catheter or arteriovenous fistula), or through the peritoneum (peritoneal dialysis). Hemodialysis is typically administered three times a week for several hours at free-standing dialysis centers, allowing recipients to lead an otherwise essentially normal life.[41]
Congenital disease
[edit]- Congenital hydronephrosis
- Congenital obstruction of urinary tract
- Duplex kidneys, or double kidneys, occur in approximately 1% of the population. This occurrence normally causes no complications, but can occasionally cause urinary tract infections.[42][43]
- Duplicated ureter occurs in approximately one in 100 live births
- Horseshoe kidney occurs in approximately one in 400 live births
- Nephroblastoma (Syndromic Wilm's tumour)
- Nutcracker syndrome
- Polycystic kidney disease
- Autosomal dominant polycystic kidney disease affects patients later in life. Approximately one in 1,000 people will develop this condition
- Autosomal recessive polycystic kidney disease is far less common, but more severe, than the dominant condition. It is apparent in utero or at birth.
- Renal agenesis. Failure of one kidney to form occurs in approximately one in 750 live births. Failure of both kidneys to form used to be fatal; however, medical advances such as amnioinfusion therapy during pregnancy and peritoneal dialysis have made it possible to stay alive until a transplant can occur.
- Renal dysplasia
- Unilateral small kidney
- Multicystic dysplastic kidney occurs in approximately one in every 2,400 live births
- Ureteropelvic junction obstruction (UPJO); although most cases are congenital, some are acquired.[44]
Diagnosis
[edit]Many renal diseases are diagnosed on the basis of a detailed medical history, and physical examination.[45] The medical history takes into account present and past symptoms, especially those of kidney disease; recent infections; exposure to substances toxic to the kidney; and family history of kidney disease.
Kidney function is tested by using blood tests and urine tests. The most common blood tests are creatinine, urea and electrolytes. Urine tests such as urinalysis can evaluate for pH, protein, glucose, and the presence of blood. Microscopic analysis can also identify the presence of urinary casts and crystals.[46] The glomerular filtration rate (GFR) can be directly measured ("measured GFR", or mGFR) but this rarely done in everyday practice. Instead, special equations are used to calculate GFR ("estimated GFR", or eGFR).[47][46]
Imaging
[edit]Renal ultrasonography is essential in the diagnosis and management of kidney-related diseases.[48] Other modalities, such as CT and MRI, should always be considered as supplementary imaging modalities in the assessment of renal disease.[48]
Biopsy
[edit]The role of the renal biopsy is to diagnose renal disease in which the etiology is not clear based upon noninvasive means (clinical history, past medical history, medication history, physical exam, laboratory studies, imaging studies). In general, a renal pathologist will perform a detailed morphological evaluation and integrate the morphologic findings with the clinical history and laboratory data, ultimately arriving at a pathological diagnosis. A renal pathologist is a physician who has undergone general training in anatomic pathology and additional specially training in the interpretation of renal biopsy specimens.
Ideally, multiple core sections are obtained and evaluated for adequacy (presence of glomeruli) intraoperatively. A pathologist/pathology assistant divides the specimen(s) for submission for light microscopy, immunofluorescence microscopy and electron microscopy.
The pathologist will examine the specimen using light microscopy with multiple staining techniques (hematoxylin and eosin/H&E, PAS, trichrome, silver stain) on multiple level sections. Multiple immunofluorescence stains are performed to evaluate for antibody, protein and complement deposition. Finally, ultra-structural examination is performed with electron microscopy and may reveal the presence of electron-dense deposits or other characteristic abnormalities that may suggest an etiology for the patient's renal disease.
Other animals
[edit]In the majority of vertebrates, the mesonephros persists into the adult, albeit usually fused with the more advanced metanephros; only in amniotes is the mesonephros restricted to the embryo. The kidneys of fish and amphibians are typically narrow, elongated organs, occupying a significant portion of the trunk. The collecting ducts from each cluster of nephrons usually drain into an archinephric duct, which is homologous with the vas deferens of amniotes. However, the situation is not always so simple; in cartilaginous fish and some amphibians, there is also a shorter duct, similar to the amniote ureter, which drains the posterior (metanephric) parts of the kidney, and joins with the archinephric duct at the bladder or cloaca. Indeed, in many cartilaginous fish, the anterior portion of the kidney may degenerate or cease to function altogether in the adult.[49]
In the most primitive vertebrates, the hagfish and lampreys, the kidney is unusually simple: it consists of a row of nephrons, each emptying directly into the archinephric duct. Invertebrates may possess excretory organs that are sometimes referred to as "kidneys", but, even in Amphioxus, these are never homologous with the kidneys of vertebrates, and are more accurately referred to by other names, such as nephridia.[49] In amphibians, kidneys and the urinary bladder harbour specialized parasites, monogeneans of the family Polystomatidae.[50]
The kidneys of reptiles consist of a number of lobules arranged in a broadly linear pattern. Each lobule contains a single branch of the ureter in its centre, into which the collecting ducts empty. Reptiles have relatively few nephrons compared with other amniotes of a similar size, possibly because of their lower metabolic rate.[49]
Birds have relatively large, elongated kidneys, each of which is divided into three or more distinct lobes. The lobes consists of several small, irregularly arranged, lobules, each centred on a branch of the ureter. Birds have small glomeruli, but about twice as many nephrons as similarly sized mammals.[49]
The human kidney is fairly typical of that of mammals. Distinctive features of the mammalian kidney, in comparison with that of other vertebrates, include the presence of the renal pelvis and renal pyramids and a clearly distinguishable cortex and medulla. The latter feature is due to the presence of elongated loops of Henle; these are much shorter in birds, and not truly present in other vertebrates (although the nephron often has a short intermediate segment between the convoluted tubules). It is only in mammals that the kidney takes on its classical "kidney" shape, although there are some exceptions, such as the multilobed reniculate kidneys of pinnipeds and cetaceans.[49]
Evolutionary adaptation
[edit]Kidneys of various animals show evidence of evolutionary adaptation and have long been studied in ecophysiology and comparative physiology. Kidney morphology, often indexed as the relative medullary thickness, is associated with habitat aridity among species of mammals[51] and diet (e.g., carnivores have only long loops of Henle).[38]
Society and culture
[edit]Significance
[edit]Egyptian
[edit]In ancient Egypt, the kidneys, like the heart, were left inside the mummified bodies, unlike other organs which were removed. Comparing this to the biblical statements, and to drawings of human body with the heart and two kidneys portraying a set of scales for weighing justice, it seems that the Egyptian beliefs had also connected the kidneys with judgement and perhaps with moral decisions.[52]
Hebrew
[edit]According to studies in modern and ancient Hebrew, various body organs in humans and animals served also an emotional or logical role, today mostly attributed to the brain and the endocrine system. The kidney is mentioned in several biblical verses in conjunction with the heart, much as the bowels were understood to be the "seat" of emotion – grief, joy and pain.[53] Similarly, the Talmud (Berakhoth 61.a) states that one of the two kidneys counsels what is good, and the other evil.
In the sacrifices offered at the biblical Tabernacle and later on at the temple in Jerusalem, the priests were instructed[54] to remove the kidneys and the adrenal gland covering the kidneys of the sheep, goat and cattle offerings, and to burn them on the altar, as the holy part of the "offering for God" never to be eaten.[55]
India: Ayurvedic system
[edit]In ancient India, according to the Ayurvedic medical systems, the kidneys were considered the beginning of the excursion channels system, the 'head' of the Mutra Srotas, receiving from all other systems, and therefore important in determining a person's health balance and temperament by the balance and mixture of the three 'Dosha's – the three health elements: Vatha (or Vata) – air, Pitta – bile, and Kapha – mucus. The temperament and health of a person can then be seen in the resulting color of the urine.[56]
Modern Ayurveda practitioners, a practice which is characterized as pseudoscience,[57] have attempted to revive these methods in medical procedures as part of Ayurveda Urine therapy.[58] These procedures have been called "nonsensical" by skeptics.[59]
Medieval Christianity
[edit]The Latin term renes is related to the English word "reins", a synonym for the kidneys in Shakespearean English (e.g. Merry Wives of Windsor 3.5), which was also the time when the King James Version of the Bible was translated. Kidneys were once popularly regarded as the seat of the conscience and reflection,[60][61] and a number of verses in the Bible (e.g. Ps. 7:9, Rev. 2:23) state that God searches out and inspects the kidneys, or "reins", of humans, together with the heart.[62]
History
[edit]Kidney stones have been identified and recorded about as long as written historical records exist.[63] The urinary tract including the ureters, as well as their function to drain urine from the kidneys, has been described by Galen in the second century AD.[64]
The first to examine the ureter through an internal approach, called ureteroscopy, rather than surgery was Hampton Young in 1929.[63] This was improved on by VF Marshall who is the first published use of a flexible endoscope based on fiber optics, which occurred in 1964.[63] The insertion of a drainage tube into the renal pelvis, bypassing the uterers and urinary tract, called nephrostomy, was first described in 1941. Such an approach differed greatly from the open surgical approaches within the urinary system employed during the preceding two millennia.[63]
Additional images
[edit]See also
[edit]References
[edit]Citations
[edit]- ^ "Kidneys: Anatomy, Function, Health & Conditions". Cleveland Clinic. Archived from the original on 2023-06-29. Retrieved 2023-07-13.
- ^ Zhou, Xin J.; Laszik, Zoltan G.; Nadasdy, Tibor; D'Agati, Vivette D. (2017-03-02). Silva's Diagnostic Renal Pathology. Cambridge University Press. p. 19. ISBN 978-1-316-61398-6. Archived from the original on 2023-04-04. Retrieved 2023-08-16.
- ^ Haschek, Wanda M.; Rousseaux, Colin G.; Wallig, Matthew A.; Bolon, Brad; Ochoa, Ricardo (2013-05-01). Haschek and Rousseaux's Handbook of Toxicologic Pathology. Academic Press. p. 1678. ISBN 978-0-12-415765-1.
- ^ Lote CJ (2012). Principles of Renal Physiology, 5th edition. Springer. p. 21.
- ^ Mescher AL (2016). Junqueira's Basic Histology, 14th edition. Lange. p. 393.
- ^ Lv JC, Zhang LX (2019). "Prevalence and Disease Burden of Chronic Kidney Disease". Renal Fibrosis: Mechanisms and Therapies. Advances in Experimental Medicine and Biology. Vol. 1165. pp. 3–15. doi:10.1007/978-981-13-8871-2_1. ISBN 978-981-13-8871-2. PMID 31399958. S2CID 199519437.
- ^ Cotran RS, Kumar V, Fausto N, Robbins SL, Abbas AK (2005). Robbins and Cotran pathologic basis of disease. St. Louis, MO: Elsevier Saunders. ISBN 978-0-7216-0187-8.
- ^ Kalantar-Zadeh K, McCullough PA, Agarwal SK, Beddhu S, Boaz M, Bruchfeld A, et al. (June 2021). "Nomenclature in nephrology: preserving 'renal' and 'nephro' in the glossary of kidney health and disease". Journal of Nephrology. 34 (3): 639–648. doi:10.1007/s40620-021-01011-3. PMC 8192439. PMID 33713333.
- ^ "HowStuffWorks How Your Kidney Works". 2001-01-10. Archived from the original on 2012-11-05. Retrieved 2012-08-09.
- ^ "Kidneys Location Stock Illustration". Archived from the original on 2013-09-27.
- ^ "Kidney". BioPortfolio Ltd. Archived from the original on 10 February 2008.
- ^ Glodny B, Unterholzner V, Taferner B, Hofmann KJ, Rehder P, Strasak A, Petersen J (December 2009). "Normal kidney size and its influencing factors - a 64-slice MDCT study of 1.040 asymptomatic patients". BMC Urology. 9 (1): 19. doi:10.1186/1471-2490-9-19. PMC 2813848. PMID 20030823.
- ^ Dragomir A, Hjortberg M, Romans GM. Bålens ytanatomy [Superficial anatomy of the trunk]. Section for human anatomy at the Department of Medical Biology, Uppsala University, Sweden (Report) (in Swedish).
- ^ "Renal system". Britannica. Archived from the original on 2022-05-31. Retrieved 2022-05-22.
- ^ Molina DK, DiMaio VJ (December 2012). "Normal organ weights in men: part II – the brain, lungs, liver, spleen, and kidneys". The American Journal of Forensic Medicine and Pathology. 33 (4): 368–372. doi:10.1097/PAF.0b013e31823d29ad. PMID 22182984. S2CID 32174574.
- ^ Molina DK, DiMaio VJ (September 2015). "Normal Organ Weights in Women: Part II-The Brain, Lungs, Liver, Spleen, and Kidneys". The American Journal of Forensic Medicine and Pathology. 36 (3): 182–187. doi:10.1097/PAF.0000000000000175. PMID 26108038. S2CID 25319215.
- ^ Emamian SA, Nielsen MB, Pedersen JF, Ytte L (January 1993). "Kidney dimensions at sonography: correlation with age, sex, and habitus in 665 adult volunteers". AJR. American Journal of Roentgenology. 160 (1): 83–86. doi:10.2214/ajr.160.1.8416654. PMID 8416654.
- ^ a b Boron WF (2004). Medical Physiology: A Cellular And Molecular Approach. Elsevier/Saunders. ISBN 978-1-4160-2328-9.
- ^ Clapp WL (2009). "Renal Anatomy". In Zhou XJ, Laszik Z, Nadasdy T, D'Agati VD, Silva FG (eds.). Silva's Diagnostic Renal Pathology. New York: Cambridge University Press. ISBN 978-0-521-87702-2.
- ^ Prothero, John William, ed. (2015), "Urinary system", The Design of Mammals: A Scaling Approach, Cambridge: Cambridge University Press, pp. 195–203, doi:10.1017/CBO9781316275108.016, ISBN 978-1-107-11047-2, archived from the original on 2018-06-17, retrieved 2022-06-25
- ^ Martini, Frederic; Tallitsch, Robert B.; Nath, Judi L. (2017). Human Anatomy (9th ed.). Pearson. p. 689. ISBN 978-0-13-432076-2.
- ^ a b c d e Bard J, Vize PD, Woolf AS (2003). The kidney: from normal development to congenital disease. Boston: Academic Press. p. 154. ISBN 978-0-12-722441-1. Archived from the original on 2023-08-17. Retrieved 2020-10-19.
- ^ Cheng, Xiaofeng; Zhang, Yongsheng; Chen, Ruixi; Qian, Shenghui; Lv, Haijun; Liu, Xiuli; Zeng, Shaoqun (December 2022). "Anatomical Evidence for Parasympathetic Innervation of the Renal Vasculature and Pelvis". Journal of the American Society of Nephrology. 33 (12): 2194–2210. doi:10.1681/ASN.2021111518. ISSN 1046-6673. PMC 9731635. PMID 36253054.
- ^ Schrier RW, Berl T (October 1972). "Mechanism of the antidiuretic effect associated with interruption of parasympathetic pathways". The Journal of Clinical Investigation. 51 (10): 2613–2620. doi:10.1172/JCI107079. PMC 332960. PMID 5056657.
- ^ Qais Al-Awqati; Juan A Oliver (1 February 2002). "Stem cells in the kidney". Kidney International. 61 (2): 387–395. doi:10.1046/J.1523-1755.2002.00164.X. ISSN 0085-2538. PMID 11849378. Wikidata Q34534499.
- ^ "The human proteome in kidney – The Human Protein Atlas". www.proteinatlas.org. Archived from the original on 2017-09-22. Retrieved 2017-09-22.
- ^ Uhlén M, Fagerberg L, Hallström BM, Lindskog C, Oksvold P, Mardinoglu A, et al. (January 2015). "Proteomics. Tissue-based map of the human proteome". Science. 347 (6220) 1260419. doi:10.1126/science.1260419. PMID 25613900. S2CID 802377.
- ^ Habuka M, Fagerberg L, Hallström BM, Kampf C, Edlund K, Sivertsson Å, et al. (2014-12-31). "The kidney transcriptome and proteome defined by transcriptomics and antibody-based profiling". PLOS ONE. 9 (12) e116125. Bibcode:2014PLoSO...9k6125H. doi:10.1371/journal.pone.0116125. PMC 4281243. PMID 25551756.
- ^ Carlson BM (2004). Human Embryology and Developmental Biology (3rd ed.). Saint Louis: Mosby. ISBN 978-0-323-03649-8.
- ^ Hall JE (2016). Guyton and Hall textbook of medical physiology (13th ed.). Philadelphia, PA: Elsevier Health Sciences. p. 1129. ISBN 978-0-323-38930-3.
- ^ Alpern, Robert J.; Caplan, Michael; Moe, Orson W. (2012-12-31). Seldin and Giebisch's The Kidney: Physiology and Pathophysiology. Academic Press. p. 1405. ISBN 978-0-12-381463-0. Archived from the original on 2023-07-22. Retrieved 2022-07-28.
- ^ "Urine 24-hour volume". mountsinai. Archived from the original on 21 November 2022. Retrieved 21 November 2022.
- ^ Kumaran GK, Hanukoglu I (2024). "Mapping the cytoskeletal architecture of renal tubules and surrounding peritubular capillaries in the kidney". Cytoskeleton (Hoboken). 81 (4–5): 227–237. doi:10.1002/cm.21809. PMID 37937511.
- ^ a b Le, Tao. First Aid for the USMLE Step 1 2013. New York: McGraw-Hill Medical, 2013. Print.
- ^ McNamara, J.; Worthley, L.I.G. (September 2001). "Acid-Base Balance: Part I. Physiology". Critical Care and Resuscitation. 3 (3): 181–187. doi:10.1016/s1441-2772(23)00613-0. ISSN 1441-2772. PMID 16573501.
- ^ Brown, Dennis; Bouley, Richard; Pǎunescu, Teodor G.; Breton, Sylvie; Lu, Hua A. J. (2012-05-15). "New insights into the dynamic regulation of water and acid-base balance by renal epithelial cells". American Journal of Physiology. Cell Physiology. 302 (10): C1421 – C1433. doi:10.1152/ajpcell.00085.2012. ISSN 0363-6143. PMC 3362000. PMID 22460710.
- ^ Weinstein AM (1994). "Mathematical models of tubular transport". Annual Review of Physiology. 56: 691–709. doi:10.1146/annurev.physiol.56.1.691. PMID 8010757.
- ^ a b Thomas SR (2005). "Modelling and simulation of the kidney". Journal of Biological Physics and Chemistry. 5 (2/3): 70–83. doi:10.4024/230503.jbpc.05.02.
- ^ Maton A, Hopkins J, McLaughlin CW, Johnson S, Warner MQ, LaHart D, Wright JD (1993). Human Biology and Health. Englewood Cliffs, New Jersey, USA: Prentice Hall. ISBN 978-0-13-981176-0.
- ^ Kalantar-Zadeh K, Jafar TH, Nitsch D, Neuen BL, Perkovic V (August 2021). "Chronic kidney disease" (PDF). Lancet. 398 (10302): 786–802. doi:10.1016/S0140-6736(21)00519-5. PMID 34175022. S2CID 235631509. Archived (PDF) from the original on 2022-05-17. Retrieved 2022-05-22.
- ^ "Dialysis". National Kidney Foundation. 2015-12-24. Archived from the original on 2017-09-26. Retrieved 8 November 2017.
- ^ Sample I (2008-02-19). "How many people have four kidneys?". The Guardian. London. Archived from the original on 2016-08-17. Retrieved 2016-12-19.
- ^ "Kidneys Fail, Girl Survives with Spare Parts". Abcnews.go.com. 2010-05-18. Archived from the original on 2010-05-21. Retrieved 2011-01-03.
- ^ Novick AC, Gill IS, Klein EA, Rackley R, Ross JH, Jones JS (2006). "Ureteropelvic Junction Obstruction". Operative Urology at the Cleveland Clinic. Vol. 8. Totowa, NJ: Humana Press. pp. S102 – S108. doi:10.1007/978-1-59745-016-4_16. ISBN 978-1-58829-081-6. PMC 4869439.
{{cite book}}:|journal=ignored (help) - ^ Gaitonde DY (15 December 2017). "Chronic Kidney Disease: Detection and Evaluation". Am Fam Physician. 12 (96): 776–783. Archived from the original on 26 February 2021. Retrieved 1 March 2021.
- ^ a b Post TW, Rose BD (December 2012). Curhan GC, Sheridan AM (eds.). "Diagnostic Approach to the Patient With Acute Kidney Injury (Acute Kidney Failure) or Chronic Kidney Disease". www.uptodate.com. Archived from the original on 2015-11-10. Retrieved 2016-12-19.
- ^ "KDIGO 2012 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease". Kidney Int Suppl. 3: 1–150. 2013. Archived from the original on 2019-05-01. Retrieved 2021-01-25.
- ^ a b Content initially copied from: Hansen KL, Nielsen MB, Ewertsen C (December 2015). "Ultrasonography of the Kidney: A Pictorial Review". Diagnostics. 6 (1): 2. doi:10.3390/diagnostics6010002. PMC 4808817. PMID 26838799. (CC-BY 4.0) Archived 2017-10-16 at the Wayback Machine
- ^ a b c d e Romer AS, Parsons TS (1977). The Vertebrate Body. Philadelphia, PA: Holt-Saunders International. pp. 367–376. ISBN 978-0-03-910284-5.
- ^ Theunissen M, Tiedt L, Du Preez LH (2014). "The morphology and attachment of Protopolystoma xenopodis (Monogenea: Polystomatidae) infecting the African clawed frog Xenopus laevis". Parasite. 21: 20. doi:10.1051/parasite/2014020. PMC 4018937. PMID 24823278.
- ^ al-Kahtani MA, Zuleta C, Caviedes-Vidal E, Garland T (2004). "Kidney mass and relative medullary thickness of rodents in relation to habitat, body size, and phylogeny" (PDF). Physiological and Biochemical Zoology. 77 (3): 346–365. CiteSeerX 10.1.1.407.8690. doi:10.1086/420941. PMID 15286910. S2CID 12420368. Archived from the original (PDF) on 2010-06-17. Retrieved 2009-03-28.
- ^ Salem ME, Eknoyan G (1999). "The kidney in ancient Egyptian medicine: where does it stand?". American Journal of Nephrology. 19 (2): 140–147. doi:10.1159/000013440. PMID 10213808. S2CID 35305403.
- ^ "Body Part Metaphors in Biblical Hebrew by David Steinberg". March 22, 2003. Archived from the original on March 22, 2003. Retrieved July 21, 2019.
- ^ Leviticus 3: 4, 10 and 15
- ^ ie Deut 3:4,9,10,15... or the Babylonian Talmud, Bechorot (39a) Ch6:Tr2...
- ^ "What is Vata Dosha? Tips and diet for balancing vata | CA College of Ayurveda". www.ayurvedacollege.com. 7 April 2010. Archived from the original on 9 November 2019. Retrieved July 21, 2019.
- ^ List of topics characterized as pseudoscience, according to the American Medical Association's Report 12 of the Council of Scientific Affairs (A-97) and claims by skeptics Archived 2016-08-10 at the Wayback Machine ('The Skeptics Dictionary' website)
- ^ Sangu PK, Kumar VM, Shekhar MS, Chagam MK, Goli PP, Tirupati PK (January 2011). "A study on Tailabindu pariksha – An ancient Ayurvedic method of urine examination as a diagnostic and prognostic tool". AYU. 32 (1): 76–81. doi:10.4103/0974-8520.85735. PMC 3215423. PMID 22131762.
- ^ Barrett S. "A Few Thoughts on Ayurvedic Mumbo-Jumbo". Archived from the original on 2020-09-29. Retrieved 2022-05-22. M.D, head of the National Council Against Health Fraud NGO and owner of the QuackWatch website.
- ^ Ramsey P, Jonsen AR, May WF (2002). The Patient as Person: Explorations in Medical Ethics (Second ed.). New Haven: Yale University Press. p. 60. ISBN 978-0-300-09396-4.
- ^ Eknoyan G, Marketos SG, De Santo NG, eds. (January 1997). History of Nephrology 2. Karger Medical and Scientific Publishers. p. 235. ISBN 978-3-8055-6499-1. International Association for the History of Nephrology Congress, Reprint of American Journal of Nephrology; v. 14, no. 4–6, 1994.
- ^ intertextual.bible/text/revelation-2.23-berakhot-119.29, archived from the original on 2022-12-15, retrieved 2022-12-15
- ^ a b c d Tefekli A, Cezayirli F (November 2013). "The history of urinary stones: in parallel with civilization". TheScientificWorldJournal. 2013 423964. doi:10.1155/2013/423964. PMC 3856162. PMID 24348156.
- ^ Nahon I, Waddington G, Dorey G, Adams R (2011). "The history of urologic surgery: from reeds to robotics". Urologic Nursing. 31 (3): 173–180. doi:10.7257/1053-816X.2011.31.3.173. PMID 21805756.
General and cited references
[edit]- Barrett KE, Barman SM, Yuan JX, Brooks H (2019). Ganong's review of medical physiology (26th ed.). New York. ISBN 978-1-260-12240-4. OCLC 1076268769.
{{cite book}}: CS1 maint: location missing publisher (link)
External links
[edit]Kidney
View on GrokipediaAnatomy
Gross anatomy
The kidneys are paired retroperitoneal organs located on the posterior abdominal wall, positioned between the T12 and L3 vertebral levels, with the right kidney slightly inferior to the left due to the influence of the liver.[2] They lie lateral to the vertebral column, anterior to the quadratus lumborum and psoas major muscles, and are partially protected by the 11th and 12th ribs.[2] Each kidney is bean-shaped, featuring a convex lateral border and a concave medial border, with typical adult dimensions of approximately 10-12 cm in length, 5-7 cm in width, and 3-5 cm in thickness.[2] The average weight is about 150-170 g per kidney in adults, with the left kidney typically 10 g heavier than the right and males having slightly heavier kidneys than females.[2] Externally, the medial concavity forms the hilum, a slit-like opening through which the renal artery enters, the renal vein and ureter exit, and nerves and lymphatics pass.[2] The hilum opens into the renal sinus, a central cavity lined by extensions of the fibrous capsule and filled with adipose tissue, calyces, and the renal pelvis.[2] The kidney is enclosed by a thin, fibrous renal capsule that adheres closely to its surface, surrounded by perirenal fat for cushioning and the renal fascia (Gerota's fascia anteriorly and Zuckerkandl's posteriorly) that anchors it to the abdominal wall.[2] Internally, a coronal section reveals an outer renal cortex and an inner renal medulla, with the medulla organized into 8-18 renal pyramids that project their apices (renal papillae) into the renal sinus.[2] Between the pyramids are the renal columns of Bertin, which are extensions of cortical tissue.[2] Urine from the pyramids drains through 7-9 minor calyces into 2-3 major calyces, which converge at the funnel-shaped renal pelvis and continue as the ureter.[2] Superiorly, each kidney is capped by an adrenal (suprarenal) gland, which is separated from the kidney by a thin layer of fat but functionally independent.[2] Anteriorly, the right kidney relates to the liver, duodenum, and ascending colon, while the left relates to the spleen, stomach, pancreas, and descending colon; posteriorly, both contact the diaphragm, transverse fascia, and subcostal vessels and nerves.[2] Anatomical variations include supernumerary kidneys, which are extremely rare additional functional kidneys arising from independent metanephric blastemas, with fewer than 100 cases reported in the medical literature.[3] Ectopic positions, such as pelvic kidneys, also occur rarely (about 1 in 12,000 births), where the kidney fails to ascend to its normal retroperitoneal location during development.[2]Microscopic anatomy
The nephron serves as the functional unit of the kidney, consisting of a renal corpuscle and an associated renal tubule.[4] The renal corpuscle, located in the renal cortex, comprises the glomerulus—a network of fenestrated capillaries—and Bowman's capsule, a double-walled epithelial cup that envelops the glomerulus.[4] Podocytes, specialized epithelial cells of Bowman's capsule, feature interdigitating foot processes that form filtration slits, contributing to the glomerular filtration barrier alongside the glomerular basement membrane (GBM).[5] The GBM is a specialized extracellular matrix composed primarily of type IV collagen, laminin, nidogen, and heparan sulfate proteoglycans, providing structural support and selective permeability.[5] The renal tubule extends from Bowman's capsule and includes the proximal convoluted tubule (PCT), loop of Henle, distal convoluted tubule (DCT), and collecting duct.[4] The PCT, lined by cuboidal epithelial cells with a prominent brush border of microvilli, occupies the renal cortex.[6] The loop of Henle descends into the medulla and ascends back to the cortex, featuring thin-walled segments in the descending and ascending limbs.[4] The DCT, with its cuboidal epithelium and fewer microvilli, connects to the collecting duct, which converges with others to form papillary ducts draining into the renal pelvis.[4] Nephrons exhibit regional variations: cortical nephrons, comprising about 85% of the total, have short loops of Henle confined mostly to the outer medulla, while juxtamedullary nephrons, located near the corticomedullary junction, possess long loops extending deep into the inner medulla.[7] These differences influence the structural organization of the renal medulla.[6] The juxtaglomerular apparatus (JGA), situated at the vascular pole of the renal corpuscle, regulates renal blood flow and includes three main components: juxtaglomerular cells (modified smooth muscle cells in the afferent arteriole that store renin), the macula densa (a plaque of tall, closely packed cells in the DCT wall sensing tubular fluid composition), and extraglomerular mesangial cells (supportive cells bridging the afferent and efferent arterioles).[8] Supporting tissues in the kidney include interstitial cells, which are fibroblast-like cells residing in the renal interstitium, providing structural support and producing extracellular matrix components.[2] Peritubular capillaries, arising from efferent arterioles, closely surround the cortical tubules to facilitate exchange, while vasa recta—specialized straight capillaries—encircle the loops of Henle and collecting ducts in the medulla, maintaining the medullary osmotic gradient.[6] Histological examination of kidney tissues often employs specific staining techniques to highlight structural details; for instance, periodic acid-Schiff (PAS) staining selectively visualizes basement membranes and brush borders by reacting with carbohydrate moieties in glycoproteins and glycoconjugates.[9]Vascular supply
The kidneys receive their arterial blood supply primarily from the renal arteries, which originate from the lateral aspect of the abdominal aorta at the level of the L1 or L2 vertebral interspace.[10] Each renal artery enters the kidney at the hilum and branches into anterior and posterior divisions, which further divide into segmental arteries supplying specific regions of the kidney.[2] These segmental arteries give rise to interlobar arteries that ascend between the renal pyramids in the renal columns, followed by arcuate arteries that arch over the bases of the pyramids at the corticomedullary junction.[2] From the arcuate arteries, interlobular arteries extend radially into the cortex, and the smallest branches, the afferent arterioles, deliver blood to the glomerular capillaries within each nephron.[2] Venous drainage follows a parallel but reversed path to the arterial supply. Blood exits the glomeruli via efferent arterioles, which lead into peritubular capillaries surrounding the cortical nephrons or vasa recta in the medullary regions.[2] These capillaries converge into venules that join the interlobular veins, arcuate veins, and interlobar veins, ultimately forming the main renal vein at the hilum.[11] The renal veins drain directly into the inferior vena cava, with the left renal vein being longer and crossing anterior to the aorta.[11] Lymphatic vessels in the kidney originate as blind-ended capillaries in the cortical interstitium and follow a drainage pattern similar to the veins, collecting in hilar lymphatics before ascending to the lumbar lymph nodes and ultimately to the cisterna chyli.[12] The renal vasculature maintains stable blood flow through intrinsic autoregulatory mechanisms involving structural elements. The myogenic response occurs in the smooth muscle of the afferent arteriole wall, which contracts in response to increased pressure to prevent excessive flow.[13] Tubuloglomerular feedback relies on the structural apposition of the macula densa cells in the distal tubule to the vascular pole of the glomerulus via the extraglomerular mesangium, allowing sensing of tubular fluid composition to influence afferent arteriole tone.[13] Anatomical variations in the renal vasculature are common, with approximately 25% of individuals having multiple renal arteries, including accessory or polar arteries that arise separately from the aorta or iliac arteries.[14] These variations can affect surgical planning but do not typically impair function. In cases of renal ischemia, such as from main renal artery occlusion, collateral circulation may develop through capsular, ureteral, gonadal, and adrenal arterial networks to supply the kidney parenchyma.[15]Neural supply
The kidney receives neural innervation primarily through the renal plexus, a network of autonomic and sensory nerves that surrounds the renal artery and enters the organ at the hilum. This plexus is formed by contributions from the celiac, aorticorenal, and intermesenteric ganglia, as well as lumbar splanchnic nerves originating from the thoracic spinal cord segments T10 to L1.[16][17] Sympathetic innervation dominates the efferent supply to the kidney, arising from preganglionic fibers in the intermediolateral cell column of the spinal cord at levels T10 to L1, which synapse in the celiac and aorticorenal ganglia. Postganglionic sympathetic fibers, releasing norepinephrine, travel via the renal plexus to target renal blood vessels, juxtaglomerular cells, and tubules, mediating vasoconstriction of arterioles to regulate renal blood flow and glomerular filtration rate. These nerves also stimulate renin release from juxtaglomerular cells via β1-adrenergic receptors and enhance sodium reabsorption in the proximal and distal tubules through α1-adrenergic receptors.[17][18][19] Parasympathetic innervation of the kidney is limited and controversial, with some anatomical evidence indicating minor contributions from preganglionic fibers of the vagus nerve that may reach the renal plexus and supply cholinergic nerves to the renal vasculature and pelvis. These fibers potentially play a minor role in modulating secretion and vasodilation via acetylcholine receptors on endothelial and smooth muscle cells, though no robust functional impact has been consistently demonstrated.[20][21] Sensory afferent innervation originates mainly from mechanoreceptors and chemoreceptors in the renal pelvis and cortex, with the highest density in the pelvic region, and projects to the spinal cord dorsal horn at levels T10 to L1 via the renal plexus and splanchnic nerves. These unmyelinated C-fibers and thinly myelinated Aδ-fibers convey sensations of pain, such as in renal colic, and detect stretch or distension to elicit reflexes, including nociceptive responses that radiate to the flanks and abdomen.[16][22][23] A key reflex arc involving renal innervation is the renorenal reflex, where activation of afferent nerves in one kidney—such as by increased pelvic pressure from mechanoreceptors—inhibits efferent sympathetic activity in the contralateral kidney, promoting sodium excretion and maintaining fluid balance. This ipsilateral excitatory and contralateral inhibitory response helps coordinate bilateral renal function.[24][25]Physiology
Glomerular filtration
Glomerular filtration is the initial process in urine formation, where blood plasma is ultrafiltered across the glomerular capillaries into Bowman's space, producing a cell-free filtrate that enters the renal tubules.[26] This process occurs in the glomerulus, a tuft of capillaries within the Bowman's capsule of each nephron, and is driven by hemodynamic forces that favor the movement of fluid from the blood into the urinary space.[13] The glomerular filtration barrier consists of three layered structures that provide selective permeability: the fenestrated endothelium of the glomerular capillaries, the glomerular basement membrane (GBM), and the filtration slits formed by podocyte foot processes.[27] The fenestrated endothelium features pores approximately 70-100 nm in diameter, allowing passage of water and solutes while restricting larger blood components.[28] The GBM, a gel-like extracellular matrix composed primarily of laminin, type IV collagen, and proteoglycans, further refines filtration by its negatively charged surface, which repels anionic molecules.[5] Podocyte slit diaphragms, bridged by proteins like nephrin, form narrow slits about 25-30 nm wide, serving as the final barrier to prevent passage of larger macromolecules.[27] Filtration across this barrier is governed by Starling forces, which determine the net movement of fluid based on hydrostatic and oncotic pressure gradients.[26] The primary driving force is the glomerular capillary hydrostatic pressure, approximately 55 mmHg, which exceeds the opposing hydrostatic pressure in Bowman's space (about 15 mmHg) and the colloid oncotic pressure in the glomerular capillaries (around 28 mmHg at the afferent arteriole, rising to 35 mmHg at the efferent arteriole due to water filtration).[26] The net filtration pressure thus averages about 17 mmHg along the capillary length, promoting ultrafiltration while oncotic pressure increases progressively to oppose further filtration near the efferent end.[13] The glomerular filtration rate (GFR) quantifies the volume of filtrate produced per unit time and is normally about 125 mL/min in healthy adults, representing roughly 20% of the renal plasma flow.[26] GFR is calculated using the clearance of an ideal marker like inulin, which is freely filtered but neither reabsorbed nor secreted, via the formula: where is the urine inulin concentration, is the urine flow rate, and is the plasma inulin concentration.[29] The filtration fraction, defined as GFR divided by renal plasma flow, is typically 20%, indicating that one-fifth of plasma entering the glomerulus is filtered, with the remainder exiting via the efferent arteriole.[13] The filtration barrier exhibits selective permeability, allowing unrestricted passage of water, ions, glucose, and other small molecules (up to about 69 kDa) while retaining proteins like albumin and all cellular elements.[27] This selectivity arises from both size restrictions and charge repulsion, as the negatively charged glycocalyx on endothelial cells, GBM heparan sulfate proteoglycans, and podocyte components deter filtration of similarly charged plasma proteins.[30] GFR is influenced by renal plasma flow and the resistance of afferent and efferent arterioles, which modulate glomerular capillary hydrostatic pressure.[13] Increased renal plasma flow enhances filtration by delivering more fluid to the glomeruli, while constriction of the afferent arteriole reduces inflow and thus GFR, and efferent arteriole constriction raises glomerular pressure to potentially increase filtration.[31] These hemodynamic adjustments help maintain stable filtration under varying conditions.[13]Tubular reabsorption and secretion
Tubular reabsorption and secretion are essential processes in the renal tubules that modify the glomerular filtrate by reclaiming vital substances and eliminating waste or xenobiotics, ensuring homeostasis of electrolytes, water, and nutrients.[32] These processes occur along the nephron segments—proximal tubule, loop of Henle, distal convoluted tubule, and collecting duct—via active and passive transport mechanisms driven by electrochemical gradients and ATP hydrolysis.[33] Approximately 99% of the filtered water and solutes are reabsorbed, with the remainder forming urine.[32] In the proximal tubule, the primary site of bulk reabsorption, about 65% of filtered sodium (Na⁺) and water are reclaimed isosmotically, along with nearly all glucose and amino acids.[33] The basolateral Na⁺/K⁺-ATPase pump extrudes Na⁺ in exchange for K⁺ using ATP, establishing a low intracellular Na⁺ concentration that drives apical entry via secondary active transporters.[33] Glucose enters via sodium-glucose linked transporters (SGLT2 in early segments and SGLT1 in later), accounting for 97% and the remainder of reabsorption, respectively, preventing glucosuria under normal conditions.[33] Amino acids are similarly reabsorbed through Na⁺-coupled cotransporters like B⁰AT1, recovering over 80% of the filtered load.[33] Bicarbonate (HCO₃⁻) reabsorption, comprising 70–90% of the filtered amount, involves apical Na⁺/H⁺ exchanger (NHE3) secreting H⁺, which combines with filtered HCO₃⁻ to form CO₂ and H₂O via luminal carbonic anhydrase IV; the CO₂ diffuses into cells for regeneration of HCO₃⁻ by intracellular carbonic anhydrase II.[33] The loop of Henle fine-tunes reabsorption and establishes the medullary osmotic gradient. In the thick ascending limb, the Na⁺/K⁺/2Cl⁻ cotransporter (NKCC2) reabsorbs 25–30% of filtered NaCl, operating with a 1:1:2 stoichiometry and powered by the Na⁺ gradient from basolateral Na⁺/K⁺-ATPase.[34] This active transport creates a lumen-positive potential that drives paracellular reabsorption of cations like Ca²⁺ and Mg²⁺, while the impermeability of this segment to water dilutes the filtrate.[32] The countercurrent multiplier system, facilitated by NKCC2 activity, generates hyperosmolarity in the medullary interstitium, essential for subsequent urine concentration.[35] In the distal convoluted tubule and collecting duct, regulated reabsorption adjusts to physiological needs. Principal cells in the cortical collecting duct reabsorb Na⁺ via apical epithelial Na⁺ channels (ENaC), stimulated by aldosterone, with basolateral Na⁺/K⁺-ATPase maintaining the gradient; this process enhances K⁺ secretion through apical ROMK channels.[32] Type A intercalated cells secrete H⁺ via apical vacuolar H⁺-ATPase and H⁺/K⁺-ATPase, reclaiming K⁺ and contributing to acid-base balance, while type B cells perform the reverse for alkalosis correction.[36] Water reabsorption here is vasopressin-dependent via aquaporin-2 channels.[32] Secretion primarily occurs in the proximal tubule via organic anion transporters (OAT1, OAT3) and organic cation transporters (OCT2), which actively export drugs, toxins, and metabolites from blood into the filtrate using the Na⁺ gradient and ATP-dependent mechanisms.[37] H⁺ secretion in distal segments, as noted, aids in organic acid handling and pH regulation.[36] Transport pathways include transcellular (across cell membranes via carriers and pumps) and paracellular (through tight junctions driven by electrochemical gradients) routes; for instance, Na⁺ and glucose use transcellular paths in the proximal tubule, while Cl⁻ and water follow paracellularly in the thick ascending limb.[38] Energy demands are met primarily by ATP for primary active pumps like Na⁺/K⁺-ATPase, which consumes ~40% of renal oxygen, with secondary active transport leveraging the resulting ion gradients for efficiency.[32]Urine concentration and excretion
The urine concentration mechanism in the kidney relies on the countercurrent multiplier system established by the loops of Henle and the countercurrent exchange in the vasa recta, which together create a hyperosmotic gradient in the renal medulla. In the descending limb of the loop of Henle, water is reabsorbed passively due to the increasing interstitial osmolality, while the ascending limb actively transports sodium chloride out of the tubule, making the tubular fluid hypoosmotic without water following. This process multiplies the osmotic gradient from the cortex (approximately 300 mOsm/L) to the inner medulla, reaching up to 1200 mOsm/L at the papillary tip in humans. The vasa recta, parallel to the loops, function as a countercurrent exchanger, preserving the medullary hyperosmolality by minimizing solute washout through blood flow.[39][40] In the collecting ducts, which traverse this gradient, the final concentration of urine occurs through regulated water reabsorption. Principal cells in the collecting duct express aquaporin-2 (AQP2) water channels on their apical membrane, whose insertion is stimulated by antidiuretic hormone (ADH, or vasopressin). ADH binds to V2 receptors, activating a cAMP-protein kinase A pathway that translocates AQP2 vesicles to the apical surface, increasing water permeability and allowing osmosis of water into the hypertonic interstitium. Basolateral aquaporins AQP3 and AQP4 facilitate water exit, enabling the tubule fluid to equilibrate with the medullary osmolality, often concentrating urine to 1200 mOsm/L or more. Without ADH, AQP2 is internalized, rendering the duct impermeable to water and producing dilute urine.[41][42] Urea recycling further enhances the medullary osmotic gradient. Urea, a major waste product, is reabsorbed in the proximal tubule and inner medullary collecting duct via urea transporters UT-A1 and UT-A3, which are upregulated by ADH. This reabsorbed urea diffuses into the interstitium, contributing up to 50% of the inner medullary osmolality, and is then taken up by the descending vasa recta or thin descending limbs of juxtamedullary nephrons for recirculation. This process traps urea in the medulla, amplifying the countercurrent system's effectiveness without additional energy expenditure.[43][44] Under normal conditions, the kidneys produce approximately 1-2 liters of urine per day in adults, representing the net excretion after reabsorption of over 99% of the glomerular filtrate. Urine is about 95% water, with the remainder consisting primarily of urea (around 2%), creatinine (0.1%), and various ions such as sodium, potassium, chloride, and sulfate. This composition reflects the kidney's role in eliminating metabolic wastes while conserving essential solutes, with output varying based on hydration status and dietary intake.[45][46] The elimination of urine occurs via the micturition reflex, triggered when bladder volume reaches 300-400 mL. Stretch receptors in the bladder wall signal the pontine micturition center, leading to parasympathetic activation that contracts the detrusor muscle (smooth muscle of the bladder wall) while inhibiting sympathetic input to relax the internal urethral sphincter. Voluntary control via somatic nerves relaxes the external urethral sphincter, allowing coordinated expulsion.[47][48] Waste excretion, particularly nitrogenous products like creatinine, serves as a marker of renal function. Creatinine clearance provides a clinical proxy for glomerular filtration rate (GFR), calculated as: where is urine creatinine concentration, is urine flow rate, and is plasma creatinine concentration. Normal values approximate 90-120 mL/min/1.73 m², reflecting the kidney's efficiency in clearing freely filtered wastes like creatinine, which is produced endogenously at a constant rate and minimally reabsorbed or secreted.[49][50]Endocrine functions
The kidneys function as endocrine organs by synthesizing and secreting hormones that regulate systemic processes such as blood pressure, erythropoiesis, mineral homeostasis, and aging.[51] These humoral factors are produced by specific renal cell types and respond to physiological cues like hypoxia or electrolyte imbalances.[52] Renin, a key enzyme, is produced and stored in juxtaglomerular cells located in the media of afferent arterioles at the glomerular entrance.[51] Upon release, renin cleaves circulating angiotensinogen, primarily from the liver, to form angiotensin I, thereby initiating the renin-angiotensin-aldosterone system (RAAS).[51] This mechanism contributes to blood pressure regulation, as detailed in the relevant section.[52] Erythropoietin (EPO) is secreted by peritubular interstitial fibroblast-like cells, primarily in the renal cortex and outer medulla, in response to hypoxia.[51] Hypoxia-inducible factor-2 (HIF-2) drives EPO gene transcription, leading to increased production that stimulates red blood cell formation in the bone marrow.[51] Calcitriol, or 1,25-dihydroxyvitamin D, is activated in the proximal tubule through hydroxylation of 25-hydroxyvitamin D by the enzyme 1-α-hydroxylase in mitochondrial membranes.[51] The precursor 25-hydroxyvitamin D is initially formed via 25-hydroxylation in the liver, with the kidney performing the final 1-α-hydroxylation step to generate the active hormone.[53][54] Calcitriol promotes intestinal calcium absorption and renal calcium reabsorption by activating vitamin D receptors.[51] Klotho, an anti-aging hormone, is primarily expressed and secreted by distal tubule epithelial cells in both membrane-bound and soluble forms.[55] The soluble form circulates systemically, functioning as a co-receptor for fibroblast growth factor 23 (FGF23) to modulate phosphate and calcium handling.[51] Prostaglandins, such as prostaglandin E2 (PGE2) and prostacyclin (PGI2), are synthesized in the renal medulla via the cyclooxygenase (COX) pathway and act as local vasodilators to maintain medullary blood flow.[52] These lipid mediators are produced by interstitial cells and contribute to renal vascular tone regulation through autocrine and paracrine effects.[56]Blood pressure regulation
The kidneys play a central role in maintaining systemic blood pressure through integrated mechanisms that respond to changes in perfusion pressure, extracellular fluid volume, and neural inputs. These processes ensure long-term homeostasis by adjusting renal blood flow, glomerular filtration rate (GFR), and sodium excretion, thereby influencing vascular resistance and fluid balance. Key pathways include hormonal cascades, local feedback loops, and neural reflexes, which collectively prevent excessive fluctuations in arterial pressure.[57] A primary mechanism is the renin-angiotensin-aldosterone system (RAAS), which is activated when renal perfusion pressure decreases, as detected by baroreceptors in the afferent arterioles of the juxtaglomerular apparatus. Renin, an enzyme secreted by juxtaglomerular cells, cleaves circulating angiotensinogen (produced by the liver) into angiotensin I, which is then converted to angiotensin II by angiotensin-converting enzyme (ACE), primarily in the lungs. Angiotensin II exerts direct vasoconstriction on systemic arterioles, increasing peripheral resistance and elevating blood pressure; it also stimulates the release of aldosterone from the adrenal cortex, which promotes sodium reabsorption in the distal tubules and collecting ducts of the kidney, thereby expanding extracellular fluid volume and further supporting blood pressure. Additionally, angiotensin II enhances sympathetic outflow and thirst, contributing to volume retention. This cascade restores pressure but can lead to sustained elevation if dysregulated.[58][59] Complementing RAAS, pressure natriuresis provides a direct physical link between arterial pressure and sodium excretion, serving as a long-term controller of extracellular fluid volume. As mean arterial pressure rises, renal interstitial hydrostatic pressure increases, reducing sodium reabsorption in the tubules and promoting natriuresis (sodium excretion in urine), which decreases plasma volume and lowers blood pressure. This relationship can be expressed as: where is urinary sodium excretion, is a proportionality constant reflecting renal sodium handling efficiency, is mean arterial pressure, and is the pressure below which natriuresis does not occur. This mechanism operates independently of hormonal input in the steady state, ensuring that sodium balance adjusts to maintain pressure.[57][60] Tubuloglomerular feedback (TGF) offers fine-tuned short-term regulation of GFR and renal blood flow to stabilize glomerular pressure against fluctuations. At the macula densa cells in the distal tubule, increased NaCl delivery (due to elevated GFR) is sensed via the Na-K-2Cl cotransporter, triggering adenosine release, which constricts the afferent arteriole and reduces GFR. Conversely, low NaCl sensing dilates the arteriole, increasing GFR. This feedback loop, oscillating at approximately 30 mHz, maintains constant tubular flow and protects against pressure-induced hyperfiltration.[61][62] Atrial natriuretic peptide (ANP), released from cardiac atria in response to volume expansion, counteracts RAAS by promoting natriuresis and diuresis while inhibiting renin and aldosterone secretion. ANP dilates afferent arterioles and constricts efferent ones, increasing GFR, and directly suppresses Na+ reabsorption in the collecting ducts via cGMP-mediated pathways, reducing blood volume and pressure. This opposition to RAAS prevents excessive vasoconstriction during high-pressure states.[63][64] Renal nerves, modulated by systemic baroreceptor reflexes, further integrate blood pressure control. Baroreceptors in the carotid sinus and aortic arch detect pressure changes and reflexively adjust renal sympathetic nerve activity (RSNA); hypotension increases RSNA, enhancing renin release and Na+ retention, while hypertension decreases RSNA, promoting natriuresis. Local renal baroreceptors in the juxtaglomerular apparatus directly sense afferent arteriolar pressure to modulate renin secretion, linking neural and hormonal pathways for rapid and sustained regulation.[65][66]Acid-base homeostasis
The kidneys play a central role in maintaining acid-base homeostasis by regulating plasma bicarbonate (HCO₃⁻) concentration and excreting excess hydrogen ions (H⁺), thereby stabilizing blood pH around 7.40. This involves two primary processes: reabsorption of filtered HCO₃⁻ to prevent its loss and generation of new HCO₃⁻ through H⁺ excretion, primarily as ammonium (NH₄⁺) and titratable acids. Under normal conditions, the kidneys handle a daily acid load of approximately 1 mEq/kg body weight, equivalent to about 70 mEq/day in a 70-kg adult, derived from dietary intake and endogenous metabolism.[67] Bicarbonate reabsorption occurs predominantly in the proximal tubule, where about 90% of the filtered load (roughly 4,500 mmol/day) is reclaimed. This process relies on the apical Na⁺/H⁺ exchanger (NHE3), which secretes H⁺ into the tubular lumen in exchange for Na⁺, combining with filtered HCO₃⁻ to form carbonic acid (H₂CO₃). Carbonic anhydrase (CA) enzymes, both luminal (CA IV) and cytosolic (CA II), catalyze the rapid conversion: The CO₂ diffuses into the tubular cell, where it is rehydrated by intracellular CA to regenerate HCO₃⁻, which exits basolaterally via the Na⁺/HCO₃⁻ cotransporter (NBC1). The remaining 10% is fine-tuned in the distal nephron through similar mechanisms but at a lower capacity.[68][67] Acid excretion primarily involves the production and secretion of NH₄⁺ and titratable acids to buffer and eliminate H⁺. In the proximal tubule, glutamine is deaminated by glutaminase to form NH₄⁺ and α-ketoglutarate, which is metabolized to generate new HCO₃⁻; this NH₄⁺ is secreted into the lumen via NHE3 and partially reabsorbed in the thick ascending limb via the Na⁺-K⁺-2Cl⁻ cotransporter (NKCC2), before final excretion in the collecting duct. Under basal conditions, NH₄⁺ accounts for 30–50 mmol/day of H⁺ excretion, increasing substantially during acidosis. Titratable acids, such as H₂PO₄⁻ (formed by H⁺ buffering phosphate), contribute an additional 20–40 mmol/day, representing about one-third to one-half of net acid excretion.[69][68] New HCO₃⁻ generation occurs mainly in the distal tubule's α-intercalated cells, where vacuolar H⁺-ATPase pumps H⁺ into the lumen, creating a new HCO₃⁻ molecule from intracellular CO₂ and H₂O via CA; this HCO₃⁻ is transported basolaterally via the Cl⁻/HCO₃⁻ exchanger (AE1). This process is crucial for net acid elimination beyond filtered HCO₃⁻ reabsorption. In response to acidosis, the kidneys enhance H⁺ excretion by upregulating ammoniagenesis, NH₄⁺ transport (e.g., via Rh glycoproteins), and H⁺-ATPase activity, potentially increasing net acid excretion to over 200 mmol/day; conversely, alkalosis suppresses these mechanisms to reduce H⁺ loss and promote HCO₃⁻ excretion. Aldosterone briefly stimulates distal H⁺ secretion, linking to broader tubular transport.[70][67][69]Osmoregulation
The kidneys play a central role in osmoregulation by maintaining plasma osmolality within a narrow range of approximately 285–295 mOsm/kg through the precise balance of water reabsorption and excretion relative to solutes.[71] This process ensures cellular function and volume stability, primarily via adjustments in the collecting ducts where water permeability is hormonally regulated.[72] Disruptions in this balance can lead to hypo- or hyperosmolality, prompting compensatory renal responses integrated with systemic signals.[73] Antidiuretic hormone (ADH), also known as vasopressin, is the primary regulator of renal water handling in response to changes in plasma osmolality.[71] Secreted from the posterior pituitary, ADH binds to V2 receptors on the basolateral membrane of principal cells in the collecting duct, activating a cAMP-mediated pathway that promotes the insertion of aquaporin-2 (AQP2) water channels into the apical membrane.[73] This increases water permeability, allowing osmotic equilibration with the hypertonic medullary interstitium and reducing urine volume to conserve water.[72] Concurrently, osmoreceptors in the hypothalamus detect elevations in plasma osmolality (typically a 1–2% increase) and trigger both ADH release and the sensation of thirst to stimulate water intake, thereby restoring osmolality through both renal and behavioral mechanisms.[74] In hypotonic states, suppressed ADH secretion minimizes AQP2 insertion, facilitating water excretion.[75] The kidney's ability to adjust urine osmolality is quantified by free water clearance (), which measures the rate of solute-free water excretion or reabsorption.[76] It is calculated as , where is urine flow rate and is osmolar clearance (urine osmolality × divided by plasma osmolality).[77] A positive indicates excretion of dilute urine during hypo-osmolality, while a negative value (often denoted as ) reflects free water reabsorption in hyperosmolar conditions.[76] In response to hypoosmolality, the kidneys produce maximally dilute urine with osmolality below 100 mOsm/L, whereas hyperosmolality elicits concentrated urine exceeding 1200 mOsm/L in humans.[39] These extremes depend on the countercurrent multiplier system, where NaCl reabsorption in the thick ascending limb establishes the initial gradient, augmented by urea recycling in the inner medulla.[78] The medullary osmotic gradient, essential for urine concentration, is primarily generated by NaCl in the outer medulla and urea in the inner medulla, reaching up to 1200 mOsm/L at the papillary tip.[40] Urea contributes significantly by being reabsorbed from the collecting duct under ADH influence via urea transporters (UT-A1/3), recycling into the interstitium to amplify the gradient without additional energy expenditure.[79] NaCl, actively transported out of the ascending limb, provides the foundational hypertonicity that drives water reabsorption in the descending limb and collecting duct.[78] Disorders such as diabetes insipidus impair osmoregulation due to ADH deficiency (central) or renal resistance (nephrogenic), resulting in excessive dilute urine output and hypernatremia if water access is limited.[80] In central diabetes insipidus, insufficient ADH prevents AQP2 insertion, abolishing the kidney's concentrating ability.[81]Development and Genetics
Embryonic development
The development of the human kidney occurs through three successive and overlapping stages derived from the intermediate mesoderm of the nephrogenic cord: the pronephros, mesonephros, and metanephros.[82][83][84] These stages represent a progression from transient, non-functional structures to the permanent organ, with the process beginning around week 4 of gestation.[85][86] The pronephros is the earliest and most rudimentary stage, forming in the cervical region during the 4th week of embryonic development. It consists of approximately 6 to 10 pairs of nephrotomes that connect to the pronephric duct, but it is non-functional in humans and rapidly regresses by the end of the 4th week, serving primarily as an inductive structure for subsequent stages.[82][83][84][85] The mesonephros follows as an intermediate stage, developing caudally to the pronephros from weeks 5 to 8 in the thoracolumbar region. It forms a more complex structure with up to 40 functional tubules and glomeruli that temporarily produce urine between weeks 6 and 10, providing limited excretory function during early gestation.[82][83][84] Most mesonephric tubules regress by the end of the second month, though portions of the mesonephric duct persist and contribute to the formation of genital ducts, such as the epididymis in males.[85][86] The ureteric bud, which arises from the mesonephric duct around week 5, plays a key role in initiating the next stage.[82][83] The metanephros represents the definitive kidney, emerging around week 5 at the sacral level from the interaction between the ureteric bud and the metanephric mesenchyme, which derives from the intermediate mesoderm. Through reciprocal induction, the ureteric bud branches repeatedly to form the collecting system, including the renal pelvis, calyces, and collecting ducts, while the metanephric mesenchyme differentiates into nephrons, starting with renal vesicles that progress to comma-shaped and S-shaped bodies.[82][83][84] This process yields over 1 million nephrons per kidney, with the organ becoming functional by week 12.[85][86] During development, the metanephric kidneys initially form in the pelvis and undergo cranial ascent to their final abdominal position between weeks 6 and 9, driven by differential body growth and medial rotation. This movement involves a shift in vascular supply from pelvic arteries to those arising from the abdominal aorta, with the right kidney typically positioned slightly lower than the left due to the liver's influence.[82][83][84] Nephrogenesis, the formation of new nephrons, continues throughout gestation and is complete by week 36, after which no additional nephrons are produced.[84][86] Genetic factors influence the induction process, though detailed molecular mechanisms are addressed elsewhere.[83]Genetic and molecular basis
The genetic foundation of kidney development and function is orchestrated by a network of transcription factors and signaling molecules that regulate nephrogenesis and cellular differentiation. Key genes such as WT1, PAX2, GDNF, and SIX2 play pivotal roles in establishing the metanephric mesenchyme and ureteric bud interactions essential for kidney formation. The WT1 gene encodes a transcription factor critical for the specification and maintenance of kidney progenitor cells, with mutations leading to developmental disorders like Wilms tumor.[87] PAX2, a paired box transcription factor, is expressed in the ureteric bud and metanephric mesenchyme, promoting branching morphogenesis and nephron induction.[88] Complementing this, GDNF (glial cell line-derived neurotrophic factor) serves as an inductive signal from the metanephric mesenchyme to the ureteric bud, initiating reciprocal signaling loops that drive kidney organogenesis.[89] Meanwhile, SIX2 maintains the self-renewal and multipotency of nephron progenitor cells in the cap mesenchyme, preventing premature differentiation and ensuring a sufficient progenitor pool for nephron formation throughout development.[90] At the protein level, the kidney exhibits spatially restricted expression patterns that underpin its filtration and transport functions. Nephrin, encoded by NPHS1, is a slit diaphragm protein selectively expressed in podocytes of the glomerulus, forming the molecular barrier that regulates selective permeability during ultrafiltration.[91] In the tubular epithelium, aquaporins facilitate water reabsorption; for instance, AQP1 is abundantly expressed in the proximal tubule and descending thin limb, while AQP2 is localized to the principal cells of the collecting duct, where it responds to vasopressin to modulate water permeability.[92] Isoforms of the Na⁺/K⁺-ATPase, the primary active transporter for sodium and potassium, show segment-specific distribution along the nephron, with α1 and β1 subunits predominant in the proximal tubule to support reabsorption, and variations in distal segments adapting to electrochemical gradients.[93] Transcriptomic analyses reveal kidney-specific gene expression profiles that highlight functional specialization across cell types. For example, proximal tubule cells exhibit high expression of solute transporters such as SLC34A1 (for phosphate) and SLC5A2 (for glucose), reflecting their role in bulk reabsorption, as identified through bulk and single-nucleus RNA sequencing datasets.[94] These patterns underscore the transcriptional diversity that enables the kidney's homeostatic roles. Epigenetic mechanisms, particularly DNA methylation, fine-tune gene expression during nephrogenesis by silencing or activating developmental loci. Dynamic DNA methylation events, mediated by DNA methyltransferases, regulate the differentiation of nephron progenitors and stromal cells, ensuring proper spatiotemporal control of genes like WT1 and SIX2.[95] Advances in single-cell RNA sequencing since 2020 have provided granular insights into kidney cellular heterogeneity, identifying over 20 distinct cell types in the human kidney, including rare populations like intercalated cells and endothelial subtypes. These studies, using techniques such as single-nucleus RNA-seq, have mapped transcriptional states in podocytes, tubular epithelia, and immune cells, revealing markers like NPHS1 for podocytes and AQP2 for principal cells, and highlighting developmental trajectories in organoids.[96][97] Mutations in kidney-related genes exemplify how genetic alterations disrupt molecular pathways. In autosomal dominant polycystic kidney disease, heterozygous loss-of-function mutations in PKD1 (encoding polycystin-1) or PKD2 (encoding polycystin-2) impair ciliary signaling and calcium homeostasis in renal epithelial cells, leading to cyst initiation and progression.[98] Over 1,250 PKD1 and 200 PKD2 variants have been documented, with PKD1 mutations associated with more severe phenotypes due to their prevalence and functional impact.[99]Postnatal maturation
The neonatal kidney exhibits immature function at birth, characterized by a low glomerular filtration rate (GFR) of approximately 20 mL/min/1.73 m², which rapidly increases to around 40 mL/min/1.73 m² by the fifth day and reaching about 60 mL/min/1.73 m² by four weeks of age.[100] This maturation continues progressively, attaining adult levels of roughly 120 mL/min/1.73 m² by 1 to 2 years of age.[101] Tubular function lags behind glomerular maturation, resulting in a temporary glomerulotubular imbalance with reduced reabsorption efficiency for solutes and water during the early postnatal period.[101] Postnatally, no new nephrons form after birth, with the total nephron number fixed at approximately 600,000 to 1.1 million per kidney, determined during fetal development.[102] Kidney growth occurs through hypertrophy and elongation of existing nephrons, with rapid size increases in the first two years of life; renal length expands from about 5 cm at birth to over 7 cm by age 2, and combined kidney weight rises from roughly 20-25 g per kidney in newborns to approximately 50-60 g by age 2, effectively more than doubling overall renal mass.[103][104] Hormonal systems mature concurrently, with the renin-angiotensin-aldosterone system (RAAS) showing heightened activity in neonates to support blood pressure and fluid balance, gradually stabilizing as renal vascular resistance decreases and angiotensin II sensitivity refines for normal function.[105] Erythropoietin (EPO) production shifts primarily to the kidney shortly after birth, with peritubular fibroblasts becoming the key site, enhancing sensitivity to hypoxia and supporting postnatal erythropoiesis.[106] Males typically have larger kidneys than females even when adjusted for body surface area, with adult male kidneys averaging 150-200 g compared to 120-150 g in females, influencing baseline renal reserve.[107] In later life, kidney function undergoes age-related decline starting around age 40, with GFR decreasing by approximately 1 mL/min/1.73 m² per year (or 10 mL/min per decade), accompanied by progressive glomerulosclerosis affecting up to 10-20% of glomeruli by age 70.[108][109] Environmental factors, particularly early postnatal nutrition, can influence renal maturation by promoting optimal nephron hypertrophy and function, as inadequate intake in preterm infants may impair long-term glomerular development and increase susceptibility to reduced renal capacity.[110]Clinical Significance
Acute and chronic kidney diseases
Acute kidney injury (AKI) is a sudden episode of kidney failure or damage that causes a buildup of waste products in the blood, leading to an abrupt decline in glomerular filtration rate (GFR).[111] AKI is classified into three main categories based on etiology: prerenal, intrinsic, and postrenal. Prerenal AKI results from hypoperfusion of the kidneys due to reduced renal blood flow, often caused by conditions such as volume depletion, hypotension, sepsis, or heart failure.[112] Intrinsic AKI involves direct damage to kidney structures, including acute tubular necrosis (ATN) from ischemia or toxins, and glomerulonephritis from immune-mediated inflammation.[113] Postrenal AKI arises from urinary tract obstruction, such as from kidney stones, tumors, or prostate enlargement, impeding urine flow and causing upstream pressure damage.[114] The Kidney Disease: Improving Global Outcomes (KDIGO) criteria define AKI stages based on changes in serum creatinine (sCr) or urine output: Stage 1 involves an sCr increase of ≥0.3 mg/dL within 48 hours or 1.5-1.9 times baseline within 7 days, with urine output <0.5 mL/kg/h for 6-12 hours; Stage 2 features a 2.0-2.9 times sCr increase and urine output <0.5 mL/kg/h for ≥12 hours; Stage 3 includes a ≥3 times sCr rise, sCr ≥4 mg/dL, or initiation of renal replacement therapy, with urine output <0.3 mL/kg/h for ≥24 hours or anuria for ≥12 hours.[115] These criteria facilitate early detection and risk stratification in clinical settings.[116] Chronic kidney disease (CKD) is a progressive condition characterized by a gradual loss of kidney function over months or years, defined by abnormalities in kidney structure or function persisting for more than three months, with implications for health.[117] CKD is staged from 1 to 5 based on estimated GFR (eGFR): Stage 1 (eGFR ≥90 mL/min/1.73 m² with evidence of kidney damage); Stage 2 (eGFR 60-89 mL/min/1.73 m²); Stage 3a (45-59), Stage 3b (30-44); Stage 4 (15-29); and Stage 5 (<15 mL/min/1.73 m², often end-stage renal disease).[118] The primary causes of CKD are diabetes mellitus and hypertension, accounting for approximately 70% of cases globally, as these conditions damage glomerular capillaries and promote sclerosis.[119] In both AKI and CKD, pathophysiology centers on inflammation, fibrosis, and structural damage. Inflammation initiates through cytokine release and immune cell infiltration, exacerbating tubular injury and interstitial changes.[120] Fibrosis, a hallmark of progression, involves excessive extracellular matrix deposition driven by transforming growth factor-β (TGF-β), which activates fibroblasts and myofibroblasts, leading to scarring and loss of functional nephrons.[121] Podocyte loss, particularly in glomerular diseases, reduces filtration barrier integrity, contributing to proteinuria and further fibrosis.[122] Epidemiologically, AKI affects 13-18% of hospitalized patients worldwide, with an estimated annual incidence exceeding 13 million cases, often linked to critical illnesses like sepsis or surgery.[123] As of 2023, CKD prevalence stands at approximately 14% among adults aged 20 and older, impacting nearly 788 million people globally, with rising trends due to aging populations and metabolic diseases.[124] Key risk factors for both AKI and CKD include advanced age, which impairs renal reserve; obesity, which promotes glomerular hyperfiltration and inflammation; and nonsteroidal anti-inflammatory drug (NSAID) use, which inhibits prostaglandins and reduces renal perfusion, significantly increasing AKI risk and accelerating CKD progression in vulnerable individuals.[125][126][127]Congenital and inherited disorders
Congenital anomalies of the kidney and urinary tract (CAKUT) represent a spectrum of structural malformations present at birth, affecting approximately 1 in 500 live births and accounting for 40-50% of end-stage renal disease cases in children.[128] These include renal agenesis, where one or both kidneys fail to develop; unilateral renal agenesis occurs in about 1 in 1,000 to 2,000 live births and is more common in males, often resulting from failure of the ureteric bud to interact with the metanephric mesenchyme during embryogenesis.[129] Horseshoe kidney, the most common renal fusion anomaly with a prevalence of 1 in 400 individuals, involves the lower poles of the kidneys fusing across the midline, typically held in place by mesenteric structures as the fetus develops.[130] Ectopic kidneys, occurring when one or both kidneys fail to ascend to their normal retroperitoneal position, are less frequent and may be located in the pelvis or abdomen, predisposing to associated urinary tract issues.[130] Inherited disorders of the kidney often stem from genetic mutations disrupting normal development or function, leading to progressive renal impairment. Autosomal dominant polycystic kidney disease (ADPKD), the most common inherited kidney disorder with a prevalence of 1 in 400 to 1,000 individuals, arises from mutations in PKD1 or PKD2 genes, causing fluid-filled cysts due to defective primary cilia in renal epithelial cells and dysregulated intracellular signaling.[131] These cysts enlarge over time, with about 50% of affected individuals progressing to end-stage renal disease (ESRD) by age 60.[131] Autosomal recessive polycystic kidney disease (ARPKD), rarer at 1 in 20,000 to 40,000 births, results from mutations in the PKHD1 gene (or occasionally DZIP1L), similarly involving ciliary dysfunction and leading to bilateral kidney enlargement with cysts originating from collecting ducts.[131] Alport syndrome, affecting around 1 in 50,000 people, is caused by mutations in COL4A3, COL4A4, or COL4A5 genes encoding type IV collagen chains essential for glomerular basement membrane integrity, manifesting initially as persistent microscopic hematuria and progressing to proteinuria and renal failure in many cases.[132] Cystic kidney diseases like ADPKD and ARPKD highlight the role of genetic defects in cystogenesis, with ADPKD cysts forming from dilated renal tubules due to ciliary signaling abnormalities that impair fluid transport and cell proliferation control.[133] In ARPKD, the cysts primarily affect the collecting ducts, often accompanied by congenital hepatic fibrosis, and the condition's severity correlates with the extent of ciliary protein dysfunction encoded by mutated genes.[134] Syndromic forms of inherited kidney disorders integrate renal anomalies with extrarenal features; for instance, branchio-oto-renal (BOR) syndrome, an autosomal dominant condition caused by heterozygous mutations in the EYA1 gene on chromosome 8q13, features branchial arch anomalies, hearing loss, and renal malformations such as collecting system duplications or agenesis in approximately 67% of cases.[135] Many congenital kidney disorders trace their embryological basis to malformations of the ureteric bud, which normally branches from the Wolffian duct around the 5th week of gestation to induce metanephric mesenchyme differentiation into nephrons; disruptions, such as failure of bud invasion or abnormal branching, underlie conditions like renal agenesis and duplicated collecting systems.[136] Prenatal screening via ultrasound detects many CAKUT anomalies, with identification rates of 60-85% when performed in the third trimester, particularly for hydronephrosis or agenesis, enabling early postnatal management.[137]Diagnostic approaches
Diagnostic approaches to kidney disease involve a combination of laboratory tests, imaging modalities, and invasive procedures to evaluate renal structure, function, and underlying pathology. Blood tests are fundamental for assessing kidney function by measuring waste products and estimating glomerular filtration rate (GFR). Serum creatinine, a byproduct of muscle metabolism filtered by the kidneys, is commonly elevated in renal impairment, with levels above 1.2 mg/dL in men and 1.1 mg/dL in women indicating potential dysfunction.[138] Blood urea nitrogen (BUN), which reflects urea from protein breakdown, typically ranges from 7 to 20 mg/dL but rises with reduced kidney clearance or dehydration.[138] The estimated GFR (eGFR) provides a more accurate assessment of filtration capacity, calculated using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation: where Scr is serum creatinine (mg/dL), κ is 0.7 for females and 0.9 for males, α is -0.329 for females and -0.411 for males, and the gender factor is 1.018 for females and 1 for males; values below 60 mL/min/1.73 m² suggest chronic kidney disease. Urine tests complement blood analyses by detecting abnormalities in composition and excretion. Urinalysis evaluates for proteinuria, an excess of protein indicating glomerular damage, and hematuria, the presence of red blood cells suggesting inflammation or stones.[138] The albumin-creatinine ratio (ACR) in a spot urine sample quantifies early albumin leakage, with levels exceeding 30 mg/g defining microalbuminuria, a key marker for incipient kidney injury particularly in diabetes.[139] Imaging techniques provide structural insights without invasion in most cases. Renal ultrasound is the initial modality of choice, non-invasively measuring kidney size (normal 10-12 cm), detecting hydronephrosis, cysts, or masses, and assessing echogenicity for parenchymal disease.[138] Computed tomography (CT) and magnetic resonance imaging (MRI) offer detailed vascular evaluation, tumor characterization, and stone detection; contrast-enhanced CT identifies renal artery stenosis, while MRI avoids radiation and excels in soft tissue delineation for complex pathologies.[138] Nuclear scintigraphy, or renography, uses radiotracers like technetium-99m mercaptoacetyltriglycine to quantify split renal function, perfusion, and drainage, aiding in differential diagnosis of obstructive versus non-obstructive issues.[140] Kidney biopsy remains the gold standard for definitive histopathological diagnosis, particularly when non-invasive tests are inconclusive. It is indicated for unexplained proteinuria, hematuria, or rapidly progressive glomerular diseases to identify specific etiologies like glomerulonephritis.[141] The percutaneous technique, guided by ultrasound, involves needle insertion through the skin to obtain cortical tissue samples, typically under local anesthesia with low complication rates (around 1-2% major bleeding).[142] Specimens are examined via light microscopy for architectural changes such as sclerosis or inflammation, immunofluorescence for immune deposits, and electron microscopy for ultrastructural details like podocyte effacement.[143] Functional tests directly measure kidney performance beyond estimates. Clearance studies, such as 24-hour creatinine clearance or inulin clearance, calculate actual GFR by comparing substance excretion rates to plasma levels, providing precise quantification when eGFR accuracy is doubted (normal GFR 90-120 mL/min/1.73 m²).[117] Renography extends this by dynamically tracking tracer uptake and excretion, enabling separate evaluation of each kidney's contribution to total function, often expressed as relative uptake percentages.[144]Therapeutic interventions
Therapeutic interventions for kidney diseases encompass a spectrum of approaches aimed at slowing disease progression, managing complications, and replacing kidney function when necessary. Conservative management focuses on non-dialytic strategies to preserve remaining kidney function, particularly in chronic kidney disease (CKD). Blood pressure control is a cornerstone, with angiotensin-converting enzyme (ACE) inhibitors recommended as first-line therapy to reduce proteinuria and slow glomerular damage. The target blood pressure is typically less than 130/80 mmHg to minimize cardiovascular risk and CKD progression. For patients with diabetic CKD, sodium-glucose cotransporter 2 (SGLT2) inhibitors such as dapagliflozin have demonstrated significant renoprotective effects, reducing the risk of CKD progression by approximately 39% in clinical trials.[145] When kidney function declines to end-stage, renal replacement therapies become essential. Hemodialysis is the most common modality, typically performed three to four times per week for 3-5 hours per session, with adequacy measured by Kt/V, targeting a value greater than 1.2 to ensure effective solute clearance and improve survival outcomes.[146] Peritoneal dialysis offers a home-based alternative, with continuous ambulatory peritoneal dialysis (CAPD) involving manual exchanges of dialysate solution three to five times daily, allowing continuous removal of waste while maintaining patient mobility.[147] Kidney transplantation provides the optimal long-term solution for eligible patients, utilizing kidneys from living or deceased donors. Post-transplant immunosuppression, primarily with calcineurin inhibitors like tacrolimus, is critical to prevent rejection, achieving one-year graft survival rates of approximately 93-98%.[148] Supportive therapies address common CKD complications; erythropoiesis-stimulating agents such as epoetin alfa are used to treat anemia due to erythropoietin deficiency, targeting hemoglobin levels of 10-11.5 g/dL to alleviate symptoms and reduce transfusion needs.[149] Phosphate binders, including calcium-based and non-calcium agents like sevelamer, are prescribed to control hyperphosphatemia by binding dietary phosphate in the gut, thereby preventing vascular calcification and bone disease.[150] Emerging interventions hold promise for targeted therapies, particularly for genetic disorders like polycystic kidney disease (PKD). CRISPR-based gene editing approaches, including base editing, have shown preclinical efficacy in correcting PKD1 mutations and reducing cyst formation in animal models during the 2020s.[151] These strategies aim to address underlying genetic defects rather than symptoms. Overall management aligns with the Kidney Disease: Improving Global Outcomes (KDIGO) 2025 guidelines, which emphasize integrated care to delay progression, optimize cardiovascular health, and personalize renal replacement options based on patient comorbidities and preferences, including updates on cystatin C use for eGFR estimation and expanded SGLT2 inhibitor recommendations for CKD.[152][153]Comparative and Evolutionary Aspects
Kidneys in non-human animals
In mammals, kidney morphology varies, with some species exhibiting multilobar structures derived from evolutionary divergence from an ancestral unilobar form. For instance, pigs possess multilobar kidneys consisting of 8 to 18 distinct renal lobes, which enhance functional compartmentalization.[154][155] Bird kidneys are typically organized into three main lobes, each comprising cortical and medullary regions with two nephron types: reptilian (loopless) and mammalian (looped), facilitating efficient filtration. These kidneys excrete nitrogenous waste primarily as uric acid, a semisoluble compound that minimizes water loss in terrestrial and arid environments by allowing concentrated urine formation without excessive hydration needs.[156][157] In reptiles and amphibians, the mesonephros persists as the primary functional kidney into adulthood in amphibians, unlike in reptiles, birds, and mammals where the metanephros becomes the adult kidney and the mesonephros regresses; reptilian kidneys often retain some mesonephric elements alongside the metanephros. These structures feature glomerular nephrons adapted to fluctuating aquatic and terrestrial osmoregulatory demands, supporting intermittent urine production tied to environmental moisture.[158][159] Fish kidneys display pronounced adaptations to habitat-specific osmoregulatory challenges. Freshwater teleosts produce copious dilute urine to counter osmotic water influx and ionic loss, with the kidney actively reabsorbing ions like sodium and chloride to maintain balance. In contrast, marine teleosts generate minimal urine volume, concentrating divalent ions (e.g., magnesium, sulfate) for excretion while relying on gill chloride cells for monovalent ion (e.g., sodium) regulation; some marine species, such as certain anguillid eels, possess aglomerular kidneys that prioritize tubular secretion over filtration for salt handling.[160][161] Among invertebrates, annelids like earthworms employ nephridia as the principal excretory organs; these are paired, segmentally distributed tubules that filter coelomic fluid, reabsorb useful solutes, and expel ammonia-rich waste to the exterior, aiding in osmoregulation within moist soils. Insects, conversely, utilize Malpighian tubules—fine, blind-ended structures extending from the gut—that actively transport potassium and water to form a fluid rich in uric acid, which is then processed in the hindgut for water reclamation and dry fecal pellet formation, enabling survival in desiccating conditions.[162]/41:_Osmotic_Regulation_and_the_Excretory_System/41.08:Excretion_Systems-_Flame_Cells_of_Planaria_and_Nephridia_of_Worms) Across mammals, kidney mass follows an allometric scaling relationship with body mass, typically comprising 0.2–1.5% of total body weight, which supports proportional adjustments in filtration capacity relative to metabolic demands.[163][164]Evolutionary adaptations
The kidneys of vertebrates originated as coelom-derived structures in deuterostomes, evolving from simple excretory organs that performed ultrafiltration for waste removal in early bilaterian ancestors approximately 500 million years ago.[165] These primitive organs, akin to the pronephros, emerged to manage osmotic balance in aquatic environments, marking a foundational adaptation for internal fluid regulation in the vertebrate lineage.[166] A pivotal evolutionary adaptation is the development of the loop of Henle in mammals and birds, which enables efficient water reabsorption and urine concentration in arid habitats.[167] This countercurrent multiplier system allows desert-adapted species, such as the kangaroo rat, to produce highly concentrated urine exceeding 6000 mOsm/L, minimizing water loss in water-scarce environments.[168] Birds and reptiles exhibit uricotelism, excreting nitrogenous waste primarily as uric acid, a strategy that avoids the toxicity of ammonia while requiring minimal water for elimination compared to the ureotelism of mammals, which relies on urea synthesis. This adaptation facilitates terrestrial life by conserving body water and reducing osmotic stress from ammonia, which is highly toxic and demands substantial dilution in aquatic settings.[169] During the marine-to-terrestrial transition, vertebrate osmoregulation underwent significant shifts, with early aquatic forms like sharks supplementing kidney function via a specialized rectal gland to excrete excess salts and maintain urea-based iso-osmolality with seawater.[170] This glandular adaptation highlights how kidneys evolved alongside auxiliary structures to handle salinity challenges before full reliance on renal mechanisms in terrestrial descendants.[171] Conservation of Hox genes across vertebrates underscores the genetic continuity in nephron patterning, where these transcription factors regulate segmental identity and organogenesis from fish to mammals.[172] Hox clusters direct the anterior-posterior organization of renal structures, ensuring adaptive nephron diversity despite environmental pressures over hundreds of millions of years.[173] Fossil evidence for early vertebrate kidneys is largely inferential, drawn from the pronephric structures preserved in extant lampreys, which represent a basal model of the simple, tubular excretory system in Cambrian-era ancestors around 500 million years ago.[166] Comparative anatomy of lamprey pronephros provides insights into the metameric, slit-bearing organs that predated more complex mesonephric and metanephric kidneys in jawed vertebrates.[174]Historical and Cultural Perspectives
Historical discoveries
The understanding of the kidney's anatomy, physiology, and pathology has evolved through key scientific milestones spanning millennia. In ancient Greece, Hippocrates (c. 460–370 BCE) documented dropsy—a swelling due to fluid accumulation—as a clinical entity potentially linked to renal impairment, marking one of the earliest descriptions of kidney-related edema in medical literature. Building on this, the Roman physician Galen (129–c. 216 CE) advanced renal concepts by theorizing that urine formed as a filtrate from blood processed through the kidneys' fine structures, a view that influenced medical thought for over a millennium. During the Renaissance, the advent of microscopy enabled more precise anatomical insights. Marcello Malpighi, in 1666, provided the first microscopic description of the nephron, identifying the Malpighian corpuscles (now known as renal corpuscles) as consisting of glomeruli and Bowman's capsules, thus revealing the kidney's glandular nature. In the 19th century, clinical-pathological correlations deepened knowledge of kidney diseases. Richard Bright, in 1827, established the link between albuminuria, edema, and renal pathology in what became known as Bright's disease, now recognized as glomerulonephritis, through systematic postmortem examinations. Complementing this, William Bowman in 1842 described the structural relationship between the glomerular tuft and Bowman's capsule, elucidating the site of blood filtration in the nephron. The 20th century brought functional advancements via experimental physiology. In the 1920s, James Wearn and Alfred Richards pioneered micropuncture techniques in amphibian kidneys, directly sampling tubular fluid to demonstrate selective reabsorption and secretion, foundational to understanding nephron transport. Homer Smith, in the 1930s, developed the concept of renal clearance, quantifying glomerular filtration rate (GFR) using substances like inulin, which provided a measurable index of kidney function and revolutionized diagnostic nephrology. Mid-century discoveries illuminated hormonal regulation and life-saving therapies. The renin-angiotensin-aldosterone system (RAAS) was elucidated in the 1940s, with key work by Irvine Page and others identifying renin's role in blood pressure control via angiotensin II and aldosterone, explaining many hypertensive and edematous states. Willem Kolff invented the first practical hemodialysis machine in 1945, successfully treating acute kidney injury and establishing dialysis as a viable therapy for end-stage renal disease. In 1954, Joseph Murray performed the first successful human kidney transplant between identical twins, overcoming immunological barriers and pioneering organ transplantation. Recent decades have refined assessment and molecular mapping. The Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation, introduced in 2009, improved GFR estimation over prior formulas by incorporating age, sex, race, and serum creatinine, enhancing early detection of chronic kidney disease. Since 2018, single-cell RNA sequencing has generated comprehensive kidney atlases, revealing cellular heterogeneity and transcriptional profiles across nephron segments, as demonstrated in landmark studies mapping over 100 cell types. In 2024, surgeons at Massachusetts General Hospital performed the first successful transplant of a genetically modified pig kidney into a living human patient, marking a breakthrough in xenotransplantation to address the global organ shortage.[175]Cultural and symbolic significance
In ancient Egyptian mummification practices, the kidneys were frequently left in situ due to their retroperitoneal location, which made them difficult to access during the evisceration process, unlike more superficial organs such as the liver or lungs that were routinely removed and preserved in canopic jars.[176] This selective preservation reflected the Egyptians' anatomical knowledge, as evidenced by examinations of mummified remains, where kidneys were often found intact or only partially disturbed. In Hebrew and Biblical traditions, the kidneys, referred to as "reins" in older translations, symbolized the innermost seat of emotions, affections, and moral judgment, representing the core of a person's vulnerability and conscience.[178] For instance, Psalm 7:9 invokes divine examination of the "hearts and reins," portraying the kidneys as the locus of deep feelings and ethical discernment, a metaphorical usage that underscores their role in spiritual introspection across ancient Semitic cultures.[179] In Indian Ayurvedic traditions, the kidneys, termed vrikka, are described as bean-shaped structures responsible for regulating fluid balance and maintaining doshic equilibrium, particularly balancing vata, pitta, and kapha to support overall vitality and prevent urinary imbalances.[180] Herbal remedies like Punarnava (Boerhavia diffusa) are employed to pacify excess kapha and vata doshas, promoting diuretic action and rejuvenation of the vrikka while aligning with Ayurveda's holistic approach to dosha harmony.[181] Greek philosophers, including Aristotle, depicted the kidneys as essential for separating surplus fluids from the blood, with ureters channeling urine to the bladder, viewing them as supportive structures that anchored major vessels and contributed to bodily equilibrium.[182] Roman medical texts built on this, describing the kidneys' bean-like form and role in urine formation as part of a philosophical framework emphasizing humoral balance and physiological symmetry.[183] During the medieval Islamic period, Avicenna's Canon of Medicine integrated the theory of four humors—blood, phlegm, yellow bile, and black bile—into discussions of kidney function, attributing renal disorders to imbalances in these humors and advocating treatments to restore aqueous humor excretion through the kidneys.[184] In parallel, medieval Christian anatomical texts in Europe, influenced by translated Islamic works, began incorporating illustrations of the kidneys in surgical and humoral contexts, marking an early shift toward visual representation in monastic and university settings.[185] In modern cultural contexts, kidneys feature in idioms symbolizing similarity or temperament, such as "of my own kidney," which denotes individuals sharing akin dispositions or character traits.[186] Organ donation, particularly of kidneys, carries symbolic weight as an act of altruism and life-giving solidarity, raising ethical discussions on dignity, consent, and the moral imperative to preserve life through transplantation.[187]References
- https://www.[researchgate](/page/ResearchGate).net/publication/13084171_The_Kidney_in_Ancient_Egyptian_Medicine_Where_Does_It_Stand