Recent from talks
All channels
Be the first to start a discussion here.
Be the first to start a discussion here.
Be the first to start a discussion here.
Be the first to start a discussion here.
Welcome to the community hub built to collect knowledge and have discussions related to Red heat.
Nothing was collected or created yet.
Red heat
View on Wikipediafrom Wikipedia
This article has multiple issues. Please help improve it or discuss these issues on the talk page. (Learn how and when to remove these messages)
|

In blacksmithing, red heat is the practice of using colours to determine the temperature of a piece of metal (usually iron or steel). Long before thermometers were widely available, it was necessary to know what state the metal was in for heat treating it, and the only way to do this was to heat it up to a colour which was known to be best for the work.
Chapman
[edit]According to Chapman's Workshop Technology, the colours which can be observed in steel are:[1]
| Colour | Temperature [°C] | Temperature [°F] | ||
|---|---|---|---|---|
| From | To | From | To | |
| Black red[note 1] | 426 | 593 | 799 | 1,100 |
| Very dark red | 594 | 704 | 1,100 | 1,299 |
| Dark red | 705 | 814 | 1,300 | 1,497 |
| Cherry red | 815 | 870 | 1,498 | 1,598 |
| Light cherry red | 871 | 981 | 1,599 | 1,798 |
| Orange | 982 | 1,092 | 1,799 | 1,998 |
| Yellow | 1,093 | 1,258 | 1,999 | 2,296 |
| Yellow white | 1,259 | 1,314 | 2,297 | 2,397 |
| White | 1,315+ | 2,397+ | ||
Stirling
[edit]In 1905, Stirling Consolidated Boiler Company published a slightly different set of values:[2]
| Colour | Temperature [°C] | Temperature [°F] |
|---|---|---|
| Red: Just visible | 525 | 977 |
| Dull red | 699 | 1,290 |
| Dull cherry red | 800 | 1,470 |
| Full cherry red | 900 | 1,650 |
| Clear cherry red | 1,000 | 1,830 |
| Deep orange | 1,100 | 2,010 |
| Clear orange | 1,200 | 2,190 |
| White heat | 1,300 | 2,370 |
| White bright | 1,400 | 2,550 |
| White dazzling | 1,500 | 2,730 |
See also
[edit]Notes
[edit]- ^ When viewed in dull light.
References
[edit]- ^ Chapman, W. A. J. (1972). Workshop Technology, Part 1 (5th ed.). Burlington, MA: Elsevier Butterworth-Heinemann. ISBN 978-0713132694.
- ^ A Book of Steam for Engineers. Stirling Consolidated Boiler Company. 1905. p. 50. ASIN B006RXDG3W.
Red heat
View on Grokipediafrom Grokipedia
Red heat is a term in metallurgy and blacksmithing referring to the temperature at which iron or steel becomes incandescent and glows red, typically in the range of 500–1000 °C (900–1800 °F), depending on the observed shade from dull red to bright red.[1] This visual cue, based on thermal radiation, has historically allowed blacksmiths to estimate metal temperature for forging operations such as bending, punching, and welding without precise instruments.[2] Shades like cherry red (around 750–850 °C) or bright red (850–900 °C) indicate suitability for specific tasks, with brighter hues corresponding to higher temperatures suitable for easier working.[3]
This progression aligns with the fundamental color-temperature correlation observed in thermal radiation from metals.[25]
The points are more widely spaced at higher temperatures, reflecting practical challenges in visual estimation under intense heat. It is ideal for teaching in simulated or controlled educational environments.[25] For the initial "black red" stage, Chapman stresses the importance of subdued lighting, such as a darkened room, to achieve reliable detection, as brighter conditions can obscure the faint glow.[25]
These assignments were derived from empirical tests on heated iron samples, which highlighted variations in color perception due to ambient lighting.[26]
The scale's point-specific temperatures supported hands-on forging tasks at lower ranges, while the upper whites facilitated high-heat applications like welding and riveting in early 20th-century industry.[26] It gained adoption in steam engineering contexts for its straightforward correlation between visible cues and operational safety thresholds.[26]
Overview
Definition
Red heat refers to the stage during the heating of iron or steel at which the metal begins to emit visible red light through incandescence, typically occurring around 500–600°C.[4]/University_Physics_III_-Optics_and_Modern_Physics(OpenStax)/06%3A_Photons_and_Matter_Waves/6.02%3A_Blackbody_Radiation) This phenomenon arises from thermal radiation, where the heated metal's atoms emit photons in the visible spectrum as their energy increases./University_Physics_III_-Optics_and_Modern_Physics(OpenStax)/06%3A_Photons_and_Matter_Waves/6.02%3A_Blackbody_Radiation) It is distinct from lower heat stages, such as blood heat, which involves warming the metal to approximately body temperature without any visible glow, often used in processes like tire setting on wheels.[5] In contrast, white heat represents a much higher temperature stage where the metal emits a white light, indicating intense incandescence beyond the red spectrum.[6] Blacksmiths utilize the progression of colors within red heat—from dark red to cherry red—to visually gauge appropriate temperatures for forging tasks, such as annealing, which softens the metal through controlled heating and slow cooling, or hardening, which involves rapid quenching after reaching the desired heat to increase strength.[7][8] This estimation method relies on subjective visual assessment under controlled forge lighting, a practice that originated before the development of accurate thermometric instruments.[4]Historical Context
The practice of red heat estimation, involving the visual assessment of heated metal's color to gauge temperature, traces its origins to ancient blacksmithing. Pre-modern smiths relied on color changes—from dull red to brighter hues—to determine when iron was malleable for forging and shaping, enabling the production of tools, weapons, and structural elements without precise instrumentation.[9] This method persisted and evolved through the medieval period in European workshops, where color observation became integral to controlling forging temperatures, as documented in analyses of Iron Age and early medieval ironworking sites that highlight visual cues for avoiding overheating or underheating during hammering and welding. By the Middle Ages, such practices were widespread across forges in Britain and continental Europe, supporting the craft's role in constructing armor, agricultural implements, and architectural ironwork.[10] The technique continued into the Industrial Revolution and the 19th century, where it remained a key empirical method in blacksmithing and early metallurgical practices.[11] Influenced by generations of hands-on experience, smiths used terms like "cherry red" as part of traditional knowledge passed through apprenticeships.[12] By the early 20th century, efforts to formalize these subjective color assessments resulted in the publication of structured scales, aiming to reduce inconsistencies and errors in heat treatment across industries.[13] For instance, the Stirling Consolidated Boiler Company in 1905 documented specific color-temperature correlations tailored to boiler and steam engineering, providing a benchmark that bridged traditional craftsmanship with growing metallurgical precision and facilitated safer, more predictable outcomes in forging operations.[13]Physics Principles
Thermal Radiation Basics
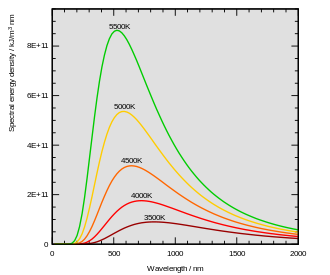
Thermal radiation, a form of electromagnetic emission from all matter above absolute zero, arises as objects convert internal thermal energy into photons across a spectrum of wavelengths.[14] For an ideal black body—an object that absorbs all incident radiation—the emitted spectrum follows Planck's law, where the total intensity increases with temperature, and the peak wavelength shifts to shorter values, as described by Wien's displacement law.[15] This shift explains why cooler objects primarily radiate in the infrared, while hotter ones extend into visible wavelengths, providing a visual cue to their thermal state.[16] In metals, incandescence occurs when heating pushes the emission peak from the infrared into the visible spectrum, beginning around 500°C with a dim red glow corresponding to wavelengths near 650 nm.[17][18] At this temperature, the black-body curve's tail enters the red portion of the visible range (approximately 620–750 nm), where longer wavelengths dominate before shorter ones become prominent at higher temperatures.[18] This transition marks the onset of visible light emission, transitioning from purely thermal infrared radiation to incandescence observable by the human eye. The total power radiated by a black body is governed by the Stefan-Boltzmann law, which states that the energy flux is proportional to the fourth power of the absolute temperature: where is the total radiated power, is the Stefan-Boltzmann constant ( W/m²K⁴), is the surface area, and is the temperature in Kelvin.[19] This dependence means that even modest temperature increases result in dramatically brighter emission, explaining why metals at higher temperatures not only change color but also intensify their glow significantly.[19] Iron and steel, particularly when rough or oxidized as in practical heating scenarios, exhibit high emissivity values (0.87–0.97), closely approximating black-body behavior in the incandescence range above 500°C.[20] This near-ideal emission allows the observed colors to reliably indicate temperature, as deviations from black-body spectra are minimal under these conditions.[20]Color-Temperature Correlation
The perceived color of heated metals correlates directly with their temperature through the principles of thermal radiation, where hotter objects emit light across a broader spectrum shifted toward shorter wavelengths. This relationship is governed by Wien's displacement law, which states that the peak wavelength of emission, , for a black body is inversely proportional to its absolute temperature : where m·K is Wien's displacement constant.[21] As temperature rises, decreases, transitioning the dominant emission from infrared wavelengths (invisible to the human eye at lower temperatures) to the visible red end of the spectrum around 600–800°C, producing the initial "red heat" glow.[22] The sequence of colors observed in heated metals reflects this spectral shift and the broadening of the emission curve. At approximately 600°C, the metal appears dull red due to longer wavelengths dominating the visible output; this progresses to cherry red around 800°C as shorter red hues intensify, then to orange near 1000°C with increased yellow-orange contributions, and finally to white-hot above 1300°C when the spectrum encompasses all visible wavelengths more uniformly. These colors arise from the black-body radiation spectrum, where the eye perceives the peak and integrated intensities across the visible range (roughly 400–700 nm).[22] Several factors influence the purity and accuracy of these perceived colors beyond ideal black-body behavior. Metal purity affects emission uniformity, as impurities can alter the spectral output or introduce non-thermal colors; for instance, trace elements in alloys may shift hues slightly from pure theoretical predictions.[23] Oxidation, particularly on steel, forms surface oxide layers (scale) that interfere with emitted light, often making the color appear duller or shifted toward darker reds due to absorption and scattering effects.[24] The human eye's detection of the initial red heat stage is facilitated by its relative sensitivity to longer wavelengths at the onset of visibility, allowing even low-intensity red emissions (near the infrared-visible boundary) to be perceptible in dim conditions before shorter-wavelength colors become evident.[22]Temperature Scales
Chapman's Scale
Chapman's Scale, introduced by W. A. J. Chapman in Workshop Technology, Part 1 (1972), serves as an educational tool for engineering students, offering a structured guide to interpreting the colors of heated metals during heat treatment processes such as forging, annealing, and tempering.[25] Designed for practical application in workshop settings, the scale correlates visual color observations with specific temperature points to ensure controlled heating without specialized equipment. The scale lists colors associated with discrete temperature points in both Celsius and Fahrenheit, often tied to specific applications, as follows:| Color | Temp (°C) | Temp (°F) | Application |
|---|---|---|---|
| Black red | 426 | 799 | Toughening carbon tool steel |
| Black red | 482 | 900 | |
| Black red | 538 | 1000 | |
| Very dark red | 593 | 1100 | Tempering high-speed steel |
| Very dark red | 648 | 1198 | |
| Dark red | 704 | 1300 | Hardening/annealing carbon tool steel |
| Cherry red | 871 | 1600 | |
| Cherry red | 926 | 1700 | |
| Light cherry red | 981 | 1800 | Hardening alloy tool steel |
| Orange red | 1036 | 1900 | |
| Yellow | 1093 | 2000 | |
| Yellow | 1149 | 2100 | |
| Yellow | 1204 | 2200 | |
| Yellow white | 1259 | 2300 | Hardening high-speed steel |
| Yellow white | 1315 | 2400 | |
| White | 1371 | 2500 | Welding |
Stirling's Scale
Stirling's Scale, introduced in 1905, originated from the industrial needs of boiler and steam engine maintenance, as outlined by the Stirling Consolidated Boiler Company in their engineering manual A Book of Steam for Engineers.[26] This scale provided a practical reference for engineers assessing metal temperatures visually during operations involving heat, such as forging and repair work in steam-powered machinery.[26] The scale categorizes heat colors from initial redness to intense white, assigning approximate temperatures based on observed glow in iron under controlled conditions. The following table summarizes the color-temperature correspondences:| Color | Temp (°C) | Temp (°F) |
|---|---|---|
| Red: Just visible | 525 | 977 |
| Dull red | 699 | 1,290 |
| Dull cherry red | 800 | 1,470 |
| Full cherry red | 900 | 1,650 |
| Clear cherry red | 1,000 | 1,830 |
| Deep orange | 1,100 | 2,010 |
| Clear orange | 1,200 | 2,190 |
| White heat | 1,300 | 2,370 |
| White bright | 1,400 | 2,550 |
| White dazzling | 1,500 | 2,730 |

