Recent from talks
Nothing was collected or created yet.
Andrussow process
View on Wikipedia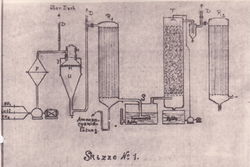
The Andrussow process is the dominant industrial process for the production of hydrogen cyanide.[1] It involves the reaction of methane, ammonia, and oxygen. The process is catalyzed by a platinum-rhodium alloy.[2]
- 2 CH4 + 2 NH3 + 3 O2 → 2 HCN + 6 H2O
Hydrogen cyanide is highly valued for the production or acrylonitrile and adiponitrile, as well as alkali metal salts such as potassium cyanide.[1]
Process details
[edit]This reaction is very exothermic. The change of enthalpy of this reaction is equal to -481.06 kJ.[3] The heat provided by the main reaction serves as a catalyst for other side reactions.
- CH4 + H2O → CO + 3 H2
- 2 CH4 + 3 O2 → 2 CO + 4 H2O
- 4 NH3 + 3 O2 → 2 N2 + 6 H2O
These side reactions can be minimized by only short exposures to the catalyst of the order of 0.0003 s.[4]
Historical articles
[edit]The process is based on a reaction that was discovered by Leonid Andrussow in 1927. In the following years he developed the process that is named after him. HCN is also produced in the BMA process.[5][6]
References
[edit]- ^ a b Gail, E.; Gos, S.; Kulzer, R.; Lorösch, J.; Rubo, A.; Sauer, M. "Cyano Compounds, Inorganic". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a08_159.pub2. ISBN 978-3-527-30673-2.
- ^ Kondratenko, V.A.; Weinberg, G.; Pohl, M.-M.; Su, D.S. (2010). "Mechanistic aspects of the Andrussow process over Pt-Rh gauzes. Effect of gauze morphology and oxygen coverage on primary O2–NH3–CH4 interactions". Applied Catalysis A: General. 381 (1–2): 66–73. Bibcode:2010AppCA.381...66K. doi:10.1016/j.apcata.2010.03.046.
- ^ Deák, Gyula (1980), Menné reakcie v organickej chémii, Bratislava: Vydavateľstvo technickej a ekonomickej literatúry, p. 14
- ^ Pirie, J M (1958). "The Manufacture of Hydrocyanic Acid by the Andrussow Process" (PDF). Platinum Metals Rev. 2 (1): 7–11. doi:10.1595/003214058X21711. Archived from the original (PDF) on 31 January 2013. Retrieved 28 March 2014.
- ^ Leonid Andrussow (1927). "Über die schnell verlaufenden katalytischen Prozesse in strömenden Gasen und die Ammoniak-Oxydation (V)". Berichte der Deutschen Chemischen Gesellschaft. 60 (8): 2005–2018. doi:10.1002/cber.19270600857.
- ^ L. Andrussow (1935). "Über die katalytische Oxydation von Ammoniak-Methan-Gemischen zu Blausäure (The catalytic oxidation of ammonia-methane-mixtures to hydrogen cyanide)". Angewandte Chemie. 48 (37): 593–595. doi:10.1002/ange.19350483702.

