Recent from talks
Nothing was collected or created yet.
Edible frog
View on Wikipedia
| Edible frog | |
|---|---|

| |
| Scientific classification | |
| Kingdom: | Animalia |
| Phylum: | Chordata |
| Class: | Amphibia |
| Order: | Anura |
| Family: | Ranidae |
| Genus: | Pelophylax |
| Species: | |
| Binomial name | |
| Pelophylax kl. esculentus | |
| Synonyms | |

The edible frog (Pelophylax kl. esculentus)[1][2] is a hybrid species of common European frog, also known as the common water frog or green frog (however, this latter term is also used for the North American species Rana clamitans).
It is used for food, particularly in France as well as Germany and Italy, for the delicacy frog legs.[3] Females are between 5 and 9 cm (2.0 and 3.5 in) long, males between 6 and 11 cm (2.4 and 4.3 in).
This widespread and common frog has many common names, including European dark-spotted frog, European black-spotted pond frog, and European black-spotted frog.
Distribution
[edit]Pelophylax esculentus is endemic to Europe. It naturally occurs from the northern half of France to western Russia, and from Estonia and Denmark to Bulgaria and northern Italy. The edible frog is introduced in Spain,[4] Norway[5] and the United Kingdom.[6] The natural range is nearly identical to that of P. lessonae.[7]
Hybridogenesis
[edit]Pelophylax kl. esculentus is the fertile hybrid of the pool frog (Pelophylax lessonae) and the marsh frog (Pelophylax ridibundus). It reproduces by hybridogenesis (hemiclonally).[8][9][10][11][12]
Hybridogenesis implies that during gametogenesis hybrids (of RL genotype) exclude one parental genome (L or R) and produce gametes with an unrecombined genome of the other parental species (R or L, respectively), instead of containing mixed recombined parental genomes.[9][10][12] The hybrid populations are usually propagated by mating (backcrosses) with a sympatric parental species – P. lessonae (LL) or P. ridibundus (RR) – providing the second, discarded parental genome (L or R respectively).[9][10][12] Hybridogenesis is thus a hemiclonal mode of reproduction; half of the genome is transmitted to the next generation clonally, unrecombined (intact); the other half sexually, recombined.[13][11][12]
For example, in the most widespread so called L–E system, edible frogs Pelophylax kl. esculentus (RE) produce gametes of the marsh frog P. ridibundus (R) and mate with coexisting pool frogs Pelophylax lessonae (L gametes) – see below in the middle.[9][12]
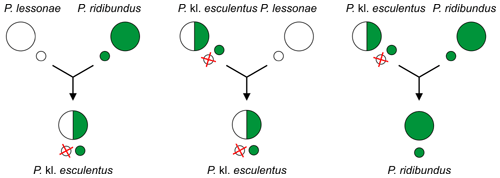
Because this hybrid requires another taxon as a sexual host to reproduce, usually one of the parental species, it is a klepton,[14][15][16] hence the addition of the "kl." (for klepton) in the species name.[17]
There are also known all-hybrid populations, where diploid hybrids (LR) coexist with triploid (LLR or LRR) hybrids, providing L or R genomes respectively. In this situation, diploid hybrids (LR) generate not only haploid R or L gametes, but also the diploid gametes (RL) needed to recreate triploids.[9][10]
-
Swimming frog
-
Attempted copulation between two males
-
Example of cannibalism
-
Close-up of head
-
Head close-up, another perspective
-
Edible frog on a human arm
-
Edible frog in pond habitat
-
Edible frog in a swamp
References
[edit]- ^ Frost, Darrel R. (2006). "Amphibian Species of the World: an Online Reference. Version 4". American Museum of Natural History, New York, USA. Retrieved 17 August 2006.
- ^ Frost, Grant, Faivovich, Bain, Haas, Haddad, de Sá, Channing, Wilkinson, Donnellan, Raxworthy, Campbell, Blotto, Moler, Drewes, Nussbaum, Lynch, Green, and Wheeler 2006. The amphibian tree of life. Bulletin of the American Museum of Natural History. Number 297. New York. Issued March 15, 2006.
- ^ Truman, Matthew (1843). "Food and its influence on food and disease". The Eclectic Magazine of Foreign Literature, Science, and Art. 1. Leavitt, Trow, & Company: 40.
- ^ Sergius Kuzmin, David Tarkhnishvili, Vladimir Ishchenko, Tatjana Dujsebayeva, Boris Tuniyev, Theodore Papenfuss, Trevor Beebee, Ismail H. Ugurtas, Max Sparreboom, Nasrullah Rastegar-Pouyani, Ahmad Mohammed Mousa Disi, Steven Anderson, Mathieu Denoël, Franco Andreone (2009). "Pelophylax ridibundus". IUCN Red List of Threatened Species. 2009 e.T58705A11825745. doi:10.2305/IUCN.UK.2009.RLTS.T58705A11825745.en. Retrieved 6 November 2023.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ "Pelophylax esculentus". artsdatabanken.no (in Norwegian). Retrieved 2022-05-10.
- ^ "Non-native amphibians". The Amphibian and Reptile Conservation Trust. Archived from the original on 9 September 2017. Retrieved 9 September 2017.
- ^ "Pelophylax esculentus, Edible Frog". AmphibiaWeb. Retrieved 28 January 2014.
- ^ Berger, L. (1970). "Some characteristics of the crossess within Rana esculenta complex in postlarval development". Ann. Zool. 27: 374–416.
- ^ a b c d e f Holsbeek, G.; Jooris, R. (2010). "Potential impact of genome exclusion by alien species in the hybridogenetic water frogs (Pelophylax esculentus complex)" (PDF). Biol Invasions. 12 (1). Springer Netherlands: 1–13. Bibcode:2010BiInv..12....1H. doi:10.1007/s10530-009-9427-2. ISSN 1387-3547. S2CID 23535815. Archived from the original (PDF) on 2019-07-13. Retrieved 2015-06-21.
- ^ a b c d Christiansen D. G. (2009). "Gamete types, sex determination and stable equilibria of all-hybrid populations of diploid and triploid edible frogs (Pelophylax esculentus) Rana esculenta as deduced from mtDNA analyses". BMC Evolutionary Biology. 9 (135): 135. doi:10.1186/1471-2148-9-135. PMC 2709657. PMID 19527499.
- ^ a b c Vorburger, Christoph; Reyer, Heinz-Ulrich (2003). "A genetic mechanism of species replacement in European waterfrogs?" (PDF). Conservation Genetics. 4 (2). Kluwer Academic Publishers: 141–155. Bibcode:2003ConG....4..141V. doi:10.1023/A:1023346824722. ISSN 1566-0621. S2CID 20453910. Retrieved 2015-06-21.
- ^ a b c d e Ragghianti M, Bucci S, Marracci S, Casola C, Mancino G, Hotz H, Guex GD, Plötner J, Uzzell T (February 2007). "Gametogenesis of intergroup hybrids of hemiclonal frogs" (PDF). Genet. Res. 89 (1): 39–45. doi:10.1017/S0016672307008610. PMID 17517158. Archived from the original on July 17, 2015. Retrieved 2012-07-25.
- ^ Simon J.-C.; Delmotte F.; Rispe C.; Crease T. (2003). "Phylogenetic relationships between parthenogens and their sexual relatives: the possible routes to parthenogenesis in animals" (PDF). Biological Journal of the Linnean Society. 79: 151–163. doi:10.1046/j.1095-8312.2003.00175.x. Retrieved 2012-07-30.
- ^ Dubois, Alain (2009). "Asexual and metasexual vertebrates. Book review". Alytes. 27 (2). ISSCA (International Society for the Study and Conservation of Amphibians): 62–66. Retrieved 2015-06-22.
John C. Avise, 2008.–Clonality. The genetics, ecology, and evolution of sexual abstinence in vertebrate animals. New York, Oxford University Press: i-xi + 1-237. ISBN 978-0-19-536967-0.
- ^ Dubois, A.; Günther, R. (1982). "Klepton and synklepton: two new evolutionary systematics categories in zoology". Zool. Jahrb. Syst. (Zoologische Jahrbücher. Abteilung für Systematik, Ökologie und Geographie der Tiere). 109. Jena; Stuttgart; New York.: Gustav Fischer Verlag: 290–305. ISSN 0044-5193.
- ^ Polls Pelaz, Manuel (October 1990). "The Biological Klepton Concept (BKC)". Alytes. 8 (3). ISSCA (International Society for the Study and Conservation of Amphibians): 75–89. Archived from the original on 2014-07-14. Retrieved 2015-06-22.
- ^ Dubois, Alain (October 1990). "Nomenclature of parthenogenetic, gynogenetic and hybridogenetic vertebrate taxons: new proposals". Alytes. 8 (3). ISSCA (International Society for the Study and Conservation of Amphibians): 61–74. Archived from the original on 2015-06-23. Retrieved 2015-06-22.
External links
[edit]- ArchéoZooThèque : Edible frog skeleton drawing Archived 2019-09-13 at the Wayback Machine
- Species account on HerpFrance.com Archived 2015-09-24 at the Wayback Machine
- Video: Pool frogs and hybrid green frogs on YouTube. Mixed mating of pool frog and edible frog; pool frog are grass green and smaller.
Edible frog
View on GrokipediaTaxonomy and nomenclature
Etymology and common names
The binomial name Pelophylax esculentus combines the genus Pelophylax, derived from the Greek words pēlos (mud) and phulax (guard or sentinel), alluding to the species' affinity for muddy aquatic environments, with the specific epithet esculentus, from Latin meaning "edible" or "fit for food," in reference to its historical exploitation for culinary purposes.[7][8] This frog was first formally described by Carl Linnaeus in 1758 under the name Rana esculenta in his seminal work Systema Naturae, where it was placed within the broad genus Rana encompassing many frog species; subsequent taxonomic revisions in the 19th and 20th centuries reclassified it to Pelophylax to reflect phylogenetic relationships, with the "kl." prefix denoting its hybridogenic status as a klepton.[1][4] In English, it is commonly known as the edible frog, common water frog, or green frog, though the latter two names can overlap with related species. Regionally, it is referred to as grenouille comestible (edible frog) or grenouille verte (green frog) in French, emphasizing its role in gastronomy where frog legs are a traditional delicacy, and as rana commestibile (edible frog) or rana verde (green frog) in Italian, similarly tied to culinary traditions in Mediterranean Europe.[9][10][11]Classification and synonyms
The edible frog, Pelophylax esculentus, belongs to the kingdom Animalia, phylum Chordata, class Amphibia, order Anura, family Ranidae, genus Pelophylax, and is designated within the P. esculentus species complex as P. kl. esculentus to denote its hybrid nature.[1][12] This taxon is recognized as a klepton, a hybrid form originating from the interspecific cross between the pool frog (Pelophylax lessonae) and the marsh frog (Pelophylax ridibundus), and it forms part of the broader Pelophylax esculentus complex that includes various hybrid lineages across Europe.[1][12] Historically, it was described under the binomial Rana esculenta by Linnaeus in 1758, which served as the original scientific name and remains a primary synonym; other informal designations include the hybrid Rana lessonae-ridibunda, though triploid variants such as P. "LLR" and P. "LRR" are not treated as full synonyms but as genotypic forms within the complex.[1] The edible frog exhibits no formally recognized subspecies, but populations display diploid (LR) and triploid (LLR, LRR) genotypes, reflecting its hybridogenetic origins where "L" denotes the genome from P. lessonae and "R" from P. ridibundus.[1] A significant taxonomic update occurred in 2006, when Frost et al. reclassified it from the genus Rana to Pelophylax based on comprehensive phylogenetic analyses of molecular and morphological data, which demonstrated the polyphyly of the broad Rana sensu lato and established Pelophylax as a monophyletic genus within Ranidae.[12]Description
Physical characteristics
The edible frog (Pelophylax kl. esculentus) is a medium-sized anuran, with adults typically attaining a snout-vent length (SVL) of 50–115 mm, with females generally larger than males; mean values are approximately 82 mm for females and 69 mm for males, and body weights reach up to 100 g or more.[13][14] The body form is robust and streamlined for aquatic life, featuring smooth skin with a granular texture, particularly on the ventral surface. Hind limbs are long and powerful, with fully webbed hind feet adapted for swimming, while forelimbs are unwebbed and shorter.[1][15] Dorsally, the coloration ranges from olive-green to brown, often marked by irregular black spots of varying size and density, accompanied by a light middorsal line extending from the snout to the cloaca in most individuals.[1] The ventral surface is pale yellow to white, sometimes with scattered dark spots. Distinctive features include prominent dorsolateral folds that run along the sides of the back and may be variably developed or colored, a strong temporal ridge above the eye, large protruding eyes with horizontal pupils, and a tympanum that lacks strong contrast with surrounding skin.[1][15] These traits serve to distinguish it from close relatives like the pool frog (Pelophylax lessonae), which exhibits less extensive spotting and a more uniformly brown dorsum. Relative to the hind foot, the inner metatarsal tubercle measures 1.73–2.89 times shorter than the first toe, and when the shins are held perpendicular to the body axis, the heels typically meet or slightly overlap.[1] Tadpoles of the edible frog are uniformly yellowish, greenish, or grayish brown dorsally and laterally, with a white belly; the tail musculature is speckled with black or white, and the fin is transparent, sometimes with gray or black spots, and eyes are white-yellow. The body is slightly depressed, eyes are small and dorsolaterally directed, the spiracle is sinistral, and the tail is approximately twice the body length with a low dorsal fin and broadly pointed tip. They reach a total length of 50–70 mm at Gosner stage 36, with body lengths around 20 mm.[3] Sexual dimorphism in adult size and proportions is notable, with females generally larger than males.[16]Sexual dimorphism and variation
Sexual dimorphism in the edible frog (Pelophylax esculentus) is most pronounced in reproductive traits and body size. Males are characterized by paired subgular vocal sacs, which inflate during calling to produce advertisement calls, and dark nuptial pads on the thumbs that develop prominently during the breeding season to facilitate amplexus.[17][18] Females lack vocal sacs and nuptial pads, enabling distinction during field identification.[3] Adult females typically exhibit larger body sizes than males, reflecting a female-biased sexual size dimorphism common in many anuran species. In central European mixed populations, mean snout-vent length (SVL) measures 74.7 ± 5.9 mm for females and 67.2 ± 6.7 mm for males, representing approximately an 11% size advantage for females; asymptotic SVL is similar between sexes at around 84 mm.[19] This dimorphism arises during ontogeny, with juveniles showing no significant size differences between sexes after initial hibernation, but adults diverging as females grow larger overall.[19] Intraspecific variation in P. esculentus is influenced by its hybridogenetic reproduction, where one parental genome (P. lessonae or P. ridibundus) is clonally transmitted, leading to phenotypic diversity tied to genome composition and population dynamics. Morphological traits such as dorsal coloration vary widely, from greyish-green to olive-green backgrounds with dark spots of differing size and density, often accompanied by a light middorsal stripe; this variability can be modulated by hybrid parentage.[20][1] Geographic patterns show body size and genetic diversity differing across regions, with northern European populations (e.g., southern Sweden) displaying higher genetic heterogeneity that may correlate with subtle morphological adaptations, while southern forms exhibit increased spotting intensity in some areas.[21] Age-related changes include smaller SVL in juveniles (around 32-41 mm post-hibernation) compared to adults, with molting contributing to skin texture variations in mature individuals.[19]Distribution and habitat
Geographic range
The edible frog (Pelophylax kl. esculentus), a hybrid species originating from the pool frog (P. lessonae) and the marsh frog (P. ridibundus), is endemic to Europe. Its native range spans western, central, and eastern regions, extending from northern France and Belgium eastward to western Russia, with northern limits in Estonia and southern boundaries in Bulgaria and northern Italy.[1] This distribution largely overlaps with those of its parental species, reflecting shared post-glacial colonization patterns from refugia in southern Europe following the Last Glacial Maximum.[22] Introduced populations have established outside this native range, notably in Spain through historical aquaculture efforts for food production, in Norway via accidental releases, and in the United Kingdom from deliberate introductions, often linked to culinary or ornamental purposes.[23] The species remains naturally absent from much of Scandinavia and the Iberian Peninsula.[1] Currently, the edible frog occupies approximately 30 European countries within its native distribution, with highest population densities recorded in central Europe, such as in Germany and Poland, where it thrives in suitable wetland networks.[1] Climate change may facilitate further range expansion northward and westward by altering temperature regimes and habitat availability, potentially increasing overlap with vulnerable native amphibians.[24]Habitat preferences
The edible frog (Pelophylax kl. esculentus) primarily inhabits permanent or semi-permanent aquatic environments such as ponds, slow-flowing rivers, ditches, and marshes, where it favors shallow, sunny waters with abundant emergent and submerged vegetation for cover and breeding.[1] These habitats typically feature overgrown edges with plants like reeds and water lilies (Nymphaea spp.), providing dense lily pads and bank vegetation that offer protection from predators and suitable sites for egg deposition.[4] The species avoids fast-flowing waters and very large open pools, as well as highly acidic conditions, preferring neutral to slightly alkaline waters with moderate dissolved oxygen levels.[1][25] Terrestrially, the edible frog utilizes adjacent open areas such as meadows, grasslands, or forest edges for foraging and dispersal, but it generally shies away from dense forest interiors or arid uplands.[1] For overwintering, individuals often burrow into mud banks or seek refuge at the bottom of water bodies, or alternatively hibernate on land in leaf litter or under vegetation when co-occurring with parental species like the pool frog (P. lessonae).[1] Populations are most common in lowlands from sea level up to 1,550 m elevation, within temperate climates characterized by mild winters and warm summers.[26] In coastal regions, the species shows some tolerance for brackish waters, occasionally entering slightly saline lagoons or estuaries up to 4-7‰ salinity.[27] As a eurythermic species, the edible frog remains active across a broad temperature range of 5-30°C, allowing it to exploit sunny, warm microhabitats during the active season while tolerating cooler conditions near hibernation thresholds.[28] This thermal flexibility, combined with its preference for vegetated shallows, enables successful colonization of both natural and anthropogenic water bodies like irrigation channels and fish ponds across its range.[1]Biology and ecology
Diet and foraging behavior
The edible frog (Pelophylax kl. esculentus) exhibits an opportunistic diet primarily composed of invertebrates, reflecting its role as a generalist predator in wetland ecosystems. Adults predominantly consume insects, which make up approximately 88% of their diet, including Hymenoptera (28%), Coleoptera (18%), Lepidoptera (17%), and Diptera (11%), along with earthworms, small crustaceans, and gastropods. Occasionally, they prey on small vertebrates such as fish, tadpoles, lizards, or even conspecifics, though these constitute a minor fraction of intake. Prey selection varies by individual size and locality, with larger P. ridibundus parental forms consuming bigger items (average length 12.24 mm, volume 1515.58 mm³) compared to smaller P. lessonae forms (average length 9.07 mm, volume 533.31 mm³), while hybrids fall intermediate (average length 10.26 mm, volume 757.27 mm³). Tadpoles of the edible frog display a herbivorous-detritivorous feeding strategy, shifting from primary consumption of algae and detritus to inclusion of small zooplankton as they develop. This larval diet supports their role as primary consumers, influencing nutrient cycling in aquatic habitats by grazing on periphyton and organic matter. Unlike adults, tadpoles do not exhibit carnivorous tendencies until metamorphosis, relying on particulate plant material and microbial films for nutrition. Foraging in adults employs a classic sit-and-wait ambush strategy, typically from emergent vegetation or perches near water edges, where the frog remains motionless until prey comes within striking distance. They project their tongue rapidly to capture items, with the tongue extending up to 1.5 times the body length to adhere to and retrieve targets using viscous saliva. Activity is both diurnal and nocturnal, though it peaks nocturnally during summer months when daytime heat limits exposure. Tadpoles forage continuously in water, filtering or scraping food from substrates without ambush tactics. Seasonal variations in diet and foraging intensity align with prey availability and environmental conditions; spring feeding emphasizes aquatic invertebrates and early-emerging insects, transitioning to more terrestrial prey like beetles and moths in summer. Vertebrate consumption increases slightly in autumn, while foraging ceases entirely during winter hibernation, when frogs fast and rely on stored energy reserves. Habitat structure, such as dense riparian vegetation, enhances prey encounter rates and supports this opportunistic approach. As a mid-level predator, the edible frog occupies an intermediate trophic position in wetland food webs, controlling invertebrate populations while serving as prey for higher predators, thereby facilitating energy transfer between aquatic and terrestrial systems.Predators and interactions
The edible frog (Pelophylax kl. esculentus) faces predation from a variety of aquatic and semi-aquatic vertebrates across its range in Europe. Avian predators include herons (such as grey herons, Ardea cinerea), ducks, and owls, which target both adults and juveniles in wetland habitats.[29][30] Reptilian predators, notably grass snakes (Natrix natrix) and other natricine snakes, frequently consume frogs near water bodies, while fish species like perch (Perca fluviatilis) and pike (Esox lucius) prey on tadpoles and smaller individuals in ponds and streams.[31] Mammalian predators such as otters (Lutra lutra) and hedgehogs (Erinaceus europaeus) occasionally take adults in riparian zones. Tadpoles are particularly vulnerable to predation by newts (e.g., Lissotriton spp. and Triturus spp.) and other amphibians, which can significantly reduce larval survival rates in shared breeding sites.[31][32] Interspecies interactions play a key role in the edible frog's ecology, often involving competition and mutualistic associations. As a hybrid species, it competes with parental species like the pool frog (Pelophylax lessonae) and marsh frog (Pelophylax ridibundus) for breeding sites and resources, which can alter local population dynamics.[33] Edible frogs also exhibit symbiosis with aquatic vegetation, such as reeds and submerged plants, which provide essential cover from predators and enhance habitat suitability in dynamic wetland environments.[1] The edible frog employs several defense mechanisms to mitigate predation risks. Its dorsal coloration, featuring green hues with dark spots, enables effective camouflage against aquatic vegetation and pond substrates, reducing visibility to visual hunters like birds and fish.[30] Upon detecting threats, individuals produce alarm calls—short, high-pitched vocalizations—to alert conspecifics and potential disrupt predators. Some populations secrete unpalatable skin compounds, including antimicrobial peptides and bradykinin-like substances from granular glands, which deter certain predators and may reduce infection risks.[34][35] Predation exerts notable population impacts on the edible frog, particularly in open habitats where cover is limited, leading to higher mortality rates among juveniles and contributing to fluctuating densities. Despite these pressures, the species serves an important ecological role as prey, supporting food webs, while its predation on insects helps regulate invertebrate populations in wetlands, indirectly benefiting biodiversity.[31] Parasites are prevalent in edible frog populations, influencing development and survival. Common helminths include nematodes and trematodes, such as those from genera Cosmocerca and Haplometra, which infect the gastrointestinal tract and can impair nutrient absorption. Protozoan parasites, including apicomplexans like Hepatozoon spp., are detected in blood samples and may weaken hosts, exacerbating vulnerability to predators; certain trematodes, such as Strigea robusta, have been linked to limb deformities (e.g., polydactyly) in water frogs (Pelophylax spp.), though prevalence varies regionally.[36][37][38][39]Reproduction
Mating and breeding
The breeding season of the edible frog (Pelophylax kl. esculentus) in temperate zones typically spans from April to July (extending to October in some areas), triggered by rising water temperatures exceeding 15°C that signal suitable conditions for reproduction.[7][40] This period aligns with post-hibernation emergence, allowing adults to migrate to aquatic habitats for mating activities. Males initiate courtship by producing advertisement calls while submerged in water, forming large choruses that amplify their signals to attract females from afar. These calls feature a series of pulses delivered at a rate of 20–40 per second, with variations influenced by genotype and environmental factors.[41][42] Females approach responsive males, assessing call quality—such as pulse structure and duration—as a cue for mate selection, which helps ensure compatibility in mixed-species environments.[43] Upon attraction, the male clasps the female in an axillary amplexus, a firm dorsal grasp that positions him to fertilize eggs externally as they are released. This embrace stimulates oviposition, during which the female deposits a single clutch of 1,000–11,000 eggs in gelatinous, spherical masses attached to aquatic vegetation just below the surface.[44][45] Clutch size can vary based on female body condition and mate preference, with undesired pairings sometimes leading to reduced egg numbers.[45] Mating is polygamous, enabling both sexes to engage in multiple pairings within a season to maximize reproductive output. Males defend small territories around calling sites using low-frequency vocalizations to deter rivals and retain access to arriving females.[46][18] This territorial behavior, combined with chorusing, facilitates intense competition and ensures gene transmission in hybrid populations.[18]Life cycle and development
The life cycle of the edible frog (Pelophylax kl. esculentus) commences with the egg stage, where fertilized eggs develop in aquatic clutches attached to vegetation. Hatching typically occurs after 10-14 days, depending on water temperature, with embryos exhibiting high sensitivity to low oxygen levels that can reduce survival rates during this vulnerable period.[47][48] Hatched tadpoles are fully aquatic, relying on external gills for respiration and adopting a primarily herbivorous diet of algae and plant matter. This larval stage endures 2–9 months depending on cohort and conditions, during which tadpoles grow and prepare for metamorphosis, culminating at Gosner stage 42 with the emergence of forelimbs, lung development, and tail resorption. In regions with variable climates, some tadpoles may overwinter in ponds if late-season hatching or cooler temperatures delay progression, resuming development and metamorphosing the following spring.[3][49][50][33] Post-metamorphosis juveniles exhibit rapid growth during their first summer, transitioning to terrestrial-aquatic habits and carnivorous feeding. Sexual maturity is attained in 1-2 years for males and up to 3 years for females, marking the onset of the adult phase.[19] Adults typically live 5-10 years in the wild, with records up to 12 years, and periodically molt their skin several times per year to renew the epidermal layer and facilitate osmoregulation. Development rates across all stages are strongly influenced by environmental temperature, with approximately 20°C optimal for efficient metamorphosis in temperate habitats.[51][52][33]Hybridogenesis
Mechanism of hybrid reproduction
The edible frog, Pelophylax kl. esculentus (genotype LR, with L from Pelophylax lessonae and R from Pelophylax ridibundus), reproduces through hybridogenesis, a form of hemiclonal reproduction that enables the hybrid form to persist across generations despite its allodiploid origin. In the common L-E system prevalent in Western Europe, diploid LR individuals eliminate the L genome during early gametogenesis in gonocytes, typically via formation of micronuclei containing the excluded chromosomes, which are then degraded. The remaining R genome undergoes endoreplication to restore a diploid state (RR), followed by standard meiosis in spermatocytes or oocytes, allowing intragenomic recombination within the R set and production of haploid, recombinant R gametes. This process ensures that the transmitted R genome incorporates genetic variation through crossing over, while the excluded L genome is not incorporated into the gametes from the hybrid parent.[53][54] The reproductive cycle relies on backcrossing with the parental pool frog (P. lessonae, genotype LL) to restore the hybrid genotype. Haploid R gametes from the LR hybrid are fertilized by haploid L gametes from P. lessonae, yielding diploid LR offspring that perpetuate the hybrid form. Without this periodic input of the L genome from the sexual parental species, the hybrid lineage could not maintain its diploid state, as self-sustaining reproduction (E-E systems) is rare and typically involves triploids. This obligatory heterospecific mating underscores the parasitic nature of hybridogenesis, where the hybrid exploits the parental species' gametes to sustain itself.[54][55] Triploid forms, such as LLR or LRR, arise occasionally from fertilization of diploid gametes and exhibit alternative reproductive modes, including gynogenesis (diploid LL or RR eggs developing after pricking by haploid sperm without genome incorporation) or androgenesis (similar process with male gametes). In LLR triploids, for instance, the single R genome is often eliminated, leading to transmission of the LL set via unrecombined diploid gametes, while LRR triploids may exclude the L genome and transmit RR. These triploids are generally less viable and fertile than diploids, with high rates of aneuploidy (up to 80% in germ cells) and degeneration, limiting their role to supporting all-hybrid populations in specific locales.[53][56] This hybridogenetic mechanism has profound evolutionary implications, allowing the edible frog to bypass true meiosis and maintain the LR genotype indefinitely without segregational loss of hybridity, though it incurs costs like partial sterility from genome elimination errors. By combining clonal transmission of one genome with sexual renewal of the other, it facilitates long-term persistence in sympatric populations while enabling occasional introgression and adaptation.[55][54] The process can be described textually as a step-by-step cycle: (1) In the LR hybrid, the L genome is excluded from gonocytes, forming a micronucleus. (2) The R genome endoreplicates to RR. (3) Meiosis produces recombinant R haploid gametes. (4) An R gamete fuses with an L gamete from P. lessonae, restoring LR. In triploids like LLR, exclusion of R yields LL diploid gametes, which may develop gynogenetically upon stimulation by a haploid sperm, producing LLR progeny if the sperm genome is incorporated.[53]Genetic structure and populations
The edible frog, Pelophylax esculentus, exhibits a complex genetic structure characterized primarily by diploid and triploid genotypes arising from its hybrid origin between Pelophylax lessonae (L genome) and Pelophylax ridibundus (R genome). The most common form is the diploid hybrid (genotype LR), which predominates in many populations across its range. Triploid forms, including LLR (two L genomes and one R) and the rarer LRR (one L and two R genomes), occur in varying proportions, with LLR being more stable and widespread in certain regions, such as all-hybrid populations in northern Europe. These ploidy variations influence reproductive success and population persistence, as triploids produce haploid gametes derived from the doubled genome set, facilitating hybrid maintenance without full recombination.[57][58] Populations of P. esculentus typically form mixed systems with one parental species, known as L-E systems (coexisting with P. lessonae) or R-E systems (coexisting with P. ridibundus), where hybrids rely on mating with the parental form to perpetuate their lineage through hybridogenesis. In L-E systems, the R genome is clonally transmitted, while in R-E systems, the L genome is clonally passed on, ensuring genome exclusion of the other parental set during gametogenesis. All-hybrid populations (E-E systems), composed solely of LR diploids and LLR/LLR triploids without parental species, are rare and geographically limited, often found in isolated habitats like the Danish archipelago. These systems highlight the dependency on parental species for long-term viability, as the absence of suitable mates can lead to population decline or extinction.[18][57] Genetic diversity within P. esculentus populations is generally low, attributable to the hemiclonal reproduction that clonally transmits one parental genome (typically L in many systems) without recombination, resulting in reduced variability compared to purely sexual species. Mitochondrial DNA diversity, however, shows evidence of contributions from both parental lineages, including heteroplasmy where individuals carry mtDNA from P. lessonae and P. ridibundus simultaneously, reflecting historical hybridization events. Studies on ploidy variation, such as those examining all-hybrid populations, reveal lower heterozygosity (e.g., expected heterozygosity H_E ≈ 0.157 for L genome) and increasing isolation by distance northward, which may enhance invasiveness in introduced ranges by allowing adaptation through clonal propagation in novel environments. Population stability hinges on the presence of parental species; declines in P. lessonae or P. ridibundus can destabilize hybrid systems, elevating extinction risks in mixed populations.[57][59]Human interactions
Culinary and economic uses
The hind legs of the edible frog (Pelophylax kl. esculentus), known as "cuisses de grenouilles" in French cuisine, are a prized delicacy, particularly in France where they feature prominently on restaurant menus and in regional dishes from areas like the Dombes plateau.[16] These legs are valued for their tender texture and mild flavor, often compared to chicken, and are consumed fresh or frozen across Europe, with notable popularity in Italy and Germany as well.[16] Nutritionally, frog legs offer a high-protein, low-fat profile, providing approximately 16 grams of protein and just 0.3 grams of fat per 100 grams, making them a lean source of essential amino acids.[60] Historically, the consumption of edible frogs traces back to Roman times, when they were prepared with salt and oil as a remedy against poisons, and their use expanded in medieval France around the 12th century, when church authorities classified frogs as fish to permit eating during Lent.[61] Demand peaked in 19th-century France amid growing urbanization and gourmet trends, though overharvesting led to domestic bans by the 1980s.[61] Today, preparation methods include sautéing in garlic butter and parsley for a Provençal style, frying after dredging in flour, boiling for soups, or incorporating into stews, often served with lemon wedges or alongside fries.[62] Cultural festivals celebrate this tradition, such as the annual Foire aux Grenouilles in Vittel, France, where up to 7 tonnes of frog legs are consumed by visitors over two days.[63] Harvesting primarily involves wild collection in southern Europe, with exports from Turkey and Albania to the EU, though domestic European stocks have declined due to protective regulations.[64] While the edible frog is harvested in Europe, much of the EU's frog legs trade involves other species from Asia. The broader EU frog legs market imports around 4,000 tonnes yearly from various species and countries including Indonesia, Vietnam, Turkey, and Albania. For the edible frog, imports are smaller, primarily from Turkey and Albania, totaling hundreds of tonnes annually (e.g., around 40-50 tonnes from each as of 2022).[65][64][66] Aquaculture supplements supply, with small-scale farms in Italy producing edible frogs for local and export markets, while larger operations in China and Southeast Asia focus on similar species for global trade, yielding millions of frogs annually.[64] The EU frog legs market holds an estimated value of €10-20 million, driven by demand in France, Belgium, and the Netherlands.[66]Conservation status and threats
The edible frog (Pelophylax kl. esculentus) is assessed as Least Concern on the global IUCN Red List, based on its wide distribution across Europe and presumed large population size, with the most recent formal evaluation dating to 2009 but reaffirmed in subsequent regional reviews.[1] However, as a hybridogenetic species reliant on co-occurrence with parental species (P. lessonae or P. ridibundus), its long-term viability can be precarious in areas where these dynamics are disrupted. Locally, populations face varying risks; for instance, in introduced regions like the United Kingdom, the species is non-native and viewed as potentially invasive rather than threatened, though some fragmented native-range habitats show localized declines warranting attention.[67] Key threats to the edible frog include habitat loss and degradation from agricultural intensification, wetland drainage, and urbanization, which reduce breeding sites such as ponds and marshes essential for its aquatic lifestyle.[68] Overharvesting for the international frogs' legs trade has historically depleted populations in source countries like Turkey and Albania, where unsustainable collection during breeding seasons exacerbates declines.[69] Emerging infectious diseases, particularly chytridiomycosis caused by the fungus Batrachochytrium dendrobatidis, pose a growing risk, with infections documented in wild populations across Europe, though the species shows some tolerance compared to more susceptible amphibians.[70] Climate change further compounds these pressures by altering temperature and precipitation patterns, potentially disrupting breeding phenology and increasing drought stress in wetland habitats.[71] In introduced ranges such as the UK, where the edible frog was established in the 20th century, it exhibits invasive potential by competing with native species like the common frog (Rana temporaria) for resources and through hybridization risks with reintroduced pool frogs (P. lessonae), prompting ongoing monitoring as a potential ecological pest.[72] Conservation measures include its listing on Annex V of the EU Habitats Directive, which regulates exploitation to prevent overharvesting while allowing sustainable management.[4] Efforts also encompass wetland restoration initiatives across Europe to bolster habitat connectivity, alongside seasonal harvest bans in countries like Turkey (May–June) and Albania's national wildlife collection moratorium from 2014 to 2025.[64] However, in August 2025, Albania lifted its long-standing national hunting ban, potentially increasing future harvest risks unless regulated sustainably.[73] Population trends indicate stability or slight increases in core central European ranges due to the hybrid's reproductive advantages, but declines occur in peripheral or fragmented areas affected by habitat loss and disease.[74] Monitoring programs, including citizen science initiatives like those coordinated by national herpetological societies, track distribution and abundance to inform targeted interventions.[75]References
- https://en.wiktionary.org/wiki/esculentus










