Recent from talks
Nothing was collected or created yet.
Isopentenyl pyrophosphate
View on Wikipedia | |
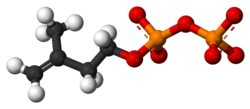 | |
| Names | |
|---|---|
| IUPAC name
(Hydroxy-(3-methylbut-3-enoxy) phosphoryl)oxyphosphonic acid
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
| MeSH | isopentenyl+pyrophosphate |
PubChem CID
|
|
CompTox Dashboard (EPA)
|
|
| |
| Properties | |
| C5H12O7P2 | |
| Molar mass | 246.092 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Isopentenyl pyrophosphate (IPP, isopentenyl diphosphate, or IDP)[1] is an isoprenoid precursor. IPP is an intermediate in the classical, HMG-CoA reductase pathway (commonly called the mevalonate pathway) and in the non-mevalonate MEP pathway of isoprenoid precursor biosynthesis. Isoprenoid precursors such as IPP, and its isomer DMAPP, are used by organisms in the biosynthesis of terpenes and terpenoids.
Biosynthesis
[edit]IPP is formed from acetyl-CoA via the mevalonate pathway (the "upstream" part), and then is isomerized to dimethylallyl pyrophosphate by the enzyme isopentenyl pyrophosphate isomerase.[2]


IPP can be synthesised via an alternative non-mevalonate pathway of isoprenoid precursor biosynthesis, the MEP pathway, where it is formed from (E)-4-hydroxy-3-methyl-but-2-enyl pyrophosphate (HMB-PP) by the enzyme HMB-PP reductase (LytB, IspH). The MEP pathway is present in many bacteria, apicomplexan protozoa such as malaria parasites, and in the plastids of higher plants.[3]
See also
[edit]References
[edit]- ^ Banerjee, A.; Sharkey, T. D. (9 July 2014). "Methylerythritol 4-phosphate (MEP) pathway metabolic regulation". Natural Product Reports. 31 (8): 1043–1055. doi:10.1039/C3NP70124G. PMID 24921065.
- ^ Chang, Wei-chen; Song, Heng; Liu, Hung-wen; Liu, Pinghua (2013). "Current development in isoprenoid precursor biosynthesis and regulation". Current Opinion in Chemical Biology. 17 (4): 571–579. doi:10.1016/j.cbpa.2013.06.020. PMC 4068245. PMID 23891475.
- ^ Wiemer, AJ; Hsiao, CH; Wiemer, DF (2010). "Isoprenoid metabolism as a therapeutic target in gram-negative pathogens". Current Topics in Medicinal Chemistry. 10 (18): 1858–71. doi:10.2174/156802610793176602. PMID 20615187.
Isopentenyl pyrophosphate
View on GrokipediaChemical Structure and Properties
Molecular Formula and Structure
Isopentenyl pyrophosphate (IPP), also known as isopentenyl diphosphate, has the molecular formula C5H12O7P2 in its neutral form, though it commonly exists as the dianionic species C5H10O7P22- under physiological conditions due to deprotonation of the phosphate groups.[8] This formula reflects a five-carbon hydrocarbon skeleton combined with a pyrophosphate moiety, where the pyrophosphate consists of two phosphate groups linked by a phosphoanhydride bond.[8] The core structure of IPP is based on a 3-methylbut-3-en-1-yl chain, featuring a primary alcohol at position 1 that is esterified to the pyrophosphate group. The carbon chain includes a terminal double bond between carbons 3 and 4, with a methyl group attached to carbon 3, giving the arrangement OP(O)(O)O-OP(O)(O)-O-CH2(1)-CH2(2)-C(3)(CH3)=CH2(4). This configuration positions the double bond in a 1,1-disubstituted alkene, characteristic of the isoprene unit. The IUPAC name is (3-methylbut-3-en-1-yl) diphosphate, and the canonical SMILES notation is CC(=C)CCOP(=O)(O)OP(=O)(O)O.[8][9] IPP is achiral, as its structure contains no tetrahedral stereocenters or other elements of chirality; the double bond geometry is fixed as the E/Z isomerism does not apply to this terminal alkene.[8] This lack of stereoisomers simplifies its role as a universal building block in biochemical pathways.[9]Physical and Chemical Properties
Isopentenyl pyrophosphate (IPP) is typically obtained as a colorless, hygroscopic solid in its salt forms, such as the triammonium or trilithium salts, due to the polar nature of its phosphate groups.[10] The molecular weight of the free acid form is 246.09 g/mol.[1] IPP exhibits high solubility in water, exceeding 10 mg/mL in aqueous buffers for the triammonium salt, attributed to the ionic phosphate moieties that facilitate hydration and dissolution.[11] However, it is unstable in aqueous solutions at neutral pH, where it undergoes hydrolysis of the pyrophosphate linkage, leading to degradation products like isopentenol and inorganic phosphate.[12] Chemically, IPP features a high-energy pyrophosphate bond, rendering it susceptible to cleavage, particularly by enzymes in biological systems.[13] This bond enables the allylic pyrophosphate (typically dimethylallyl pyrophosphate, the isomer of IPP) to generate a carbocation that is attacked by the double bond of IPP in condensation reactions catalyzed by prenyltransferases. The phosphate groups have multiple pKa values that influence its ionization and reactivity across pH ranges. IPP is sensitive to metal ions such as Mg²⁺ or Ca²⁺, which can catalyze non-enzymatic hydrolysis.[14] For long-term storage, it requires conditions like pH 11.5 and -100°C to prevent degradation.[12]Biosynthesis Pathways
Mevalonate Pathway
The mevalonate pathway, also known as the classical mevalonate route, is the primary biosynthetic route for producing isopentenyl pyrophosphate (IPP) in eukaryotes, archaea, and some bacteria, beginning with the condensation of acetyl-CoA units and proceeding through a series of enzymatic steps in the cytosol.[15] This pathway generates IPP, the universal five-carbon building block for isoprenoids, via key intermediates including acetoacetyl-CoA, 3-hydroxy-3-methylglutaryl-CoA (HMG-CoA), mevalonate, 5-phosphomevalonate, and 5-diphosphomevalonate.[15] In animals, the pathway operates mainly in the cytosol with HMG-CoA reductase localized to the endoplasmic reticulum, while in plants, the initial steps occur in the cytosol and the final phosphorylation and decarboxylation steps take place in peroxisomes.[15][16] The pathway consists of six committed enzymatic reactions:- Two molecules of acetyl-CoA are condensed to form acetoacetyl-CoA by acetoacetyl-CoA thiolase (EC 2.3.1.9), a reversible Claisen condensation that does not require additional cofactors beyond the substrates.[15]
- Acetoacetyl-CoA then reacts with a third acetyl-CoA to produce HMG-CoA, catalyzed by HMG-CoA synthase (EC 2.3.3.10), an irreversible aldol condensation also occurring without external cofactors.[15]
- HMG-CoA is reduced to mevalonate by HMG-CoA reductase (HMGR; EC 1.1.1.34), the rate-limiting and highly regulated step that consumes two molecules of NADPH and two protons.[15]
- Mevalonate is phosphorylated to 5-phosphomevalonate by mevalonate kinase (EC 2.7.1.36), utilizing one molecule of ATP.[15]
- 5-Phosphomevalonate is further phosphorylated to 5-diphosphomevalonate by phosphomevalonate kinase (EC 2.7.4.2), requiring another ATP molecule.[15]
- Finally, 5-diphosphomevalonate undergoes ATP-dependent decarboxylation to yield IPP, catalyzed by mevalonate diphosphate decarboxylase (EC 4.1.1.33), which involves a concerted elimination of CO₂ and proton loss facilitated by a Mg²⁺-ATP complex.[15]

