Recent from talks
Nothing was collected or created yet.
Algae
View on Wikipedia
| Algae | |
|---|---|
| Organisms that perform oxygenic photosynthesis, except land plants | |
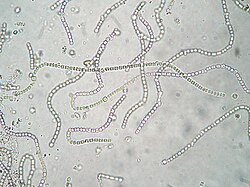
 Marine algae growing on the sea bed in shallow waters | |
 Freshwater microscopic unicellular and colonial algae | |
| Traditional algal divisions[1][2] | |
| Prokaryotic | Cyanobacteria |
| Eukaryotic (primary endosymbiosis) | Glaucophyta, Rhodophyta, Prasinodermophyta, Chlorophyta, Charophyta* |
| Eukaryotic (secondary endosymbiosis) | Chlorarachniophyta, Chromeridophyta, Cryptophyta, Dinoflagellata, Euglenophyta (partially), Haptophyta, Heterokontophyta |
| *paraphyletic, it excludes land plants | |
| Diversity | |
| Living | 50,605 species |
| Fossil | 10,556 species |
Algae (/ˈældʒiː/ ⓘ AL-jee,[3] UK also /ˈælɡiː/ AL-ghee; sg.: alga /ˈælɡə/ ⓘ AL-gə) is an informal term for any organisms of a large and diverse group of photosynthetic organisms that are not land plants, and includes species from multiple distinct clades. Such organisms range from unicellular microalgae, such as cyanobacteria,[a] Chlorella, and diatoms, to multicellular macroalgae such as kelp or brown algae which may grow up to 50 metres (160 ft) in length. Most algae are aquatic organisms and lack many of the distinct cell and tissue types, such as stomata, xylem, and phloem that are found in land plants. The largest and most complex marine algae are called seaweeds. In contrast, the most complex freshwater forms are the Charophyta, a division of green algae which includes, for example, Spirogyra and stoneworts. Algae that are carried passively by water are plankton, specifically phytoplankton.
Algae constitute a polyphyletic group[4] because they do not include a common ancestor, and although eukaryotic algae with chlorophyll-bearing plastids seem to have a single origin (from symbiogenesis with cyanobacteria),[5] they were acquired in different ways. Green algae are a prominent example of algae that have primary chloroplasts derived from endosymbiont cyanobacteria. Diatoms and brown algae are examples of algae with secondary chloroplasts derived from endosymbiotic red algae, which they acquired via phagocytosis.[6] Algae exhibit a wide range of reproductive strategies, from simple asexual cell division to complex forms of sexual reproduction via spores.[7]
Algae lack the various structures that characterize plants (which evolved from freshwater green algae), such as the phyllids (leaf-like structures) and rhizoids of bryophytes (non-vascular plants), and the roots, leaves and other xylemic/phloemic organs found in tracheophytes (vascular plants). Most algae are autotrophic, although some are mixotrophic, deriving energy both from photosynthesis and uptake of organic carbon either by osmotrophy, myzotrophy or phagotrophy. Some unicellular species of green algae, many golden algae, euglenids, dinoflagellates, and other algae have become heterotrophs (also called colorless or apochlorotic algae), sometimes parasitic, relying entirely on external energy sources and have limited or no photosynthetic apparatus.[8][9][10] Some other heterotrophic organisms, such as the apicomplexans, are also derived from cells whose ancestors possessed chlorophyllic plastids, but are not traditionally considered as algae. Algae have photosynthetic machinery ultimately derived from cyanobacteria that produce oxygen as a byproduct of splitting water molecules, unlike other organisms that conduct anoxygenic photosynthesis such as purple and green sulfur bacteria. Fossilized filamentous algae from the Vindhya basin have been dated to 1.6 to 1.7 billion years ago.[11]
Because of the wide range of types of algae, there is a correspondingly wide range of industrial and traditional applications in human society. Traditional seaweed farming practices have existed for thousands of years and have strong traditions in East Asian food cultures. More modern algaculture applications extend the food traditions for other applications, including cattle feed, using algae for bioremediation or pollution control, transforming sunlight into algae fuels or other chemicals used in industrial processes, and in medical and scientific applications. A 2020 review found that these applications of algae could play an important role in carbon sequestration to mitigate climate change while providing lucrative value-added products for global economies.[12]
Etymology and study
[edit]The singular alga is the Latin word for 'seaweed' and retains that meaning in English.[13] The etymology is obscure. Although some speculate that it is related to Latin algēre, 'be cold',[14] no reason is known to associate seaweed with temperature. A more likely source is alliga, 'binding, entwining'.[15]
The Ancient Greek word for 'seaweed' was φῦκος (phŷkos), which could mean either the seaweed (probably red algae) or a red dye derived from it. The Latinization, fūcus, meant primarily the cosmetic rouge. The etymology is uncertain, but a strong candidate has long been some word related to the Biblical פוך (pūk), 'paint' (if not that word itself), a cosmetic eye-shadow used by the ancient Egyptians and other inhabitants of the eastern Mediterranean. It could be any color: black, red, green, or blue.[16]
The study of algae is most commonly called phycology (from Greek phykos 'seaweed'); the term algology is falling out of use.[17]
Description
[edit]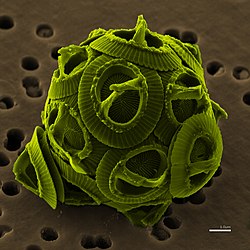
The algae are a heterogeneous group of mostly photosynthetic organisms that produce oxygen and lack the reproductive features and structural complexity of land plants. This concept includes the cyanobacteria, which are prokaryotes, and all photosynthetic protists, which are eukaryotes. They contain chlorophyll a as their primary photosynthetic pigment, and generally inhabit aquatic environments.[18][19]
However, there are many exceptions to this definition. Many non-photosynthetic protists are included in the study of algae, such as the heterotrophic relatives of euglenophytes[19] or the numerous species of colorless algae that have lost their chlorophyll during evolution (e.g., Prototheca). Some exceptional species of algae tolerate dry terrestrial habitats, such as soil, rocks, or caves hidden from light sources, although they still need enough moisture to become active.[19]
Morphology
[edit]
A range of algal morphologies is exhibited, and convergence of features in unrelated groups is common. The only groups to exhibit three-dimensional multicellular thalli are the reds and browns, and some chlorophytes.[20] Apical growth is constrained to subsets of these groups: the florideophyte reds, various browns, and the charophytes.[20] The form of charophytes is quite different from those of reds and browns, because they have distinct nodes, separated by internode 'stems'; whorls of branches reminiscent of the horsetails occur at the nodes.[20] Conceptacles are another polyphyletic trait; they appear in the coralline algae and the Hildenbrandiales, as well as the browns.[20]
Most of the simpler algae are unicellular flagellates or amoeboids, but colonial and nonmotile forms have developed independently among several of the groups. Some of the more common organizational levels, more than one of which may occur in the lifecycle of a species, are
- Colonial: small, regular groups of motile cells
- Capsoid: individual non-motile cells embedded in mucilage
- Coccoid: individual non-motile cells with cell walls
- Palmelloid: nonmotile cells embedded in mucilage
- Filamentous: a string of connected nonmotile cells, sometimes branching
- Parenchymatous: cells forming a thallus with partial differentiation of tissues
In three lines, even higher levels of organization have been reached, with full tissue differentiation. These are the brown algae,[21]—some of which may reach 50 m in length (kelps)[22]—the red algae,[23] and the green algae.[24] The most complex forms are found among the charophyte algae (see Charales and Charophyta), in a lineage that eventually led to the higher land plants. The innovation that defines these nonalgal plants is the presence of female reproductive organs with protective cell layers that protect the zygote and developing embryo. Hence, the land plants are referred to as the Embryophytes.
Turfs
[edit]The term algal turf is commonly used but poorly defined. Algal turfs are thick, carpet-like beds of seaweed that retain sediment and compete with foundation species like corals and kelps, and they are usually less than 15 cm tall. Such a turf may consist of one or more species, and will generally cover an area in the order of a square metre or more. Some common characteristics are listed:[25]
- Algae that form aggregations that have been described as turfs include diatoms, cyanobacteria, chlorophytes, phaeophytes and rhodophytes. Turfs are often composed of numerous species at a wide range of spatial scales, but monospecific turfs are frequently reported.[25]
- Turfs can be morphologically highly variable over geographic scales and even within species on local scales and can be difficult to identify in terms of the constituent species.[25]
- Turfs have been defined as short algae, but this has been used to describe height ranges from less than 0.5 cm to more than 10 cm. In some regions, the descriptions approached heights which might be described as canopies (20 to 30 cm).[25]
Physiology
[edit]Many algae, particularly species of the Characeae,[26] have served as model experimental organisms to understand the mechanisms of the water permeability of membranes, osmoregulation, salt tolerance, cytoplasmic streaming, and the generation of action potentials. Plant hormones are found not only in higher plants, but in algae, too.[27]
Life cycle
[edit]Rhodophyta, Chlorophyta, and Heterokontophyta, the three main algal divisions, have life cycles which show considerable variation and complexity. In general, an asexual phase exists where the seaweed's cells are diploid, a sexual phase where the cells are haploid, followed by fusion of the male and female gametes. Asexual reproduction permits efficient population increases, but less variation is possible. Commonly, in sexual reproduction of unicellular and colonial algae, two specialized, sexually compatible, haploid gametes make physical contact and fuse to form a zygote. To ensure a successful mating, the development and release of gametes is highly synchronized and regulated; pheromones may play a key role in these processes.[28] Sexual reproduction allows for more variation and provides the benefit of efficient recombinational repair of DNA damages during meiosis, a key stage of the sexual cycle.[29] However, sexual reproduction is more costly than asexual reproduction.[30] Meiosis has been shown to occur in many different species of algae.[31]
Diversity
[edit]The most recent estimate (as of January 2024) documents 50,605 living and 10,556 fossil algal species, according to the online database AlgaeBase.[b] They are classified into 15 phyla or divisions. Some phyla are not photosynthetic, namely Picozoa and Rhodelphidia, but they are included in the database due to their close relationship with red algae.[1][35]
| phylum (division) | described genera |
described species | ||
|---|---|---|---|---|
| living | fossil | total | ||
| "Charophyta" (Streptophyta without land plants) | 236 | 4,940 | 704 | 5,644 |
| Chlorarachniophyta | 10[b] | 16[b] | 0 | 16[b] |
| Chlorophyta | 1,513 | 6,851 | 1,083 | 7,934 |
| Chromeridophyta | 6 | 8 | 0 | 8 |
| Cryptophyta | 44 | 245 | 0 | 245 |
| Cyanobacteria | 866 | 4,669 | 1,054 | 5,723 |
| Dinoflagellata (Dinophyta) | 710 | 2,956 | 955 | 3,911 |
| Euglenophyta (not all species are algae) | 164 | 2,037 | 20 | 2,057 |
| Glaucophyta | 8 | 25 | 0 | 25 |
| Haptophyta | 391 | 517 | 1205 | 1,722 |
| Heterokontophyta | 1,781 | 21,052 | 2,262 | 23,314 |
| Picozoa (Picobiliphyta) | 1 | 1 | 0 | 1 |
| Prasinodermophyta | 5 | 10 | 0 | 10 |
| Rhodelphidia | 1 | 2 | 0 | 2 |
| Rhodophyta | 1,094 | 7,276 | 278 | 7,554 |
| Incertae sedis fossils | 887 | 0 | 2,995 | 2,995 |
| Total | 7,717 | 50,605 | 10,556 | 61,161 |
The various algal phyla can be differentiated according to several biological traits. They have distinct morphologies, photosynthetic pigmentation, storage products, cell wall composition,[19] and mechanisms of carbon concentration.[36] Some phyla have unique cellular structures.[19]
Prokaryotic algae
[edit]Among prokaryotes, five major groups of bacteria have evolved the ability to photosynthesize, including heliobacteria, green sulfur and nonsulfur bacteria and proteobacteria.[38] However, the only lineage where oxygenic photosynthesis has evolved is in the cyanobacteria,[39] named for their blue-green (cyan) coloration and often known as blue-green algae.[40] They are classified as the phylum Cyanobacteriota or Cyanophyta. However, this phylum also includes two classes of non-photosynthetic bacteria: Melainabacteria[41] (also called Vampirovibrionia[42] or Vampirovibrionophyceae)[43] and Sericytochromatia[44] (also known as Blackallbacteria).[45] A third class contains the photosynthetic ones, known as Cyanophyceae[43] (also called Cyanobacteriia[42] or Oxyphotobacteria).[44]
As bacteria, their cells lack membrane-bound organelles, with the exception of thylakoids. Like other algae, cyanobacteria have chlorophyll a as their primary photosynthetic pigment. Their accessory pigments include phycobilins (phycoerythrobilin and phycocyanobilin), carotenoids and, in some cases, b, d, or f chlorophylls, generally distributed in phycobilisomes found in the surface of thylakoids. They display a variety of body forms, such as single cells, colonies, and unbranched or branched filaments. Their cells are commonly covered in a sheath of mucilage, and they also have a typical gram-negative bacterial cell wall composed largely of peptidoglycan. They have various storage particles, including cyanophycin as aminoacid and nitrogen reserves, "cyanophycean starch" (similar to plant amylose) for carbohydrates, and lipid droplets. Their Rubisco enzymes are concentrated in carboxysomes. They occupy a diverse array of aquatic and terrestrial habitats, including extreme environments from hot springs to polar glaciers. Some are subterranean, living via hydrogen-based lithoautotrophy instead of photosynthesis.[40]
Three lineages of cyanobacteria, Prochloraceae, Prochlorothrix and Prochlorococcus, independently evolved to have chlorophylls a and b instead of phycobilisomes. Due to their different pigmentation, they were historically grouped in a separate division, Prochlorophyta, as this is the typical pigmentation seen in green algae (e.g., chlorophytes). Eventually, this classification became obsolete, as it is a polyphyletic grouping.[46][47]
Cyanobacteria are included as algae by most phycological sources[18][19][1] and by the International Code of Nomenclature for algae, fungi, and plants,[48] although a few authors exclude them from the definition of algae and reserve the term for eukaryotes only.[4][49]
Eukaryotic algae
[edit]Eukaryotic algae contain chloroplasts that are similar in structure to cyanobacteria. Chloroplasts contain circular DNA like that in cyanobacteria and are interpreted as representing reduced endosymbiotic cyanobacteria. However, the exact origin of the chloroplasts is different among separate lineages of algae, reflecting their acquisition during different endosymbiotic events. Many groups contain some members that are no longer photosynthetic. Some retain plastids, but not chloroplasts, while others have lost plastids entirely.[50]
Primary algae
[edit]These algae, grouped in the clade Archaeplastida (meaning 'ancient plastid'), have "primary chloroplasts", i.e. the chloroplasts are surrounded by two membranes and probably developed through a single endosymbiotic event with a cyanobacterium. The chloroplasts of red algae have chlorophylls a and c (often), and phycobilins, while those of green algae have chloroplasts with chlorophyll a and b without phycobilins. Land plants are pigmented similarly to green algae and probably developed from them, thus the Chlorophyta is a sister taxon to the plants; sometimes the Chlorophyta, the Charophyta, and land plants are grouped together as the Viridiplantae.[citation needed]
There is also a minor group of algae with primary plastids of different origin than the chloroplasts of the archaeplastid algae. The photosynthetic plastid of three species of the genus Paulinella (Rhizaria – Cercozoa – Euglyphida), often referred to as a 'cyanelle', was originated in the endosymbiosis of a α-cyanobacterium (probably an ancestral member of Chroococcales).[51][52]
Secondary algae
[edit]These algae appeared independently in various distantly related lineages after acquiring a chloroplast derived from another eukaryotic alga. Two lineages of secondary algae, chlorarachniophytes and euglenophytes have "green" chloroplasts containing chlorophylls a and b.[53] Their chloroplasts are surrounded by four and three membranes, respectively, and were probably retained from ingested green algae.[54][55][56]
- Chlorarachniophytes, which belong to the phylum Cercozoa, contain a small nucleomorph, which is a relict of the algae's nucleus.[57]
- Euglenophytes, which belong to the phylum Euglenozoa, live primarily in fresh water and have chloroplasts with only three membranes. The endosymbiotic green algae may have been acquired through myzocytosis rather than phagocytosis.[58]
- Another group with green algae endosymbionts is the dinoflagellate genus Lepidodinium, which has replaced its original endosymbiont of red algal origin with one of green algal origin. A nucleomorph is present, and the host genome still have several red algal genes acquired through endosymbiotic gene transfer. Also, the euglenid and chlorarachniophyte genome contain genes of apparent red algal ancestry.[59][60][61]
Other groups have "red" chloroplasts containing chlorophylls a and c, and phycobilins. The shape can vary; they may be of discoid, plate-like, reticulate, cup-shaped, spiral, or ribbon shaped. They have one or more pyrenoids to preserve protein and starch. The latter chlorophyll type is not known from any prokaryotes or primary chloroplasts, but genetic similarities with red algae suggest a relationship there.[62] In some of these groups, the chloroplast has four membranes, retaining a nucleomorph in cryptomonads, and they likely share a common pigmented ancestor, although other evidence casts doubt on whether the heterokonts, Haptophyta, and cryptomonads are in fact more closely related to each other than to other groups.[63][64]
The typical dinoflagellate chloroplast has three membranes, but considerable diversity exists in chloroplasts within the group, and a number of endosymbiotic events apparently occurred.[5] The Apicomplexa, a group of closely related parasites, also have plastids called apicoplasts, which are not photosynthetic.[5] The Chromerida are the closest relatives of apicomplexans, and some have retained their chloroplasts.[65] The three alveolate groups evolved from a common myzozoan ancestor that obtained chloroplasts.[66]
History of classification
[edit]
Linnaeus, in Species Plantarum (1753),[67] the starting point for modern botanical nomenclature, recognized 14 genera of algae, of which only four are currently considered among algae.[68] In Systema Naturae, Linnaeus described the genera Volvox and Corallina, and a species of Acetabularia (as Madrepora), among the animals.
In 1768, Samuel Gottlieb Gmelin (1744–1774) published the Historia Fucorum, the first work dedicated to marine algae and the first book on marine biology to use the then new binomial nomenclature of Linnaeus. It included elaborate illustrations of seaweed and marine algae on folded leaves.[69][70]
W. H. Harvey (1811–1866) and Lamouroux (1813)[71] were the first to divide macroscopic algae into four divisions based on their pigmentation. This is the first use of a biochemical criterion in plant systematics. Harvey's four divisions are: red algae (Rhodospermae), brown algae (Melanospermae), green algae (Chlorospermae), and Diatomaceae.[72][73]
At this time, microscopic algae were discovered and reported by a different group of workers (e.g., O. F. Müller and Ehrenberg) studying the Infusoria (microscopic organisms). Unlike macroalgae, which were clearly viewed as plants, microalgae were frequently considered animals because they are often motile.[71] Even the nonmotile (coccoid) microalgae were sometimes merely seen as stages of the lifecycle of plants, macroalgae, or animals.[74][75]
Although used as a taxonomic category in some pre-Darwinian classifications, e.g., Linnaeus (1753),[76] de Jussieu (1789),[77] Lamouroux (1813), Harvey (1836), Horaninow (1843), Agassiz (1859), Wilson & Cassin (1864),[76] in further classifications, the "algae" are seen as an artificial, polyphyletic group.[78]
Throughout the 20th century, most classifications treated the following groups as divisions or classes of algae: cyanophytes, rhodophytes, chrysophytes, xanthophytes, bacillariophytes, phaeophytes, pyrrhophytes (cryptophytes and dinophytes), euglenophytes, and chlorophytes. Later, many new groups were discovered (e.g., Bolidophyceae), and others were splintered from older groups: charophytes and glaucophytes (from chlorophytes), many heterokontophytes (e.g., synurophytes from chrysophytes, or eustigmatophytes from xanthophytes), haptophytes (from chrysophytes), and chlorarachniophytes (from xanthophytes).[79]
With the abandonment of plant-animal dichotomous classification, most groups of algae (sometimes all) were included in Protista, later also abandoned in favour of Eukaryota. However, as a legacy of the older plant life scheme, some groups that were also treated as protozoans in the past still have duplicated classifications (see ambiregnal protists).[80]
Some parasitic algae (e.g., the green algae Prototheca and Helicosporidium, parasites of metazoans, or Cephaleuros, parasites of plants) were originally classified as fungi, sporozoans, or protistans of incertae sedis,[81] while others (e.g., the green algae Phyllosiphon and Rhodochytrium, parasites of plants, or the red algae Pterocladiophila and Gelidiocolax mammillatus, parasites of other red algae, or the dinoflagellates Oodinium, parasites of fish) had their relationship with algae conjectured early. In other cases, some groups were originally characterized as parasitic algae (e.g., Chlorochytrium), but later were seen as endophytic algae.[82] Some filamentous bacteria (e.g., Beggiatoa) were originally seen as algae. Furthermore, groups like the apicomplexans are also parasites derived from ancestors that possessed plastids, but are not included in any group traditionally seen as algae.[83][84]
Evolution
[edit]Origin of oxygenic photosynthesis
[edit]Prokaryotic algae, i.e., cyanobacteria, are the only group of organisms where oxygenic photosynthesis has evolved. The oldest undisputed fossil evidence of cyanobacteria is dated at 2100 million years ago,[85] although stromatolites, associated with cyanobacterial biofilms, appear as early as 3500 million years ago in the fossil record.[86]
First endosymbiosis
[edit]Eukaryotic algae are polyphyletic thus their origin cannot be traced back to single hypothetical common ancestor. It is thought that they came into existence when photosynthetic coccoid cyanobacteria got phagocytized by a unicellular heterotrophic eukaryote (a protist),[87] giving rise to double-membranous primary plastids. Such symbiogenic events (primary symbiogenesis) are believed to have occurred more than 1.5 billion years ago during the Calymmian period, early in Boring Billion, but it is difficult to track the key events because of so much time gap.[88] Primary symbiogenesis gave rise to three divisions of archaeplastids, namely the Viridiplantae (green algae and later plants), Rhodophyta (red algae) and Glaucophyta ("grey algae"), whose plastids further spread into other protist lineages through eukaryote-eukaryote predation, engulfments and subsequent endosymbioses (secondary and tertiary symbiogenesis).[88] This process of serial cell "capture" and "enslavement" explains the diversity of photosynthetic eukaryotes.[87] The oldest undisputed fossil evidence of eukaryotic algae is Bangiomorpha pubescens, a red alga found in rocks around 1047 million years old.[89][90]
Consecutive endosymbioses
[edit]
Recent genomic and phylogenomic approaches have significantly clarified plastid genome evolution, the horizontal movement of endosymbiont genes to the "host" nuclear genome, and plastid spread throughout the eukaryotic tree of life.[87] It is accepted that both euglenophytes and chlorarachniophytes obtained their chloroplasts from chlorophytes that became endosymbionts.[93] In particular, euglenophyte chloroplasts share the most resemblance with the genus Pyramimonas.[94]
However, there is still no clear order in which the secondary and tertiary endosymbioses occurred for the "chromist" lineages (ochrophytes, cryptophytes, haptophytes and myzozoans).[95] Two main models have been proposed to explain the order, both of which agree that cryptophytes obtained their chloroplasts from red algae. One model, hypothesized in 2014 by John W. Stiller and coauthors,[96] suggests that a cryptophyte became the plastid of ochrophytes, which in turn became the plastid of myzozoans and haptophytes. The other model, suggested by Andrzej Bodył and coauthors in 2009,[97] describes that a cryptophyte became the plastid of both haptophytes and ochrophytes, and it is a haptophyte that became the plastid of myzozoans instead.[92] In 2024, a third model by Filip Pietluch and coauthors proposed that there were two independent endosymbioses with red algae: one that originated the cryptophyte plastids (as in the previous models), and subsequently the haptophyte plastids; and another that originated the ochrophyte plastids, where the myzozoans obtained theirs.[91]
Relationship to land plants
[edit]Fossils of isolated spores suggest land plants may have been around as long as 475 million years ago (mya) during the Late Cambrian/Early Ordovician period,[98][99] from sessile shallow freshwater charophyte algae much like Chara,[100] which likely got stranded ashore when riverine/lacustrine water levels dropped during dry seasons.[101] These charophyte algae probably already developed filamentous thalli and holdfasts that superficially resembled plant stems and roots, and probably had an isomorphic alternation of generations. They perhaps evolved some 850 mya[102] and might even be as early as 1 Gya during the late phase of the Boring Billion.[103]
Distribution
[edit]The distribution of algal species has been fairly well studied since the founding of phytogeography in the mid-19th century.[104] Algae spread mainly by the dispersal of spores analogously to the dispersal of cryptogamic plants by spores. Spores can be found in a variety of environments: fresh and marine waters, air, soil, and in or on other organisms.[104] Whether a spore is to grow into an adult organism depends on the species and the environmental conditions where the spore lands.
The spores of freshwater algae are dispersed mainly by running water and wind, as well as by living carriers.[104] However, not all bodies of water can carry all species of algae, as the chemical composition of certain water bodies limits the algae that can survive within them.[104] Marine spores are often spread by ocean currents. Ocean water presents many vastly different habitats based on temperature and nutrient availability, resulting in phytogeographic zones, regions, and provinces.[105]
To some degree, the distribution of algae is subject to floristic discontinuities caused by geographical features, such as Antarctica, long distances of ocean or general land masses. It is, therefore, possible to identify species occurring by locality, such as "Pacific algae" or "North Sea algae". When they occur out of their localities, hypothesizing a transport mechanism is usually possible, such as the hulls of ships. For example, Ulva reticulata and U. fasciata travelled from the mainland to Hawaii in this manner.
Mapping is possible for select species only: "there are many valid examples of confined distribution patterns."[106] For example, Clathromorphum is an arctic genus and is not mapped far south of there.[where?][107] However, scientists regard the overall data as insufficient due to the "difficulties of undertaking such studies."[108]
Regional algae checklists
[edit]
The Algal Collection of the US National Herbarium (located in the National Museum of Natural History) consists of approximately 320,500 dried specimens, which, although not exhaustive (no exhaustive collection exists), gives an idea of the order of magnitude of the number of algal species (that number remains unknown).[109] Estimates vary widely. For example, according to one standard textbook,[110] in the British Isles, the UK Biodiversity Steering Group Report estimated there to be 20,000 algal species in the UK. Another checklist reports only about 5,000 species. Regarding the difference of about 15,000 species, the text concludes: "It will require many detailed field surveys before it is possible to provide a reliable estimate of the total number of species ..."
Regional and group estimates have been made, as well:
- 5,000–5,500 species of red algae worldwide[citation needed]
- "some 1,300 in Australian Seas"[111]
- 400 seaweed species for the western coastline of South Africa,[112] and 212 species from the coast of KwaZulu-Natal.[113] Some of these are duplicates, as the range extends across both coasts, and the total recorded is probably about 500 species. Most of these are listed in List of seaweeds of South Africa. These exclude phytoplankton and crustose corallines.
- 669 marine species from California (US)[114]
- 642 in the check-list of Britain and Ireland[115]
and so on, but lacking any scientific basis or reliable sources, these numbers have no more credibility than the British ones mentioned above. Most estimates also omit microscopic algae, such as phytoplankton.[citation needed]
Ecology
[edit]
Algae are prominent in bodies of water, common in terrestrial environments, and are found in unusual environments, such as on snow and ice. Seaweeds grow mostly in shallow marine waters, less than100 m (330 ft) deep; however, some such as Navicula pennata have been recorded to a depth of 360 m (1,180 ft).[116] A type of algae, Ancylonema nordenskioeldii, was found in Greenland in areas known as the 'Dark Zone', which caused an increase in the rate of melting ice sheet.[117] The same algae was found in the Italian Alps, after pink ice appeared on parts of the Presena glacier.[118]
The various sorts of algae play significant roles in aquatic ecology. Microscopic forms that live suspended in the water column (phytoplankton) provide the food base for most marine food chains. In very high densities (algal blooms), these algae may discolor the water and outcompete, poison, or asphyxiate other life forms.[119]
Algae can be used as indicator organisms to monitor pollution in various aquatic systems.[120] In many cases, algal metabolism is sensitive to various pollutants. Due to this, the species composition of algal populations may shift in the presence of chemical pollutants.[120] To detect these changes, algae can be sampled from the environment and maintained in laboratories with relative ease.[120]
On the basis of their habitat, algae can be categorized as: aquatic (planktonic, benthic, marine, freshwater, lentic, lotic),[121] terrestrial, aerial (subaerial),[122] lithophytic, halophytic (or euryhaline), psammon, thermophilic, cryophilic, epibiont (epiphytic, epizoic), endosymbiont (endophytic, endozoic), parasitic, calcifilic or lichenic (phycobiont).[123]
Symbiotic algae
[edit]Some species of algae form symbiotic relationships with other organisms. In these symbioses, the algae supply photosynthates (organic substances) to the host organism providing protection to the algal cells. The host organism derives some or all of its energy requirements from the algae.[citation needed] Examples are:
Lichens
[edit]
Lichens are defined by the International Association for Lichenology to be "an association of a fungus and a photosynthetic symbiont resulting in a stable vegetative body having a specific structure".[124] The fungi, or mycobionts, are mainly from the Ascomycota with a few from the Basidiomycota. In nature, they do not occur separate from lichens. It is unknown when they began to associate.[125] One or more[126] mycobiont associates with the same phycobiont species, from the green algae, except that alternatively, the mycobiont may associate with a species of cyanobacteria (hence "photobiont" is the more accurate term). A photobiont may be associated with many different mycobionts or may live independently; accordingly, lichens are named and classified as fungal species.[127] The association is termed a morphogenesis because the lichen has a form and capabilities not possessed by the symbiont species alone (they can be experimentally isolated). The photobiont possibly triggers otherwise latent genes in the mycobiont.[128]
Trentepohlia is an example of a common green alga genus worldwide that can grow on its own or be lichenised. Lichen thus share some of the habitat and often similar appearance with specialized species of algae (aerophytes) growing on exposed surfaces such as tree trunks and rocks and sometimes discoloring them.[citation needed]
Animal symbioses
[edit]
Coral reefs are accumulated from the calcareous exoskeletons of marine invertebrates of the order Scleractinia (stony corals). These animals metabolize sugar and oxygen to obtain energy for their cell-building processes, including secretion of the exoskeleton, with water and carbon dioxide as byproducts. Dinoflagellates (algal protists) are often endosymbionts in the cells of the coral-forming marine invertebrates, where they accelerate host-cell metabolism by generating sugar and oxygen immediately available through photosynthesis using incident light and the carbon dioxide produced by the host. Reef-building stony corals (hermatypic corals) require endosymbiotic algae from the genus Symbiodinium to be in a healthy condition.[129] The loss of Symbiodinium from the host is known as coral bleaching, a condition which leads to the deterioration of a reef.
Endosymbiontic green algae live close to the surface of some sponges, for example, breadcrumb sponges (Halichondria panicea). The alga is thus protected from predators; the sponge is provided with oxygen and sugars which can account for 50 to 80% of sponge growth in some species.[130]
In human culture
[edit]In classical Chinese, the word 藻 is used both for "algae" and (in the modest tradition of the imperial scholars) for "literary talent". The third island in Kunming Lake beside the Summer Palace in Beijing is known as the Zaojian Tang Dao (藻鑒堂島), which thus simultaneously means "Island of the Algae-Viewing Hall" and "Island of the Hall for Reflecting on Literary Talent".[citation needed]
Cultivation
[edit]

Algaculture is a form of aquaculture involving the farming of species of algae.[131]
The majority of algae that are intentionally cultivated fall into the category of microalgae (also referred to as phytoplankton, microphytes, or planktonic algae). Macroalgae, commonly known as seaweed, also have many commercial and industrial uses, but due to their size and the specific requirements of the environment in which they need to grow, they do not lend themselves as readily to cultivation (this may change, however, with the advent of newer seaweed cultivators, which are basically algae scrubbers using upflowing air bubbles in small containers, known as tumble culture).[132]
Commercial and industrial algae cultivation has numerous uses, including production of nutraceuticals such as omega-3 fatty acids (as algal oil)[133][134][135] or natural food colorants and dyes, food, fertilizers, bioplastics, chemical feedstock (raw material), protein-rich animal/aquaculture feed, pharmaceuticals, and algal fuel,[136] and can also be used as a means of pollution control and natural carbon sequestration.[137]
Global production of farmed aquatic plants, overwhelmingly dominated by seaweeds, grew in output volume from 13.5 million tonnes in 1995, to just over 30 million tonnes in 2016 and 37.8 million tonnes in 2022.[138][139] This increase was the result of production expansions led by China, followed by Malaysia, the Philippines, the United Republic of Tanzania, and the Russian Federation.[138]
Cultured microalgae already contribute to a wide range of sectors in the emerging bioeconomy.[140] Research suggests there are large potentials and benefits of algaculture for the development of a future healthy and sustainable food system.[141][137]Seaweed farming
[edit]

Seaweed farming or kelp farming is the practice of cultivating and harvesting seaweed. In its simplest form farmers gather from natural beds, while at the other extreme farmers fully control the crop's life cycle.
The seven most cultivated taxa are Eucheuma spp., Kappaphycus alvarezii, Gracilaria spp., Saccharina japonica, Undaria pinnatifida, Pyropia spp., and Sargassum fusiforme. Eucheuma and K. alvarezii are attractive for carrageenan (a gelling agent); Gracilaria is farmed for agar; the rest are eaten after limited processing.[142] Seaweeds are different from mangroves and seagrasses, as they are photosynthetic algal organisms[143] and are non-flowering.[142]
The largest seaweed-producing countries as of 2022 are China (58.62%) and Indonesia (28.6%); followed by South Korea (5.09%) and the Philippines (4.19%). Other notable producers include North Korea (1.6%), Japan (1.15%), Malaysia (0.53%), Zanzibar (Tanzania, 0.5%), and Chile (0.3%).[144][145] Seaweed farming has frequently been developed to improve economic conditions and to reduce fishing pressure.[146]
The Food and Agriculture Organization (FAO) reported that world production in 2019 was over 35 million tonnes. North America produced some 23,000 tonnes of wet seaweed. Alaska, Maine, France, and Norway each more than doubled their seaweed production since 2018. As of 2019, seaweed represented 30% of marine aquaculture.[147] In 2023, the global seaweed extract market was valued at $16.5 billion, with strong projected growth.[148]
Seaweed farming is a carbon negative crop, with a high potential for climate change mitigation.[149][150] The IPCC Special Report on the Ocean and Cryosphere in a Changing Climate recommends "further research attention" as a mitigation tactic.[151] World Wildlife Fund, Oceans 2050, and The Nature Conservancy publicly support expanded seaweed cultivation.[147]Bioreactors
[edit]
Uses
[edit]
Biofuel
[edit]To be competitive and independent from fluctuating support from (local) policy on the long run, biofuels should equal or beat the cost level of fossil fuels. Here, algae-based fuels hold great promise,[153][154] directly related to the potential to produce more biomass per unit area in a year than any other form of biomass. The break-even point for algae-based biofuels is estimated to occur by 2025.[155]
Fertilizer
[edit]
For centuries, seaweed has been used as a fertilizer; George Owen of Henllys writing in the 16th century referring to drift weed in South Wales:[156]
This kind of ore they often gather and lay on great heapes, where it heteth and rotteth, and will have a strong and loathsome smell; when being so rotten they cast on the land, as they do their muck, and thereof springeth good corn, especially barley ... After spring-tydes or great rigs of the sea, they fetch it in sacks on horse backes, and carie the same three, four, or five miles, and cast it on the lande, which doth very much better the ground for corn and grass.
Today, algae are used by humans in many ways; for example, as fertilizers, soil conditioners, and livestock feed.[157] Aquatic and microscopic species are cultured in clear tanks or ponds and are either harvested or used to treat effluents pumped through the ponds. Algaculture on a large scale is an important type of aquaculture in some places. Maerl is commonly used as a soil conditioner.[158]
Food industry
[edit]Algae are used as foods in many countries: China consumes more than 70 species, including fat choy, a cyanobacterium considered a vegetable; Japan, over 20 species such as nori and aonori;[159] Ireland, dulse; Chile, cochayuyo.[160] Laver is used to make laverbread in Wales, where it is known as bara lawr. In Korea, green laver is used to make gim.[161]
Three forms of algae used as food:
- Chlorella: This form of alga is found in freshwater and contains photosynthetic pigments in its chloroplasts.[162]
- Klamath AFA: A subspecies of Aphanizomenon flos-aquae found wild in many bodies of water worldwide but harvested only from Upper Klamath Lake, Oregon.[163]
- Spirulina: Known otherwise as a cyanobacterium (a prokaryote or a "blue-green alga")[164]
The oils from some algae have high levels of unsaturated fatty acids. Some varieties of algae favored by vegetarianism and veganism contain the long-chain, essential omega-3 fatty acids, docosahexaenoic acid (DHA) and eicosapentaenoic acid (EPA).[165] Fish oil contains the omega-3 fatty acids, but the original source is algae (microalgae in particular), which are eaten by marine life such as copepods and are passed up the food chain.[165]
The natural pigments (carotenoids and chlorophylls) produced by algae can be used as alternatives to chemical dyes and coloring agents.[166] The presence of some individual algal pigments, together with specific pigment concentration ratios, are taxon-specific: analysis of their concentrations with various analytical methods, particularly high-performance liquid chromatography, can therefore offer deep insight into the taxonomic composition and relative abundance of natural algae populations in sea water samples.[167][168]
Carrageenan, from the red alga Chondrus crispus, is used as a stabilizer in milk products.[citation needed]
Gelling agents
[edit]Agar, a gelatinous substance derived from red algae, has a number of commercial uses.[169] It is a good medium on which to grow bacteria and fungi, as most microorganisms cannot digest agar.[170]
Alginic acid, or alginate, is extracted from brown algae. Its uses range from gelling agents in food, to medical dressings. Alginic acid also has been used in the field of biotechnology as a biocompatible medium for cell encapsulation and cell immobilization. Molecular cuisine is also a user of the substance for its gelling properties, by which it becomes a delivery vehicle for flavours.[171]
Between 100,000 and 170,000 wet tons of Macrocystis are harvested annually in New Mexico for alginate extraction and abalone feed.[172][173]
Pollution control and bioremediation
[edit]- Sewage can be treated with algae,[174] reducing the use of large amounts of toxic chemicals that would otherwise be needed.
- Algae can be used to capture fertilizers in runoff from farms. When subsequently harvested, the enriched algae can be used as fertilizer.[175]
- Aquaria and ponds can be filtered using algae, which absorb nutrients from the water in a device called an algae scrubber, also known as an algae turf scrubber.[176][177]
Agricultural Research Service scientists found that 60–90% of nitrogen runoff and 70–100% of phosphorus runoff can be captured from manure effluents using a horizontal algae scrubber, also called an algal turf scrubber (ATS). Scientists developed the ATS, which consists of shallow, 100-foot raceways of nylon netting where algae colonies can form, and studied its efficacy for three years. They found that algae can readily be used to reduce the nutrient runoff from agricultural fields and increase the quality of water flowing into rivers, streams, and oceans. Researchers collected and dried the nutrient-rich algae from the ATS and studied its potential as an organic fertilizer. They found that cucumber and corn seedlings grew just as well using ATS organic fertilizer as they did with commercial fertilizers.[178] Algae scrubbers, using bubbling upflow or vertical waterfall versions, are now also being used to filter aquaria and ponds.[citation needed]
The alga Stichococcus bacillaris has been seen to colonize silicone resins used at archaeological sites; biodegrading the synthetic substance.[179]
Bioplastics
[edit]Various polymers can be created from algae, which can be especially useful in the creation of bioplastics. These include hybrid plastics, cellulose-based plastics, poly-lactic acid, and bio-polyethylene.[180] Several companies have begun to produce algae polymers commercially, including for use in flip-flops[181] and in surf boards.[182] Even algae is also used to prepare various polymeric resins suitable for coating applications.[183][184][185]
Additional images
[edit]See also
[edit]Notes
[edit]- ^ Some botanists restrict the name algae to eukaryotes, which does not include cyanobacteria, which are prokaryotes.[citation needed]
- ^ a b c d Chlorarachniophytes were omitted from the 2024 AlgaeBase species report. The numbers shown here for the order Chlorarachniales were obtained from the 13th edition of Syllabus der Pflanzenfamilien (2015), where it contains 8 genera and 14 species total.[32] The two remaining chlorarachniophyte genera, Minorisa and Rhabdamoeba, have one species each.[33][34]
References
[edit]- ^ a b c Guiry, Michael D. (2024). "How many species of algae are there? A reprise. Four kingdoms, 14 phyla, 63 classes and still growing". Journal of Phycology. 60 (2): 214–228. Bibcode:2024JPcgy..60..214G. doi:10.1111/jpy.13431. PMID 38245909.
- ^ Guiry, M.D. & Guiry, G.M. 2025. AlgaeBase. World-wide electronic publication, University of Galway. https://www.algaebase.org; searched on 25 May 2025.
- ^ "ALGAE | English meaning - Cambridge Dictionary". Retrieved 6 April 2023.
- ^ a b Nabors, Murray W. (2004). Introduction to Botany. San Francisco: Pearson Education, Inc. ISBN 978-0-8053-4416-5.
- ^ a b c Keeling, Patrick J. (2004). "Diversity and evolutionary history of plastids and their hosts". American Journal of Botany. 91 (10): 1481–1493. Bibcode:2004AmJB...91.1481K. doi:10.3732/ajb.91.10.1481. PMID 21652304.
- ^ Palmer, J. D.; Soltis, D. E.; Chase, M. W. (2004). "The plant tree of life: an overview and some points of view". American Journal of Botany. 91 (10): 1437–1445. doi:10.3732/ajb.91.10.1437. PMID 21652302.
- ^ Smithsonian National Museum of Natural History; Department of Botany. "Algae Research". Archived from the original on 2 July 2010. Retrieved 25 August 2010.
- ^ Pringsheim, E. G. 1963. Farblose Algen. Ein beitrag zur Evolutionsforschung. Gustav Fischer Verlag, Stuttgart. 471 pp., species:Algae#Pringsheim (1963).
- ^ Tartar, A.; Boucias, D. G.; Becnel, J. J.; Adams, B. J. (2003). "Comparison of plastid 16S rRNA (rrn 16) genes from Helicosporidium spp.: Evidence supporting the reclassification of Helicosporidia as green algae (Chlorophyta)". International Journal of Systematic and Evolutionary Microbiology. 53 (Pt 6): 1719–1723. doi:10.1099/ijs.0.02559-0. PMID 14657099.
- ^ Figueroa-Martinez, F.; Nedelcu, A. M.; Smith, D. R.; Reyes-Prieto, A. (2015). "When the lights go out: the evolutionary fate of free-living colorless green algae". New Phytologist. 206 (3): 972–982. Bibcode:2015NewPh.206..972F. doi:10.1111/nph.13279. PMC 5024002. PMID 26042246.
- ^ Bengtson, S.; Belivanova, V.; Rasmussen, B.; Whitehouse, M. (2009). "The controversial 'Cambrian' fossils of the Vindhyan are real but more than a billion years older". Proceedings of the National Academy of Sciences of the United States of America. 106 (19): 7729–7734. Bibcode:2009PNAS..106.7729B. doi:10.1073/pnas.0812460106. PMC 2683128. PMID 19416859.
- ^ Paul, Vishal; Chandra Shekharaiah, P. S.; Kushwaha, Shivbachan; Sapre, Ajit; Dasgupta, Santanu; Sanyal, Debanjan (2020). "Role of Algae in CO2 Sequestration Addressing Climate Change: A Review". In Deb, Dipankar; Dixit, Ambesh; Chandra, Laltu (eds.). Renewable Energy and Climate Change. Smart Innovation, Systems and Technologies. Vol. 161. Singapore: Springer. pp. 257–265. doi:10.1007/978-981-32-9578-0_23. ISBN 978-981-329-578-0. S2CID 202902934.
- ^ "alga, algae". Webster's Third New International Dictionary of the English Language Unabridged with Seven Language Dictionary. Vol. 1. Encyclopædia Britannica, Inc. 1986.
- ^ Partridge, Eric (1983). "algae". Origins. Greenwich House. ISBN 9780517414255.
- ^ Lewis, Charlton T.; Short, Charles (1879). "Alga". A Latin Dictionary. Oxford: Clarendon Press. Retrieved 31 December 2017.
- ^ Cheyne, Thomas Kelly; Black, John Sutherland (1902). Encyclopædia biblica: A critical dictionary of the literary, political and religious history, the archæology, geography, and natural history of the Bible. Macmillan Company. p. 3525.
- ^ Lee, Robert Edward, ed. (2008), "Basic characteristics of the algae", Phycology (4 ed.), Cambridge: Cambridge University Press, pp. 3–30, doi:10.1017/CBO9780511812897.002, ISBN 978-1-107-79688-1, retrieved 13 September 2023
- ^ a b Lee, Robert Edward (2008). Phycology. Cambridge University Press. ISBN 9780521367448.
- ^ a b c d e f Graham, Linda E.; Graham, James M.; Wilcox, Lee W.; Cook, Martha E. (2022). "Chapter 1. Introduction to the Algae". Algae (4th ed.). LJLM Press. ISBN 978-0-9863935-4-9.
- ^ a b c d Xiao, S.; Knoll, A. H.; Yuan, X.; Pueschel, C. M. (2004). "Phosphatized multicellular algae in the Neoproterozoic Doushantuo Formation, China, and the early evolution of florideophyte red algae". American Journal of Botany. 91 (2): 214–227. doi:10.3732/ajb.91.2.214. PMID 21653378.
- ^ Waggoner, Ben (1994–2008). "Introduction to the Phaeophyta: Kelps and brown "Algae"". University of California Museum of Palaeontology (UCMP). Archived from the original on 21 December 2008. Retrieved 19 December 2008.
- ^ Thomas, D. N. (2002). Seaweeds. London: The Natural History Museum. ISBN 978-0-565-09175-0.
- ^ Waggoner, Ben (1994–2008). "Introduction to the Rhodophyta, the red 'algae'". University of California Museum of Palaeontology (UCMP). Archived from the original on 18 December 2008. Retrieved 19 December 2008.
- ^ "Introduction to the Green Algae". berkeley.edu. Archived from the original on 13 February 2007. Retrieved 15 February 2007.
- ^ a b c d Connell, Sean; Foster, M.S.; Airoldi, Laura (9 January 2014). "What are algal turfs? Towards a better description of turfs". Marine Ecology Progress Series. 495: 299–307. Bibcode:2014MEPS..495..299C. doi:10.3354/meps10513.
- ^ Tazawa, Masashi (2010). "Sixty Years Research with Characean Cells: Fascinating Material for Plant Cell Biology". Progress in Botany 72. Vol. 72. Springer. pp. 5–34. doi:10.1007/978-3-642-13145-5_1. ISBN 978-3-642-13145-5. Retrieved 7 October 2012.
- ^ Tarakhovskaya, E. R.; Maslov, Yu. I.; Shishova, M. F. (April 2007). "Phytohormones in algae". Russian Journal of Plant Physiology. 54 (2): 163–170. Bibcode:2007RuJPP..54..163T. doi:10.1134/s1021443707020021. S2CID 27373543.
- ^ Frenkel, J.; Vyverman, W.; Pohnert, G. (2014). "Pheromone signaling during sexual reproduction in algae". Plant J. 79 (4): 632–644. Bibcode:2014PlJ....79..632F. doi:10.1111/tpj.12496. PMID 24597605.
- ^ Bernstein, Harris; Byerly, Henry C.; Hopf, Frederic A.; Michod, Richard E. (20 September 1985). "Genetic Damage, Mutation, and the Evolution of Sex". Science. 229 (4719): 1277–1281. Bibcode:1985Sci...229.1277B. doi:10.1126/science.3898363. ISSN 0036-8075. PMID 3898363.
- ^ Otto, S. P. (2009). "The evolutionary enigma of sex". Am. Nat. 174 (Suppl 1): S1 – S14. Bibcode:2009ANat..174S...1O. doi:10.1086/599084. PMID 19441962. S2CID 9250680. Archived from the original on 9 April 2017.
- ^ Heywood, P.; Magee, P. T. (1976). "Meiosis in protists: Some structural and physiological aspects of meiosis in algae, fungi, and protozoa". Bacteriol Rev. 40 (1): 190–240. doi:10.1128/MMBR.40.1.190-240.1976. PMC 413949. PMID 773364.
- ^ Kawai, Hiroshi; Nakayama, Takeshi (2015). "Division Chlorarachniophyta D.J.Hibberd & R.E.Norris / Cercozoa Cavalier-Smith". In Frey, Wolfgang (ed.). Syllabus of Plant Families: A. Engler's Syllabus der Pflanzenfamilien. Part 2/1: Photoautotrophic eukaryotic Algae: Glaucocystophyta, Cryptophyta, Dinophyta/Dinozoa, Haptophyta, Heterokontophyta/Ochrophyta, Chlorarachniophyta/Cercozoa, Euglenophyta/Euglenozoa, Chlorophyta, Streptophyta p.p. Stuttgart: Gebr. Borntraeger Verlagsbuchhandlung. ISBN 978-3-443-01083-6.
- ^ del Campo, Javier; Not, Fabrice; Forn, Irene; Sieracki, Michael E; Massana, Ramon (1 February 2013). "Taming the smallest predators of the oceans" (PDF). The ISME Journal. 7 (2): 351–358. Bibcode:2013ISMEJ...7..351D. doi:10.1038/ismej.2012.85. ISSN 1751-7362. PMC 3554395. PMID 22810060. Retrieved 20 May 2025.
- ^ Shiratori, Takashi; Ishida, Ken-ichiro (8 November 2023). "Rhabdamoeba marina is a heterotrophic relative of chlorarachnid algae". Journal of Eukaryotic Microbiology. 71 (2) e13010. doi:10.1111/jeu.13010. ISSN 1066-5234. PMID 37941507.
- ^ Guiry, M.D. & Guiry, G.M. 2025. AlgaeBase. World-wide electronic publication, University of Galway. https://www.algaebase.org; searched on 4 June 2025.
- ^ Graham, Linda E.; Graham, James M.; Wilcox, Lee W.; Cook, Martha E. (2022). "Chapter 2. The Roles of Algae in Biochemistry". Algae (4th ed.). LJLM Press. ISBN 978-0-9863935-4-9.
- ^ Fidor, Anna; Konkel, Robert; Mazur-Marzec, Hanna (29 September 2019). "Bioactive Peptides Produced by Cyanobacteria of the Genus Nostoc: A Review". Marine Drugs. 17 (10): 561. doi:10.3390/md17100561. ISSN 1660-3397. PMC 6835634. PMID 31569531.
- ^ Gupta, Radhey S. (2003). "Evolutionary relationships among photosynthetic bacteria". Photosynthesis Research. 76 (1–3): 173–183. Bibcode:2003PhoRe..76..173G. doi:10.1023/A:1024999314839. PMID 16228576.
- ^ Castenholz, Richard W. (14 September 2015). "Oxygenic Photosynthetic Bacteria". Bergey's Manual of Systematics of Archaea and Bacteria. John Wiley & Sons, Inc., in association with Bergey's Manual Trust. p. 1. doi:10.1002/9781118960608.cbm00020. ISBN 9781118960608.
- ^ a b Graham, Linda E.; Graham, James M.; Wilcox, Lee W.; Cook, Martha E. (2022). "Chapter 6. Cyanobacteria". Algae (4th ed.). LJLM Press. ISBN 978-0-9863935-4-9.
- ^ Matheus Carnevali, Paula B.; Schulz, Frederik; Castelle, Cindy J.; Kantor, Rose S.; Shih, Patrick M.; Sharon, Itai; Santini, Joanne M.; Olm, Matthew R.; Amano, Yuki; Thomas, Brian C.; Anantharaman, Karthik; Burstein, David; Becraft, Eric D.; Stepanauskas, Ramunas; Woyke, Tanja; Banfield, Jillian F. (28 January 2019). "Hydrogen-based metabolism as an ancestral trait in lineages sibling to the Cyanobacteria" (PDF). Nature Communications. 10 (1): 463. Bibcode:2019NatCo..10..463M. doi:10.1038/s41467-018-08246-y. ISSN 2041-1723. PMC 6349859. PMID 30692531. Retrieved 21 May 2025.
- ^ a b Soo, Rochelle M.; Hemp, James; Hugenholtz, Philip (2019). "Evolution of photosynthesis and aerobic respiration in the cyanobacteria". Free Radical Biology and Medicine. 140: 200–205. doi:10.1016/j.freeradbiomed.2019.03.029. PMID 30930297.
- ^ a b Strunecký, Otakar; Ivanova, Anna Pavlovna; Mareš, Jan (2023). "An updated classification of cyanobacterial orders and families based on phylogenomic and polyphasic analysis". Journal of Phycology. 59 (1): 12–51. Bibcode:2023JPcgy..59...12S. doi:10.1111/jpy.13304. ISSN 0022-3646. PMID 36443823.
- ^ a b Soo, Rochelle M.; Hemp, James; Parks, Donovan H.; Fischer, Woodward W.; Hugenholtz, Philip (31 March 2017). "On the origins of oxygenic photosynthesis and aerobic respiration in Cyanobacteria". Science. 355 (6332): 1436–1440. Bibcode:2017Sci...355.1436S. doi:10.1126/science.aal3794. ISSN 0036-8075. PMID 28360330.
- ^ Pinevich, Alexander; Averina, Svetlana (2021). "New life for old discovery: amazing story about how bacterial predation on Chlorella resolved a paradox of dark cyanobacteria an gave the key to early history of oxygenic photosynthesis and aerobic respiration". Protistology. 15 (3): 107–126. doi:10.21685/1680-0826-2021-15-3-2.
- ^ Sciuto, Katia; Moro, Isabella (2015). "Cyanobacteria: the bright and dark sides of a charming group". Biodiversity and Conservation. 24 (4): 711–738. Bibcode:2015BiCon..24..711S. doi:10.1007/s10531-015-0898-4. ISSN 0960-3115.
- ^ Singh, K. (2021). "Salient features of Protochlorophyta". Innovative Research Thoughts. 7 (4): 70–77.
- ^ Turland, Nicholas J.; Wiersema, John H.; Barrie, Fred R.; Greuter, Werner; Hawksworth, David L.; Herendeen, Patrick S.; Knapp, Sandra; Kusber, Wolf-Henning; Li, De-Zhu; Marhold, Karol; May, Tom W.; McNeill, John; Monro, Anna M.; Prado, Jefferson; Price, Michelle J.; Smith, Gideon F., eds. (2018). International Code of Nomenclature for algae, fungi, and plants (Shenzhen Code) adopted by the Nineteenth International Botanical Congress Shenzhen, China, July 2017. Regnum Vegetabile. Vol. 159. Glashütten: Koeltz Botanical Books. doi:10.12705/Code.2018. hdl:10141/622572. ISBN 978-3-946583-16-5. Retrieved 21 May 2025. Preamble, paragraph 8:
The provisions of this Code apply to all organisms traditionally treated as algae, fungi, or plants, whether fossil or non-fossil, including blue-green algae (Cyanobacteria)
- ^ Allaby, M., ed. (1992). "Alga". The Concise Dictionary of Botany. Oxford University Press.
- ^ Sato, Naoki (27 May 2021). "Are Cyanobacteria an Ancestor of Chloroplasts or Just One of the Gene Donors for Plants and Algae?". Genes. 12 (6): 823. doi:10.3390/genes12060823. ISSN 2073-4425. PMC 8227023. PMID 34071987.
- ^ Gabr, Arwa; Grossman, Arthur R.; Bhattacharya, Debashish (August 2020). "Paulinella, a model for understanding plastid primary endosymbiosis". Journal of Phycology. 56 (4): 837–843. Bibcode:2020JPcgy..56..837G. doi:10.1111/jpy.13003. ISSN 1529-8817. PMC 7734844. PMID 32289879.
- ^ Delaye, Luis; Valadez-Cano, Cecilio; Pérez-Zamorano, Bernardo (2016). "How Really Ancient Is Paulinella Chromatophora?". PLOS Currents. 8. doi:10.1371/currents.tol.e68a099364bb1a1e129a17b4e06b0c6b. ISSN 2157-3999. PMC 4866557. PMID 28515968.
- ^ Losos, Jonathan B.; Mason, Kenneth A.; Singer, Susan R. (2007). Biology (8 ed.). McGraw-Hill. ISBN 978-0-07-304110-0.
- ^ Bicudo, Carlos E. de M.; Menezes, Mariângela (16 March 2016). "Phylogeny and Classification of Euglenophyceae: A Brief Review". Frontiers in Ecology and Evolution. 4: 17. Bibcode:2016FrEEv...4...17B. doi:10.3389/fevo.2016.00017.
- ^ Kalina, T (2001). "The origin of chloroplasts and the position of eukaryotic algae in the six- kingdom system of life". Czech Phycology. 1 (1): 1-4.
- ^ McFadden, Geoffrey I.; Gilson, Paul R.; Hofmann, Claudia J. B. (1997). "Division Chlorarachniophyta". Origins of Algae and their Plastids. Plant Systematics and Evolution. Vol. 11. pp. 175–185. doi:10.1007/978-3-7091-6542-3_10. ISBN 978-3-211-83035-2.
- ^ Ishida, K; Green, B R; Cavalier-Smith, T (1999). "Diversification of a Chimaeric Algal Group, the Chlorarachniophytes: Phylogeny of Nuclear and Nucleomorph Small-Subunit rRNA Genes". Molecular Biology &Evolution. 16 (3): 321-331. doi:10.1093/oxfordjournals.molbev.a026113.
- ^ Archibald, J. M.; Keeling, P. J. (November 2002). "Recycled plastids: A 'green movement' in eukaryotic evolution". Trends in Genetics. 18 (11): 577–584. doi:10.1016/S0168-9525(02)02777-4. PMID 12414188.
- ^ O'Neill, Ellis C.; Trick, Martin; Henrissat, Bernard; Field, Robert A. (2015). "Euglena in time: Evolution, control of central metabolic processes and multi-domain proteins in carbohydrate and natural product biochemistry". Perspectives in Science. 6: 84–93. Bibcode:2015PerSc...6...84O. doi:10.1016/j.pisc.2015.07.002.
- ^ Ponce-Toledo, Rafael I.; López-García, Purificación; Moreira, David (October 2019). "Horizontal and endosymbiotic gene transfer in early plastid evolution". New Phytologist. 224 (2): 618–624. Bibcode:2019NewPh.224..618P. doi:10.1111/nph.15965. ISSN 0028-646X. PMC 6759420. PMID 31135958.
- ^ Ponce-Toledo, Rafael I; Moreira, David; López-García, Purificación; Deschamps, Philippe (19 June 2018). "Secondary Plastids of Euglenids and Chlorarachniophytes Function with a Mix of Genes of Red and Green Algal Ancestry". Molecular Biology and Evolution. 35 (9): 2198–2204. doi:10.1093/molbev/msy121. ISSN 0737-4038. PMC 6949139. PMID 29924337.
- ^ Janson, Sven; Graneli, Edna (September 2003). "Genetic analysis of the psbA gene from single cells indicates a cryptomonad origin of the plastid in Dinophysis (Dinophyceae)". Phycologia. 42 (5): 473–477. Bibcode:2003Phyco..42..473J. doi:10.2216/i0031-8884-42-5-473.1. ISSN 0031-8884. S2CID 86730888.
- ^ Wegener Parfrey, Laura; Barbero, Erika; Lasser, Elyse; Dunthorn, Micah; Bhattacharya, Debashish; Patterson, David J.; Katz, Laura A (December 2006). "Evaluating Support for the Current Classification of Eukaryotic Diversity". PLOS Genetics. 2 (12): e220. doi:10.1371/journal.pgen.0020220. PMC 1713255. PMID 17194223.
- ^ Burki, F.; Shalchian-Tabrizi, K.; Minge, M.; Skjæveland, Å.; Nikolaev, S. I.; et al. (2007). Butler, Geraldine (ed.). "Phylogenomics Reshuffles the Eukaryotic Supergroups". PLOS ONE. 2 (8): e790. Bibcode:2007PLoSO...2..790B. doi:10.1371/journal.pone.0000790. PMC 1949142. PMID 17726520.
- ^ Moore RB; Oborník M; Janouskovec J; Chrudimský T; Vancová M; Green DH; Wright SW; Davies NW; et al. (February 2008). "A photosynthetic alveolate closely related to apicomplexan parasites". Nature. 451 (7181): 959–963. Bibcode:2008Natur.451..959M. doi:10.1038/nature06635. PMID 18288187. S2CID 28005870.
- ^ Janouškovec, J.; Tikhonenkov, D.V.; Burki, F.; Howe, A.T.; Kolísko, M.; Mylnikov, A.P.; Keeling, P.J. (2015). "Factors mediating plastid dependency and the origins of parasitism in apicomplexans and their close relatives". PNAS. 112 (33): 10200–10207. Bibcode:2015PNAS..11210200J. doi:10.1073/pnas.1423790112. PMC 4547307. PMID 25717057.
- ^ Linnæus, Caroli (1753). Species Plantarum. Vol. 2. Impensis Laurentii Salvii. p. 1131.
- ^ Sharma, O. P. (1 January 1986). Textbook of Algae. Tata McGraw-Hill. p. 22. ISBN 9780074519288.
- ^ Gmelin, S. G. (1768). Historia Fucorum. St. Petersburg: Ex typographia Academiae scientiarum – via Google Books.
- ^ Silva, P. C.; Basson, P. W.; Moe, R. L. (1996). Catalogue of the Benthic Marine Algae of the Indian Ocean. University of California Press. ISBN 9780520915817 – via Google Books.
- ^ a b Medlin, Linda K.; Kooistra, Wiebe H. C. F.; Potter, Daniel; Saunders, Gary W.; Anderson, Robert A. (1997). "Phylogenetic relationships of the 'golden algae' (haptophytes, heterokont chromophytes) and their plastids" (PDF). Plant Systematics and Evolution: 188. Archived (PDF) from the original on 5 October 2013.
- ^ Dixon, P. S. (1973). Biology of the Rhodophyta. Edinburgh: Oliver & Boyd. p. 232. ISBN 978-0-05-002485-0.
- ^ Harvey, D. (1836). "Algae" (PDF). In Mackay, J. T. (ed.). Flora hibernica comprising the Flowering Plants Ferns Characeae Musci Hepaticae Lichenes and Algae of Ireland arranged according to the natural system with a synopsis of the genera according to the Linnaean system. pp. 157–254. Archived (PDF) from the original on 9 October 2022. Retrieved 31 December 2017..
- ^ Braun, A. Algarum unicellularium genera nova et minus cognita, praemissis observationibus de algis unicellularibus in genere (New and less known genera of unicellular algae, preceded by observations respecting unicellular algae in general) Archived 20 April 2016 at the Wayback Machine. Lipsiae, Apud W. Engelmann, 1855. Translation at: Lankester, E. & Busk, G. (eds.). Quarterly Journal of Microscopical Science, 1857, vol. 5, (17), 13–16 Archived 4 March 2016 at the Wayback Machine; (18), 90–96 Archived 5 March 2016 at the Wayback Machine; (19), 143–149 Archived 4 March 2016 at the Wayback Machine.
- ^ Siebold, C. Th. v. "Ueber einzellige Pflanzen und Thiere (On unicellular plants and animals) Archived 26 November 2014 at the Wayback Machine". In: Siebold, C. Th. v. & Kölliker, A. (1849). Zeitschrift für wissenschaftliche Zoologie, Bd. 1, p. 270. Translation at: Lankester, E. & Busk, G. (eds.). Quarterly Journal of Microscopical Science, 1853, vol. 1, (2), 111–121 Archived 4 March 2016 at the Wayback Machine; (3), 195–206 Archived 4 March 2016 at the Wayback Machine.
- ^ a b Ragan, Mark (3 June 2010). "On the delineation and higher-level classification of algae". European Journal of Phycology. 33 (1): 1–15. doi:10.1080/09670269810001736483. Retrieved 16 February 2024.
- ^ de Jussieu, Antoine Laurent (1789). Genera plantarum secundum ordines naturales disposita. Parisiis, Apud Viduam Herissant et Theophilum Barrois. p. 6.
- ^ Khan, Amna Komal; Kausar, Humera; Jaferi, Syyada Samra; et al. (6 November 2020). "An Insight into the Algal Evolution and Genomics". Biomolecules. 10 (11): 1524. doi:10.3390/biom10111524. PMC 7694994. PMID 33172219.
- ^ "Compte rendu du premier colloque de l'association des Diatomistes de Langue Française. Paris, 25 janvier 1980". Cryptogamie. Algologie. 1 (1): 67–74. 1980. Bibcode:1980CrypA...1...67.. doi:10.5962/p.308988. ISSN 0181-1568.
- ^ Corliss, J O (1995). "The ambiregnal protists and the codes of nomenclature: a brief review of the problem and of proposed solutions". The Bulletin of Zoological Nomenclature. 52: 11–17. doi:10.5962/bhl.part.6717. ISSN 0007-5167.
- ^ Williams, B. A.; Keeling, P. J. (2003). "Cryptic organelles in parasitic protists and fungi". In Littlewood, D. T. J. (ed.). The Evolution of Parasitism. London: Elsevier Academic Press. p. 46. ISBN 978-0-12-031754-7.
- ^ Round (1981). pp. 398–400, Round, F. E. (8 March 1984). The Ecology of Algae. CUP Archive. ISBN 9780521269063. Retrieved 6 February 2015..
- ^ Grabda, Jadwiga; Grabda, Jadwiga (1991). Marine fish parasitology: an outline. Weinheim: VCH-Verl.-Ges. ISBN 978-0-89573-823-3.
- ^ Smith, David Roy; Keeling, Patrick J. (8 September 2016). "Protists and the Wild, Wild West of Gene Expression: New Frontiers, Lawlessness, and Misfits". Annual Review of Microbiology. 70 (1): 161–178. doi:10.1146/annurev-micro-102215-095448. ISSN 0066-4227. PMID 27359218.
- ^ Schirrmeister BE, de Vos JM, Antonelli A, Bagheri HC (January 2013). "Evolution of multicellularity coincided with increased diversification of cyanobacteria and the Great Oxidation Event". Proceedings of the National Academy of Sciences of the United States of America. 110 (5): 1791–1796. Bibcode:2013PNAS..110.1791S. doi:10.1073/pnas.1209927110. PMC 3562814. PMID 23319632.
- ^ Baumgartner, Raphael J.; Van Kranendonk, Martin J.; Wacey, David; Fiorentini, Marco L.; Saunders, Martin; Caruso, Stefano; Pages, Anais; Homann, Martin; Guagliardo, Paul (November 2019). "Nano−porous pyrite and organic matter in 3.5-billion-year-old stromatolites record primordial life". Geology. 47 (11): 1039–1043. Bibcode:2019Geo....47.1039B. doi:10.1130/G46365.1.
- ^ a b c Reyes-Prieto, Adrian; Weber, Andreas P.M.; Bhattacharya, Debashish (2007). "The Origin and Establishment of the Plastid in Algae and Plants". Annual Review of Genetics. 41: 147–168. doi:10.1146/annurev.genet.41.110306.130134. PMID 17600460. Retrieved 3 December 2023.
- ^ a b Khan, Amna Komal; Kausar, Humera; Jaferi, Syyada Samra; Drouet, Samantha; Hano, Christophe; Abbasi, Bilal Haider; Anjum, Sumaira (6 November 2020). "An Insight into the Algal Evolution and Genomics". Biomolecules. 10 (11): 1524. doi:10.3390/biom10111524. PMC 7694994. PMID 33172219.
- ^ Butterfield, N. J. (2000). "Bangiomorpha pubescens n. gen., n. sp.: Implications for the evolution of sex, multicellularity, and the Mesoproterozoic/Neoproterozoic radiation of eukaryotes". Paleobiology. 26 (3): 386–404. Bibcode:2000Pbio...26..386B. doi:10.1666/0094-8373(2000)026<0386:BPNGNS>2.0.CO;2. ISSN 0094-8373. S2CID 36648568. Archived from the original on 7 March 2007.
- ^ T.M. Gibson (2018). "Precise age of Bangiomorpha pubescens dates the origin of eukaryotic photosynthesis". Geology. 46 (2): 135–138. Bibcode:2018Geo....46..135G. doi:10.1130/G39829.1.
- ^ a b Pietluch, Filip; Mackiewicz, Paweł; Ludwig, Kacper; Gagat, Przemysław (3 September 2024). "A New Model and Dating for the Evolution of Complex Plastids of Red Alga Origin". Genome Biology and Evolution. 16 (9: evae192) evae192. doi:10.1093/gbe/evae192. PMC 11413572. PMID 39240751.
- ^ a b Strassert, Jürgen F. H.; Irisarri, Iker; Williams, Tom A.; Burki, Fabien (25 March 2021). "A molecular timescale for eukaryote evolution with implications for the origin of red algal-derived plastids" (PDF). Nature Communications. 12 (1): 1879. Bibcode:2021NatCo..12.1879S. doi:10.1038/s41467-021-22044-z. ISSN 2041-1723. PMC 7994803. PMID 33767194. Retrieved 13 May 2025.
- ^ Keeling, Patrick J. (2017). "Chlorarachniophytes". In Archibald, John M.; Simpson, Alastair G.B.; Slamovits, Claudio H. (eds.). Handbook of the Protists. Vol. 1 (2nd ed.). Springer. pp. 765–781. doi:10.1007/978-3-319-28149-0_34. ISBN 978-3-319-28147-6.
- ^ Bicudo, Carlos E. de M.; Menezes, Mariângela (16 March 2016). "Phylogeny and Classification of Euglenophyceae: A Brief Review". Frontiers in Ecology and Evolution. 4: 17. Bibcode:2016FrEEv...4...17B. doi:10.3389/fevo.2016.00017. ISSN 2296-701X.
- ^ Eliáš, Marek (2021). "Protist diversity: Novel groups enrich the algal tree of life". Current Biology. 31 (11): R733 – R735. Bibcode:2021CBio...31.R733E. doi:10.1016/j.cub.2021.04.025. PMID 34102125. Retrieved 13 May 2025.
- ^ Stiller, John W.; Schreiber, John; Yue, Jipei; Guo, Hui; Ding, Qin; Huang, Jinling (10 December 2014). "The evolution of photosynthesis in chromist algae through serial endosymbioses" (PDF). Nature Communications. 5 (1): 5764. Bibcode:2014NatCo...5.5764S. doi:10.1038/ncomms6764. ISSN 2041-1723. PMC 4284659. PMID 25493338. Retrieved 13 May 2025.
- ^ Bodył, Andrzej; Stiller, John W.; Mackiewicz, Paweł (2009). "Chromalveolate plastids: direct descent or multiple endosymbioses?". Trends in Ecology & Evolution. 24 (3): 119–121. Bibcode:2009TEcoE..24..119B. doi:10.1016/j.tree.2008.11.003. PMID 19200617. Retrieved 13 May 2025.
- ^ Noble, Ivan (18 September 2003). "When plants conquered land". BBC. Archived from the original on 11 November 2006.
- ^ Wellman, C. H.; Osterloff, P. L.; Mohiuddin, U. (2003). "Fragments of the earliest land plants". Nature. 425 (6955): 282–285. Bibcode:2003Natur.425..282W. doi:10.1038/nature01884. PMID 13679913. S2CID 4383813. Archived from the original on 30 August 2017.
- ^ Kenrick, P.; Crane, P.R. (1997). The origin and early diversification of land plants. A cladistic study. Washington: Smithsonian Institution Press. ISBN 978-1-56098-729-1.
- ^ Raven, J.A.; Edwards, D. (2001). "Roots: evolutionary origins and biogeochemical significance". Journal of Experimental Botany. 52 (90001): 381–401. doi:10.1093/jexbot/52.suppl_1.381. PMID 11326045.
- ^ Knauth, L. Paul; Kennedy, Martin J. (2009). "The late Precambrian greening of the Earth". Nature. 460 (7256): 728–732. Bibcode:2009Natur.460..728K. doi:10.1038/nature08213. PMID 19587681. S2CID 4398942.
- ^ Strother, Paul K.; Battison, Leila; Brasier, Martin D.; Wellman, Charles H. (2011). "Earth's earliest non-marine eukaryotes". Nature. 473 (7348): 505–509. Bibcode:2011Natur.473..505S. doi:10.1038/nature09943. PMID 21490597. S2CID 4418860.
- ^ a b c d Round, F. E. (1981). "Chapter 8, Dispersal, continuity and phytogeography". The ecology of algae. CUP Archive. pp. 357–361. ISBN 9780521269063 – via Google Books.
- ^ Round (1981), p. 362.
- ^ Round (1981), p. 357.
- ^ Round (1981), p. 371.
- ^ Round (1981), p. 366.
- ^ "Algae Herbarium". National Museum of Natural History, Department of Botany. 2008. Archived from the original on 1 December 2008. Retrieved 19 December 2008.
- ^ John (2002), p. 1.
- ^ Huisman (2000), p. 25.
- ^ Stegenga (1997).
- ^ Clerck, Olivier (2005). Guide to the seaweeds of KwaZulu-Natal. National Botanic Garden of Belgium. ISBN 978-90-72619-64-8.
- ^ Abbott and Hollenberg (1976), p. 2.
- ^ Hardy and Guiry (2006).
- ^ Round (1981), p. 176.
- ^ "Greenland Has a Mysterious 'Dark Zone' — And It's Getting Even Darker". Space.com. 10 April 2018.
- ^ "Alpine glacier turning pink due to algae that accelerates climate change, scientists say". Sky News. 6 July 2020.
- ^ Smayda, Theodore J. (2014). "What is a bloom? A commentary". Limnology and Oceanography. 42 (5part2): 1132–1136. doi:10.4319/lo.1997.42.5_part_2.1132. ISSN 0024-3590.
- ^ a b c Omar, Wan Maznah Wan (December 2010). "Perspectives on the Use of Algae as Biological Indicators for Monitoring and Protecting Aquatic Environments, with Special Reference to Malaysian Freshwater Ecosystems". Trop Life Sci Res. 21 (2): 51–67. PMC 3819078. PMID 24575199.
- ^ Necchi Jr., O. (ed.) (2016). River Algae. Springer, Necchi, Orlando J. R. (2 June 2016). River Algae. Springer. ISBN 9783319319841..
- ^ Johansen, J. R. (2012). "The Diatoms: Applications for the Environmental and Earth Sciences". In Smol, J. P.; Stoermer, E. F. (eds.). Diatoms of aerial habitats (2nd ed.). Cambridge University Press. pp. 465–472. ISBN 9781139492621 – via Google Books.
- ^ Sharma, O. P. (1986). pp. 2–6, [1].
- ^ Brodo, Irwin M.; Sharnoff, Sylvia Duran; Sharnoff, Stephen; Laurie-Bourque, Susan (2001). Lichens of North America. New Haven: Yale University Press. p. 8. ISBN 978-0-300-08249-4.
- ^ Pearson, Lorentz C. (1995). The Diversity and Evolution of Plants. CRC Press. p. 221. ISBN 978-0-8493-2483-3.
- ^ Tuovinen, Veera; Ekman, Stefan; Thor, Göran; Vanderpool, Dan; Spribille, Toby; Johannesson, Hanna (17 January 2019). "Two Basidiomycete Fungi in the Cortex of Wolf Lichens". Current Biology. 29 (3): 476–483.e5. Bibcode:2019CBio...29E.476T. doi:10.1016/j.cub.2018.12.022. ISSN 0960-9822. PMID 30661799.
- ^ Brodo et al. (2001), p. 6: "A species of lichen collected anywhere in its range has the same lichen-forming fungus and, generally, the same photobiont. (A particular photobiont, though, may associate with scores of different lichen fungi)."
- ^ Brodo et al. (2001), p. 8.
- ^ Taylor, Dennis L. (1983). "The coral-algal symbiosis". In Goff, Lynda J. (ed.). Algal Symbiosis: A Continuum of Interaction Strategies. CUP Archive. pp. 19–20. ISBN 978-0-521-25541-7.
- ^ Knight, Susan (Fall 2001). "Are There Sponges in Your Lake?" (PDF). Lake Tides. 26 (4). Wisconsin Lakes Partnership: 4–5. Archived from the original (PDF) on 2 July 2007. Retrieved 4 August 2007 – via UWSP.edu.
- ^ Huesemann, M.; Williams, P.; Edmundson, Scott J.; Chen, P.; Kruk, R.; Cullinan, V.; Crowe, B.; Lundquist, T. (September 2017). "The laboratory environmental algae pond simulator (LEAPS) photobioreactor: Validation using outdoor pond cultures of Chlorella sorokiniana and Nannochloropsis salina". Algal Research. 26: 39–46. Bibcode:2017AlgRe..26...39H. doi:10.1016/j.algal.2017.06.017. ISSN 2211-9264. OSTI 1581797.
- ^ "Seaweed Aquaculture | California Sea Grant". caseagrant.ucsd.edu. Retrieved 15 December 2024.
- ^ Lane, Katie; Derbyshire, Emma; Li, Weili; Brennan, Charles (January 2014). "Bioavailability and Potential Uses of Vegetarian Sources of Omega-3 Fatty Acids: A Review of the Literature". Critical Reviews in Food Science and Nutrition. 54 (5): 572–579. doi:10.1080/10408398.2011.596292. PMID 24261532. S2CID 30307483.
- ^ Winwood, R.J. (2013). "Algal oil as a source of omega-3 fatty acids". Food Enrichment with Omega-3 Fatty Acids. Woodhead Publishing Series in Food Science, Technology and Nutrition. pp. 389–404. doi:10.1533/9780857098863.4.389. ISBN 978-0-85709-428-5.
- ^ Lenihan-Geels, Georgia; Bishop, Karen; Ferguson, Lynnette (18 April 2013). "Alternative Sources of Omega-3 Fats: Can We Find a Sustainable Substitute for Fish?". Nutrients. 5 (4): 1301–1315. doi:10.3390/nu5041301. PMC 3705349. PMID 23598439.
- ^ Venkatesh, G. (1 March 2022). "Circular Bio-economy—Paradigm for the Future: Systematic Review of Scientific Journal Publications from 2015 to 2021". Circular Economy and Sustainability. 2 (1): 231–279. Bibcode:2022CirES...2..231V. doi:10.1007/s43615-021-00084-3. ISSN 2730-5988. S2CID 238768104.
- ^ a b Diaz, Crisandra J.; Douglas, Kai J.; Kang, Kalisa; Kolarik, Ashlynn L.; Malinovski, Rodeon; Torres-Tiji, Yasin; Molino, João V.; Badary, Amr; Mayfield, Stephen P. (2023). "Developing algae as a sustainable food source". Frontiers in Nutrition. 9. doi:10.3389/fnut.2022.1029841. ISSN 2296-861X. PMC 9892066. PMID 36742010.
- ^ a b The State of World Fisheries and Aquaculture 2024. FAO. 7 June 2024. doi:10.4060/cd0683en. ISBN 978-92-5-138763-4.
- ^ In brief, The State of World Fisheries and Aquaculture, 2018 (PDF). FAO. 2018.
- ^ Verdelho Vieira, Vítor; Cadoret, Jean-Paul; Acien, F. Gabriel; Benemann, John (January 2022). "Clarification of Most Relevant Concepts Related to the Microalgae Production Sector". Processes. 10 (1): 175. doi:10.3390/pr10010175. hdl:10835/13146. ISSN 2227-9717.
- ^ Greene, Charles; Scott-Buechler, Celina; Hausner, Arjun; Johnson, Zackary; Lei, Xin Gen; Huntley, Mark (2022). "Transforming the Future of Marine Aquaculture: A Circular Economy Approach". Oceanography: 26–34. doi:10.5670/oceanog.2022.213. ISSN 1042-8275.
- News article about the study: "Nutrient-rich algae could help meet global food demand: Cornell researchers". CTVNews. 20 October 2022. Retrieved 17 November 2022.
- ^ a b Reynolds, Daman; Caminiti, Jeff; Edmundson, Scott; Gao, Song; Wick, Macdonald; Huesemann, Michael (12 July 2022). "Seaweed proteins are nutritionally valuable components in the human diet". The American Journal of Clinical Nutrition. 116 (4): 855–861. doi:10.1093/ajcn/nqac190. ISSN 0002-9165. PMID 35820048.
- ^ "Seaweeds: Plants or Algae?". Point Reyes National Seashore Association. Retrieved 1 December 2018.
- ^ Zhang, Lizhu; Liao, Wei; Huang, Yajun; Wen, Yuxi; Chu, Yaoyao; Zhao, Chao (13 October 2022). "Global seaweed farming and processing in the past 20 years". Food Production, Processing and Nutrition. 4 (1). doi:10.1186/s43014-022-00103-2.
- ^ Buschmann, Alejandro H.; Camus, Carolina; Infante, Javier; Neori, Amir; Israel, Álvaro; Hernández-González, María C.; Pereda, Sandra V.; Gomez-Pinchetti, Juan Luis; Golberg, Alexander; Tadmor-Shalev, Niva; Critchley, Alan T. (2 October 2017). "Seaweed production: overview of the global state of exploitation, farming and emerging research activity". European Journal of Phycology. 52 (4): 391–406. Bibcode:2017EJPhy..52..391B. doi:10.1080/09670262.2017.1365175. ISSN 0967-0262. S2CID 53640917.
- ^ Ask, E.I (1990). Cottonii and Spinosum Cultivation Handbook. Philippines: FMC BioPolymer Corporation. p. 52.
- ^ a b Jones, Nicola (15 March 2023). "Banking on the Seaweed Rush". Hakai Magazine. Retrieved 19 March 2023.
- ^ "Global Seaweed Extract Market Report 2024 - Seaweed Extract Market was Valued at $16.5 Billion in 2023 and is Projected to Grow to $20.9 Billion by 2029" (Press release). Research and Markets. 24 September 2024. Retrieved 26 January 2025 – via GlobeNewswire News Room.
- ^ Wang, Taiping; Yang, Zhaoqing; Davis, Jonathan; Edmundson, Scott J. (1 May 2022). Quantifying Nitrogen Bioextraction by Seaweed Farms – A Real-time Modeling-Monitoring Case Study in Hood Canal, WA (Technical report). Office of Scientific and Technical Information. doi:10.2172/1874372.
- ^ Duarte, Carlos M.; Wu, Jiaping; Xiao, Xi; Bruhn, Annette; Krause-Jensen, Dorte (2017). "Can Seaweed Farming Play a Role in Climate Change Mitigation and Adaptation?". Frontiers in Marine Science. 4: 100. Bibcode:2017FrMaS...4..100D. doi:10.3389/fmars.2017.00100. hdl:10754/623247. ISSN 2296-7745.
- ^ Bindoff, N. L.; Cheung, W. W. L.; Kairo, J. G.; Arístegui, J.; et al. (2019). "Chapter 5: Changing Ocean, Marine Ecosystems, and Dependent Communities" (PDF). IPCC Special Report on the Ocean and Cryosphere in a Changing Climate. pp. 447–587.
- ^ Zhu, Yunhua; Schmidt, Andrew J.; Valdez, Peter J.; Snowden-Swan, Lesley J.; Edmundson, Scott J. (21 March 2022). Hydrothermal Liquefaction and Upgrading of Wastewater-Grown Microalgae: 2021 State of Technology (Report). Pacific Northwest National Lab. (PNNL), Richland, WA (United States). doi:10.2172/1855835. OSTI 1855835.
- ^ Chisti, Y. (May–June 2007). "Biodiesel from microalgae". Biotechnology Advances. 25 (3): 294–306. doi:10.1016/j.biotechadv.2007.02.001. PMID 17350212. S2CID 18234512.
- ^ Yang, Z. K.; Niu, Y. F.; Ma, Y. H.; Xue, J.; Zhang, M. H.; Yang, W. D.; Liu, J. S.; Lu, S. H.; Guan, Y.; Li, H. Y. (4 May 2013). "Molecular and cellular mechanisms of neutral lipid accumulation in diatom following nitrogen deprivation". Biotechnology for Biofuels. 6 (1): 67. Bibcode:2013BB......6...67Y. doi:10.1186/1754-6834-6-67. PMC 3662598. PMID 23642220.
- ^ Wijffels, René H.; Barbosa, Maria J. (2010). "An Outlook on Microalgal Biofuels". Science. 329 (5993): 796–799. Bibcode:2010Sci...329..796W. doi:10.1126/science.1189003. PMID 20705853. S2CID 206526311.
- ^ Read, Clare Sewell (1849). "On the Farming of South Wales: Prize Report". Journal of the Royal Agricultural Society of England. 10: 142–143.
- ^ McHugh, Dennis J. (2003). "9, Other Uses of Seaweeds". A Guide to the Seaweed Industry: FAO Fisheries Technical Paper 441. Rome: Fisheries and Aquaculture Department, Food and Agriculture Organization (FAO) of the United Nations. ISBN 978-92-5-104958-7. Archived from the original on 28 December 2008.
- ^ Wilson, Sian; Blake, Charmaine; Berges, John A; Maggs, Christine A (1 November 2004). "Environmental tolerances of free-living coralline algae (maerl): implications for European marine conservation". Biological Conservation. 120 (2): 279–289. Bibcode:2004BCons.120..279W. doi:10.1016/j.biocon.2004.03.001. ISSN 0006-3207.
- ^ Mondragón, Jennifer; Mondragón, Jeff (2003). Seaweeds of the Pacific Coast. Monterey, California: Sea Challengers Publications. ISBN 978-0-930118-29-7.
- ^ "Durvillaea antarctica (Chamisso) Hariot". AlgaeBase.
- ^ "Laver Archives". Kimchimari. Retrieved 15 December 2024.
- ^ "Clorella Description and Uses". Britannica. Retrieved 27 July 2025.
- ^ "AFA". Klamathafa. Retrieved 27 July 2025.
- ^ Brown, Wyatt; Jantz, Katie; Milazzo, Nick (fact checker). "Spirulina". Examine. Retrieved 27 July 2025.
- ^ a b "Vegetarian EPA DHA and Essential Fats". 22 May 2013. Archived from the original on 22 May 2013. Retrieved 15 December 2024.
- ^ Arad, Shoshana; Spharim, Ishai (1998). "Production of Valuable Products from Microalgae: An Emerging Agroindustry". In Altman, Arie (ed.). Agricultural Biotechnology. Books in Soils, Plants, and the Environment. Vol. 61. CRC Press. p. 638. ISBN 978-0-8247-9439-2.
- ^ Rathbun, C.; Doyle, A.; Waterhouse, T. (June 1994). "Measurement of Algal Chlorophylls and Carotenoids by HPLC" (PDF). Joint Global Ocean Flux Study Protocols. 13: 91–96. Archived from the original (PDF) on 4 March 2016. Retrieved 7 July 2014.
- ^ Latasa, M.; Bidigare, R. (1998). "A comparison of phytoplankton populations of the Arabian Sea during the Spring Intermonsoon and Southwest Monsoon of 1995 as described by HPLC-analyzed pigments". Deep-Sea Research Part II. 45 (10–11): 2133–2170. Bibcode:1998DSRII..45.2133L. doi:10.1016/S0967-0645(98)00066-6.
- ^ Lewis, J. G.; Stanley, N. F.; Guist, G. G. (1988). "9. Commercial production of algal hydrocolloides". In Lembi, C. A.; Waaland, J. R. (eds.). Algae and Human Affairs. Cambridge University Press. ISBN 978-0-521-32115-0.
- ^ Roy, Anupam; Shrivastava, Shanker Lal; Mandal, Santi M. (1 January 2016), Grumezescu, Alexandru Mihai (ed.), "5 - Self-assembled carbohydrate nanostructures: synthesis strategies to functional application in food", Novel Approaches of Nanotechnology in Food, Nanotechnology in the Agri-Food Industry, Academic Press, pp. 133–164, ISBN 978-0-12-804308-0, retrieved 26 June 2025
- ^ Holdt, Susan Løvstad; Kraan, Stefan (2011). "Bioactive compounds in seaweed: functional food applications and legislation". Journal of Applied Phycology. 23 (3): 543–597. Bibcode:2011JAPco..23..543H. doi:10.1007/s10811-010-9632-5. ISSN 0921-8971.
- ^ "Macrocystis C. Agardh 1820: 46". AlgaeBase. Archived from the original on 4 January 2009. Retrieved 28 December 2008.
- ^ "Secondary Products of Brown Algae". Algae Research. Smithsonian National Museum of Natural History. Archived from the original on 13 April 2009. Retrieved 29 December 2008.
- ^ "Re-imagining algae". Australian Broadcasting Corporation. 12 October 2016. Archived from the original on 2 February 2017. Retrieved 26 January 2017.
- ^ Gassner, Vesela Tanaskovic; Symeonidis, Dimitris; Koukaras, Konstantinos (2024), Stefanakis, Alexandros; Oral, Hasan Volkan; Calheiros, Cristina; Carvalho, Pedro (eds.), "Harvesting of Agricultural Nutrient Runoff with Algae, to Produce New Soil Amendments for Urban and Peri-urban Olive Tree Agroforestry Systems in Southern Europe", Nature-based Solutions for Circular Management of Urban Water, Cham: Springer International Publishing, pp. 405–441, doi:10.1007/978-3-031-50725-0_23, ISBN 978-3-031-50725-0, retrieved 26 June 2025
- ^ Morrissey, J.; Jones, M. S.; Harriott, V. (1988). "Nutrient cycling in the Great Barrier Reef Aquarium – Proceedings of the 6th International Coral Reef Symposium, Australia". ReefBase. Archived from the original on 23 February 2015.
- ^ Veraart, Annelies J.; Romaní, Anna M.; Tornés, Elisabet; Sabater, Sergi (2008). "Algal Response to Nutrient Enrichment in Forested Oligotrophic Stream". Journal of Phycology. 44 (3): 564–572. Bibcode:2008JPcgy..44..564V. doi:10.1111/j.1529-8817.2008.00503.x. PMID 27041416. S2CID 2040067. Archived from the original on 1 October 2010.
- ^ "Algae: A Mean, Green Cleaning Machine". USDA Agricultural Research Service. 7 May 2010. Archived from the original on 19 October 2010.
- ^ Cappitelli, Francesca; Sorlini, Claudia (2008). "Microorganisms Attack Synthetic Polymers in Items Representing Our Cultural Heritage". Applied and Environmental Microbiology. 74 (3): 564–569. Bibcode:2008ApEnM..74..564C. doi:10.1128/AEM.01768-07. PMC 2227722. PMID 18065627.
- ^ "Algae Biopolymers, Companies, Production, Market – Oilgae – Oil from Algae". oilgae.com. Retrieved 18 November 2017.
- ^ "Renewable flip flops: scientists produce the 'No. 1' footwear in the world from algae". ZME Science. 9 October 2017. Retrieved 18 November 2017.
- ^ "World's First Algae Surfboard Makes Waves in San Diego". Energy.gov. Retrieved 18 November 2017.
- ^ Chandrashekhar K Patil, Harishchandra D Jirimali, Jayasinh S Paradeshi, Bhushan L Chaudhari, Prakash K Alagi, Sung Chul Hong, Vikas V Gite, Synthesis of biobased polyols using algae oil for multifunctional polyurethane coatings, Volume 6 Issue 4, December 2018, pp. 165–177, https://doi.org/10.1680/jgrma.18.00046
- ^ CK Patil, HD Jirimali, JS Paradeshi, BL Chaudhari, VV Gite, Functional antimicrobial and anticorrosive polyurethane composite coatings from algae oil and silver doped egg shell hydroxyapatite for sustainable development, Progress in Organic Coatings 128, 127–136, https://doi.org/10.1016/j.porgcoat.2018.11.002
- ^ Chandrashekhar K Patil, Harishchandra D Jirimali, Jayasinh S Paradeshi, Bhushan L Chaudhari, Prakash K Alagi, Pramod P Mahulikar, Sung Chul Hong, Vikas V Gite, Chemical transformation of renewable algae oil to polyetheramide polyols for polyurethane coatings, Progress in Organic Coatings 151, 106084, https://doi.org/10.1016/j.porgcoat.2020.106084
Bibliography
[edit]General
[edit]- Chapman, V.J. (1980) [1st Pub. 1950]. Seaweeds and their Uses. London: Methuen. ISBN 978-0-412-15740-0.
- Fritsch, F. E. (1945) [1935]. The Structure and Reproduction of the Algae. Vol. I & II. Cambridge University Press.
- van den Hoek, C.; Mann, D. G.; Jahns, H. M. (1995). Algae: An Introduction to Phycology. Cambridge University Press.
- Kassinger, Ruth (2020). Slime: How Algae Created Us, Plague Us, and Just Might Save Us. Mariner.
- Lembi, C. A.; Waaland, J.R. (1988). Algae and Human Affairs. Cambridge University Press. ISBN 978-0-521-32115-0.
- Mumford, T. F.; Miura, A. (1988). "Porphyra as food: cultivation and economic". In Lembi, C. A.; Waaland, J. R. (eds.). Algae and Human Affairs. Cambridge University Press. pp. 87–117. ISBN 978-0-521-32115-0..
- Round, F. E. (1981). The Ecology of Algae. London: Cambridge University Press. ISBN 978-0-521-22583-0.
- Smith, G. M. (1938). Cryptogamic Botany. Vol. I. New York: McGraw-Hill.
- Ask, E.I (1990). Cottonii and Spinosum Cultivation Handbook. FMC BioPolymer Corporation.Philippines.
Regional
[edit]Britain and Ireland
[edit]- Brodie, Juliet; Burrows, Elsie M.; Chamberlain, Yvonne M.; Christensen, Tyge; Dixon, Peter Stanley; Fletcher, R. L.; Hommersand, Max H.; Irvine, Linda M.; Maggs, Christine A. (1977–2003). Seaweeds of the British Isles: A Collaborative Project of the British Phycological Society and the British Museum (Natural History). London / Andover: British Museum of Natural History, HMSO / Intercept. ISBN 978-0-565-00781-2.
- Cullinane, John P. (1973). Phycology of the South Coast of Ireland. Cork: Cork University Press.
- Hardy, F. G.; Aspinall, R. J. (1988). An Atlas of the Seaweeds of Northumberland and Durham. The Hancock Museum, University Newcastle upon Tyne: Northumberland Biological Records Centre. ISBN 978-0-9509680-5-6.
- Hardy, F. G.; Guiry, Michael D.; Arnold, Henry R. (2006). A Check-list and Atlas of the Seaweeds of Britain and Ireland (Revised ed.). London: British Phycological Society. ISBN 978-3-906166-35-3.
- John, D. M.; Whitton, B. A.; Brook, J. A. (2002). The Freshwater Algal Flora of the British Isles. Cambridge / New York: Cambridge University Press. ISBN 978-0-521-77051-4.
- Knight, Margery; Parke, Mary W. (1931). Manx Algae: An Algal Survey of the South End of the Isle of Man. Liverpool Marine Biology Committee Memoirs on Typical British Marine Plants & Animals. Vol. XXX. Liverpool: University Press.
- Morton, Osborne (1994). Marine Algae of Northern Ireland. Belfast: Ulster Museum. ISBN 978-0-900761-28-7.
- Morton, Osborne (1 December 2003). "The Marine Macroalgae of County Donegal, Ireland". Bulletin of the Irish Biogeographical Society. 27: 3–164.
Australia
[edit]- Huisman, J. M. (2000). Marine Plants of Australia. University of Western Australia Press. ISBN 978-1-876268-33-6.
New Zealand
[edit]- Chapman, Valentine Jackson; Lindauer, VW; Aiken, M.; Dromgoole, F. I. (1970) [1900, 1956, 1961, 1969]. The Marine algae of New Zealand. London / Lehre, Germany: Linnean Society of London / Cramer.
Europe
[edit]- Cabioc'h, Jacqueline; Floc'h, Jean-Yves; Le Toquin, Alain; Boudouresque, Charles-François; Meinesz, Alexandre; Verlaque, Marc (1992). Guide des algues des mers d'Europe: Manche/Atlantique-Méditerranée (in French). Lausanne, Suisse: Delachaux et Niestlé. ISBN 978-2-603-00848-5.
- Gayral, Paulette (1966). Les Algues de côtes françaises (manche et atlantique), notions fondamentales sur l'écologie, la biologie et la systématique des algues marines (in French). Paris: Doin, Deren et Cie.
- Guiry, Michael. D.; Blunden, G. (1991). Seaweed Resources in Europe: Uses and Potential. John Wiley & Sons. ISBN 978-0-471-92947-5.
- Míguez Rodríguez, Luís (1998). Algas mariñas de Galicia: Bioloxía, gastronomía, industria (in Galician). Vigo: Edicións Xerais de Galicia. ISBN 978-84-8302-263-4.
- Otero, J. (2002). Guía das macroalgas de Galicia (in Galician). A Coruña: Baía Edicións. ISBN 978-84-89803-22-0.
- Bárbara, I.; Cremades, J. (1993). Guía de las algas del litoral gallego (in Spanish). A Coruña: Concello da Coruña – Casa das Ciencias.
Arctic
[edit]- Kjellman, Frans Reinhold (1883). The algae of the Arctic Sea: A survey of the species, together with an exposition of the general characters and the development of the flora. Vol. 20. Stockholm: Kungl. Svenska vetenskapsakademiens handlingar. pp. 1–350.
Greenland
[edit]- Lund, Søren Jensen (1959). The Marine Algae of East Greenland. Kövenhavn: C.A. Reitzel. 9584734.
Faroe Islands
[edit]- Børgesen, Frederik (1970) [1903]. "Marine Algae". In Warming, Eugene (ed.). Botany of the Faröes Based Upon Danish Investigations, Part II. Copenhagen: Det nordiske Forlag. pp. 339–532..
Canary Islands
[edit]- Børgesen, Frederik (1936) [1925, 1926, 1927, 1929, 1930]. Marine Algae from the Canary Islands. Copenhagen: Bianco Lunos.
Morocco
[edit]- Gayral, Paulette (1958). Algues de la côte atlantique marocaine (in French). Casablanca: Rabat [Société des sciences naturelles et physiques du Maroc].
South Africa
[edit]- Stegenga, H.; Bolton, J. J.; Anderson, R. J. (1997). Seaweeds of the South African West Coast. Bolus Herbarium, University of Cape Town. ISBN 978-0-7992-1793-3.
North America
[edit]- Abbott, I. A.; Hollenberg, G. J. (1976). Marine Algae of California. California: Stanford University Press. ISBN 978-0-8047-0867-8.
- Greeson, Phillip E. (1982). An annotated key to the identification of commonly occurring and dominant genera of Algae observed in the Phytoplankton of the United States. Washington DC: US Department of the Interior, Geological Survey. Retrieved 19 December 2008.
- Taylor, William Randolph (1969) [1937, 1957, 1962]. Marine Algae of the Northeastern Coast of North America. Ann Arbor: University of Michigan Press. ISBN 978-0-472-04904-2.
- Wehr, J. D.; Sheath, R. G. (2003). Freshwater Algae of North America: Ecology and Classification. Academic Press. ISBN 978-0-12-741550-5.
External links
[edit]- Guiry, Michael; Guiry, Wendy. "AlgaeBase". – a database of all algal names including images, nomenclature, taxonomy, distribution, bibliography, uses, extracts
- "Algae – Cell Centered Database". CCDb.UCSD.edu. San Diego: University of California.
- Anderson, Don; Keafer, Bruce; Kleindinst, Judy; Shaughnessy, Katie; Joyce, Katherine; Fino, Danielle; Shepherd, Adam (2007). "Harmful Algae". US National Office for Harmful Algal Blooms. Archived from the original on 5 December 2008. Retrieved 19 December 2008.
- "About Algae". NMH.ac.uk. Natural History Museum, United Kingdom.
Algae
View on GrokipediaAlgae comprise a polyphyletic assemblage of primarily aquatic, photosynthetic eukaryotes that perform oxygenic photosynthesis without the specialized vascular tissues, roots, stems, or leaves of embryophytes (land plants), ranging from unicellular microalgae to multicellular macroalgae including seaweeds.[1][2] These organisms thrive in marine, freshwater, and damp terrestrial settings, where they function as foundational primary producers, converting solar energy into biomass via chloroplasts containing chlorophyll.[3][4] Oceanic phytoplankton algae generate roughly half of Earth's atmospheric oxygen and underpin aquatic food webs by supporting higher trophic levels through nutrient cycling and carbon fixation.[5][6] Evolutionarily ancient, algae oxygenated the primordial atmosphere billions of years ago, facilitating the rise of complex aerobic life, though certain species trigger harmful blooms that deplete oxygen and release toxins, disrupting ecosystems and human health.[6][7] Algae also engage in symbioses, such as providing photosynthesis in lichens or coral reefs, and hold potential for biotechnology in biofuels and remediation due to their rapid growth and metabolic versatility.[4][8]
Definition and Etymology
Historical and Linguistic Origins
The term algae derives from the Latin plural algae, with singular alga signifying "seaweed" and first attested in English contexts around the mid-16th century.[9] The precise etymology of alga is uncertain, potentially linked to Latin ulva ("grass-like or leafy seaweed") or speculatively to algēre ("to be cold"), though no causal connection to temperature explains seaweed's connotation.[9] [10] Classical Latin usage appears in Pliny the Elder's Naturalis Historia (c. 77 AD), where algae denotes marine plants such as phycitis algae, a type of seaweed, reflecting early descriptive rather than systematic categorization.[11] In parallel ancient Greek literature, equivalents like phŷkos ("seaweed") occur as early as Homer's Iliad (c. 8th century BCE), often denoting marine vegetation or derived products like dyes, without formal biological grouping. Theophrastus (c. 371–287 BCE), in works like Enquiry into Plants, described marine herbaceous plants akin to modern algae among broader plant categories—trees, shrubs, and herbs—but emphasized environmental dependencies like salinity without distinct algal taxonomy.[12] Linnaeus elevated Algae to a formal taxonomic class in Species Plantarum (1753), classifying flowerless, seedless aquatic organisms—chiefly marine seaweeds—as cryptogams within his botanical system, marking the term's adoption in systematic biology.[13] This Linnaean framework persisted into the 18th century, influencing works like Johann Friedrich Gmelin's Historia Fucorum (1768), which detailed seaweed morphology. The English plural algae emerged scientifically by 1794, while the discipline of phycology—study of algae—stems from Greek phŷkos.[10] [14]Contemporary Taxonomic Definition
In contemporary taxonomy, algae are regarded as an informal, polyphyletic grouping of primarily aquatic organisms capable of oxygenic photosynthesis, encompassing both prokaryotic and eukaryotic lineages but excluding embryophytes (land plants), which possess protected embryos, vascular tissues, and specialized organs such as roots, stems, and leaves.[15][16] This definition emphasizes functional and ecological convergence rather than shared ancestry, as algal lineages derive from multiple independent evolutionary origins, including primary endosymbiosis of cyanobacteria in the Archaeplastida supergroup (yielding green algae, red algae, and glaucophytes) and secondary or tertiary endosymbioses in diverse protist groups.[15][17] Prokaryotic algae, specifically cyanobacteria (formerly blue-green algae), are included due to their photosynthetic role and superficial resemblance to eukaryotic algae, though they lack nuclei and organelles; these organisms represent an ancient lineage dating back over 2.4 billion years, foundational to global oxygen production.[16] Eukaryotic algae span at least 14 phyla across kingdoms such as Plantae, Chromista, and Protozoa, with pigmentation (e.g., chlorophylls a and b in green algae, chlorophyll c in stramenopiles) and plastid structure serving as key diagnostic traits, but molecular phylogenetics has revealed their non-monophyletic nature, rendering traditional divisions like divisions or classes artificial for cladistic purposes.[16][18] Modern classifications employ a polyphasic approach integrating morphology, ultrastructure, biochemistry, and genomic data, recognizing algae as a pragmatic assemblage for phycological study rather than a formal taxon; this shift, accelerated since the 1990s with ribosomal RNA sequencing, underscores that no single clade unites all algae, as they are interspersed across the tree of life with non-photosynthetic relatives.[15][18] For instance, green algae (Chlorophyta and charophytes) form a paraphyletic grade sister to embryophytes within Streptophyta, while ochrophytes (e.g., diatoms, brown algae) belong to the SAR clade, highlighting convergent adaptations to aquatic niches over deep phylogenetic divergence.[19][16]Morphology and Physiology
Cellular Structure and Morphology
Algae exhibit a wide range of cellular structures, predominantly eukaryotic, characterized by membrane-bound organelles including a nucleus enclosing linear chromosomes, chloroplasts derived from endosymbiotic cyanobacteria, and mitochondria for respiration.[20] Unlike animal cells, algal cells typically possess a cell wall external to the plasma membrane, providing structural support and protection, with composition varying by taxonomic group to adapt to aquatic environments.[21] Chloroplasts in most algae feature thylakoid membranes stacked into grana for efficient light harvesting, surrounded by a double envelope membrane, and contain pyrenoids in some species for carbon fixation enhancement.[22] Cell wall architecture differs significantly across algal divisions: green algae (Chlorophyta) often feature cellulose microfibrils embedded in pectin-like matrices or hydroxyproline-rich glycoproteins, enabling flexibility in unicellular and filamentous forms.[20] In brown algae (Phaeophyceae), walls consist primarily of alginates—copolymers of mannuronic and guluronic acids—cross-linked with fucose-containing sulfated polysaccharides, contributing to the mechanical strength of large multicellular thalli up to 60 meters in kelp species.[23] Red algae (Rhodophyta) walls include semicrystalline cellulose fibrils interwoven with sulfated glucans, mannans, and glucomannans, often impregnated with calcium carbonate for rigidity in coralline forms.[21] Diatoms (Bacillariophyta) possess unique silica-based frustules—two overlapping valves formed via specialized vesicles—providing precise geometric shapes for buoyancy and protection.[24] Morphologically, algae span unicellular forms (e.g., Chlorella with spherical cells 2–10 μm in diameter) to complex multicellular organizations, reflecting evolutionary adaptations for nutrient uptake and reproduction in aquatic niches.[25] Unicellular types include motile flagellates like Chlamydomonas, equipped with two anterior flagella and an eyespot for phototaxis, and non-motile coccoids or amoeboids.[26] Colonial morphologies aggregate cells into spheres (e.g., Volvox with somatic and reproductive cells) or plates, maintaining division of labor without true tissues.[27] Filamentous algae form unbranched (e.g., Spirogyra) or branched chains, while multicellular macroalgae develop differentiated structures: holdfasts for attachment, stipes for support, and blades for photosynthesis, as in Sargassum with air bladders for flotation.[25] These forms lack vascular tissues but achieve size through coenocytic growth or apical meristems in advanced groups, with cell sizes ranging from 1 μm in microalgae to centimeters in macroalgal blades.[1]Photosynthetic Processes
Algae perform oxygenic photosynthesis, utilizing water as an electron donor to generate oxygen, ATP, and NADPH through two linked photosystems, photosystem II (PSII) and photosystem I (PSI).[28] This process mirrors that in cyanobacteria and higher plants, where light energy drives electron transport chains embedded in thylakoid membranes, ultimately reducing NADP+ and producing O2 from water oxidation at PSII. In prokaryotic algae such as cyanobacteria, thylakoids occur freely in the cytoplasm, whereas eukaryotic algae house them within chloroplasts derived from endosymbiotic events.[29] The carbon fixation phase follows the Calvin-Benson-Bassham cycle in the stroma or cytoplasm, converting CO2 into carbohydrates.[30] All algae contain chlorophyll a as the primary pigment, absorbing light maximally at wavelengths around 430 nm and 680 nm to initiate charge separation in reaction centers.[31] Accessory pigments expand the spectral range: chlorophyll b in green algae enhances absorption in the 450-500 nm and 600-650 nm regions; chlorophyll c in chromalveolates like diatoms and brown algae targets blue-green light; while phycobilins in red algae and cyanobacteria capture green to orange wavelengths (500-650 nm) via phycobilisomes attached to thylakoids.[32] These pigments funnel energy to reaction centers via light-harvesting complexes (LHCs), with green algae employing LHCII trimers similar to plants, and ochrophytes using fucoxanthin-chlorophyll a/ c proteins (FCPs) optimized for underwater light penetration.[33] Carotenoids like beta-carotene and fucoxanthin provide photoprotection and additional light harvesting, dissipating excess energy as heat under high irradiance.[34] Algal photosynthesis contributes approximately 50% of global primary production, predominantly from marine phytoplankton, due to their vast oceanic distribution and efficient light capture in low-light aquatic environments.[35] Adaptations include state transitions regulating LHC distribution between photosystems for balanced electron flow, and non-photochemical quenching mechanisms that prevent photodamage, varying by algal group—e.g., diatom FCPs exhibit rapid energy-dependent quenching.[36] In cyanobacteria, phycobilisomes enable complementary chromatic adaptation, adjusting pigment ratios to ambient light quality.[37] These processes underscore algae's role in oxygenating Earth's atmosphere since their emergence around 2.5 billion years ago.[38]Metabolic and Reproductive Cycles
Algae exhibit diverse metabolic processes dominated by oxygenic photosynthesis, in which light energy drives the splitting of water molecules to produce oxygen, electrons for NADPH reduction, and ATP via photophosphorylation, ultimately fixing carbon dioxide through the Calvin-Benson-Bassham cycle in chloroplasts or thylakoids.[39] [30] This process supports primary production, with algae contributing approximately 50-85% of Earth's oxygen and fixing vast amounts of carbon, though rates vary by species and environmental conditions such as light intensity and CO2 availability.[39] Many algae, including green algae like Chlamydomonas, also perform aerobic respiration to break down carbohydrates and lipids for energy under dark conditions, balancing photosynthetic gains, while some switch to anaerobic fermentation producing lactate or ethanol when oxygen is limited.[40] [41] Nutrient metabolism in algae involves active uptake of macronutrients like nitrogen (often as nitrate or ammonium) and phosphorus (as phosphate), which are assimilated into amino acids, nucleic acids, and phospholipids essential for growth and division.[42] Optimal nitrogen-to-phosphorus ratios, typically around 16:1 by atoms (Redfield ratio), maximize biomass accumulation, but deficiencies redirect metabolism toward lipid or carbohydrate storage, enhancing resilience to environmental stress.[42] [43] Carbon metabolism features high flux through pathways like phosphoenolpyruvate (PEP) carboxylase, exceeding rates in higher plants and supporting rapid protein synthesis and metabolite production.[44] Green algae often employ CO2-concentrating mechanisms (CCMs) to enhance Rubisco efficiency in low-CO2 environments, involving carbonic anhydrases and inorganic carbon transporters.[45] Reproductive cycles in algae encompass both asexual and sexual modes, enabling adaptation to fluctuating conditions; asexual reproduction predominates in favorable environments for rapid population growth, while sexual reproduction promotes genetic diversity during stress.[46] Asexual mechanisms include binary fission in unicellular forms like Chlorella, fragmentation in filamentous species such as Spirogyra, and spore formation (zoospores or aplanospores) that germinate into new individuals without genetic recombination.[47] Sexual reproduction involves gamete fusion, ranging from isogamy (equal flagellated gametes in Chlamydomonas) to oogamy (large non-motile eggs and small sperm in Oedogonium), with zygotes often developing protective walls to overwinter.[48] Algal life cycles vary across taxa: haplontic cycles feature haploid dominance with zygotic meiosis post-fertilization (e.g., many green algae), diplontic cycles maintain diploidy except for gametes via gametic meiosis (e.g., some brown algae), and isomorphic or heteromorphic alternation of generations occurs in groups like red algae, where haploid gametophytes alternate with diploid sporophytes producing spores via meiotic reduction.[48] The interplay of these cycles influences population dynamics, with asexual phases favoring clonal expansion and sexual phases countering inbreeding through outcrossing, though the balance shifts based on density, nutrient availability, and predation pressures.[46] In cyanobacteria (prokaryotic algae), reproduction is strictly asexual via binary fission or akinete formation, lacking true sexual cycles but exhibiting genetic exchange via conjugation-like processes.[47]Classification and Diversity
Prokaryotic Algae
Prokaryotic algae consist of the cyanobacteria, a phylum of photosynthetic bacteria in the domain Bacteria that conduct oxygenic photosynthesis, splitting water to release oxygen and fix carbon dioxide. These organisms, often called blue-green algae due to their pigmentation, represent the only prokaryotes capable of this process, distinguishing them from other bacteria and aligning them traditionally with algal groups despite their prokaryotic nature lacking nuclei and membrane-bound organelles.[49][50] Cyanobacteria display morphological diversity including unicellular, colonial, and multicellular filamentous forms, with photosynthetic apparatus organized in thylakoids containing chlorophyll a and accessory phycobiliproteins for light harvesting. Many species form heterocysts, specialized cells that provide an anaerobic microenvironment for nitrogenase activity, enabling biological nitrogen fixation from atmospheric N₂ even in oxygen-rich settings. This capability supports their role in nutrient cycling, as heterocysts separate oxygenic photosynthesis from nitrogen fixation to protect the enzyme.[51][52] Taxonomically, the phylum Cyanobacteria encompasses numerous lineages, with genomic analyses revealing 18 orders and 42 families as of 2024, reflecting high genetic and functional diversity adapted to aquatic, terrestrial, and extreme environments. This diversity underpins their ecological significance as primary producers in oceans and lakes, where blooms can contribute substantially to carbon sequestration and nitrogen inputs, though excessive growth may lead to toxic cyanotoxin production affecting water quality.[53][54] Evolutionarily, cyanobacteria diverged from other bacteria around 3.4 billion years ago, with their oxygenic photosynthesis driving the Great Oxidation Event circa 2.4 billion years ago, which oxygenated Earth's atmosphere and enabled aerobic life while reshaping biogeochemical cycles. Fossil evidence and molecular clocks indicate their ancient origins, with multicellularity emerging in parallel with diversification that amplified their environmental impact.[55][56]Eukaryotic Algae Groups
Eukaryotic algae form a polyphyletic group of photosynthetic protists spanning multiple eukaryotic supergroups, distinguished by plastids acquired through primary endosymbiosis in Archaeplastida or secondary/tertiary endosymbioses in other lineages such as stramenopiles and alveolates. Primary plastids, bounded by two membranes, characterize Archaeplastida, while secondary plastids typically feature three or four membranes from engulfed red or green algae. These organisms range from unicellular microalgae to complex multicellular seaweeds, contributing substantially to global primary production.[57] The Rhodophyta, or red algae, encompass approximately 5,000 to 6,000 species, predominantly marine and multicellular, with phycoerythrins enabling absorption of blue-green light in deeper waters. Lacking flagella in most stages and containing unstacked thylakoids, they produce polysaccharides like agar and carrageenan in cell walls, supporting roles in food, industry, and coral reef calcification.[58][57] Chlorophyta and Charophyta constitute the green algal lineages within Archaeplastida, with Chlorophyta including over 4,500 described species (potentially up to 100,000 total) of unicellular to filamentous forms storing starch and featuring chlorophylls a and b akin to land plants. Charophyta, with fewer species, include conjugating algae and charophytes, the sister group to embryophytes, exhibiting oogamous reproduction and phragmoplasts. These groups inhabit freshwater, marine, and terrestrial environments, with some forming symbiotic lichens.[59][60][57] Stramenopile algae, part of the Heterokontophyta, feature secondary plastids from red algae and include Phaeophyceae (brown algae) with about 1,500 to 2,000 species of large, multicellular marine forms pigmented by fucoxanthin and storing laminarin; notable examples are kelp (Laminariales) forming underwater forests up to 50 meters tall. Bacillariophyta (diatoms), exceeding 20,000 described species, possess silica-impregnated frustules for structural support, dominate phytoplankton biomass, and undergo auxospore formation for size restoration in asexual divisions.[16][57][60] Dinophyta (dinoflagellates), within Alveolata, comprise around 2,000 photosynthetic species among 2,500 total, characterized by two dissimilar flagella in transverse and longitudinal grooves enabling spinning motility, cellulose thecal plates, and peridinin-chlorophyll proteins. Many are marine plankton, with some producing toxins causing red tides and paralytic shellfish poisoning, as in genera like Alexandrium and Gonyaulax.[61][57] Additional groups include Haptophyta (e.g., coccolithophores with calcium carbonate scales) and Cryptophyta, both with secondary red-derived plastids and mixotrophic capabilities, alongside excavate-derived Euglenophyta featuring green secondary plastids in flexible, euglenoid cells. These diverse lineages reflect multiple endosymbiotic events shaping algal evolution.[57]00604-6)Historical Shifts in Classification
In 1753, Carl Linnaeus included algae within the plant kingdom in Species Plantarum, classifying them under the class Cryptogamia alongside other non-flowering plants, based primarily on reproductive characteristics rather than phylogenetic relationships.[62] This approach treated algae as a heterogeneous assemblage of thalloid organisms lacking vascular tissue. Subsequent 19th-century classifications expanded on pigmentation and morphology, establishing major divisions such as Chlorophyceae (green algae), Phaeophyceae (brown algae), and Rhodophyceae (red algae), as proposed by botanists like William Henry Harvey in 1836, who arranged them into color-based groups reflecting dominant pigments like chlorophyll, fucoxanthin, and phycoerythrins.[61] These systems were artificial, prioritizing observable traits over evolutionary descent, and initially focused on macroscopic marine forms while gradually incorporating freshwater and unicellular species.[63] Mid-20th-century advancements in microscopy revealed fundamental cellular differences, prompting the separation of prokaryotic "blue-green algae" from eukaryotic algae. Electron micrographs in the 1950s demonstrated the absence of membrane-bound organelles in blue-green forms, leading Roger Stanier and C.B. van Niel to redefine bacteria in 1962 to encompass these organisms, reclassifying them as cyanobacteria within the prokaryotic domain rather than algae.[64] This shift, formalized in subsequent taxonomic works like Bergey's Manual, excluded prokaryotes from algal groupings, recognizing cyanobacteria's closer relation to bacteria based on cell division, peptidoglycan walls, and 70S ribosomes.[65] By the 1970s, five-kingdom systems by Robert Whittaker further delineated algae as eukaryotic protists, emphasizing their polyphyletic origins across multiple lineages.00553-3) The advent of molecular phylogenetics in the 1980s and 1990s revolutionized algal taxonomy through ribosomal RNA sequencing and gene analyses, overturning morphology-based schemes. Carl Woese's work confirmed cyanobacteria's bacterial affinity, while small subunit rRNA trees revealed eukaryotic algae's dispersal among protist supergroups: green algae (Chlorophyta and Streptophyta) as sister to land plants, red algae (Rhodophyta) in Archaeplastida, and brown algae (Phaeophyceae) within Stramenopiles.[66] These data highlighted algae's non-monophyly, with groups like diatoms and dinoflagellates deriving from secondary endosymbioses, prompting revisions such as the dissolution of chromalveolate hypotheses and recognition of diverse clades like Haptophyta and Cryptophyta.[67] By the 2000s, genomic studies refined these relationships, emphasizing endosymbiotic events and gene transfers as drivers of diversity, though debates persist on deep-branching resolutions due to long-branch attraction artifacts.[68] Contemporary classifications prioritize clade-based systems, continuously updated with multi-gene phylogenies to reflect evolutionary history over traditional convenience groupings.[66]Evolutionary History
Origins of Oxygenic Photosynthesis
Oxygenic photosynthesis, characterized by the splitting of water molecules to generate oxygen as a byproduct while fixing carbon dioxide into organic compounds, first evolved in cyanobacteria, a group of prokaryotic photoautotrophs. This innovation combined two photosystems—Photosystem I and II—allowing the use of abundant water as an electron donor rather than scarce reductants like hydrogen sulfide or iron used in earlier anoxygenic forms. Phylogenetic analyses indicate that the lineage leading to cyanobacteria diverged from other bacteria approximately 3.4 billion years ago, with the core machinery of oxygenic photosynthesis assembling prior to the last common ancestor of extant cyanobacteria.[55][69] Molecular clock estimates place the emergence of oxygenic photosynthesis between 3.5 and 2.7 billion years ago during the Archean Eon, supported by genomic comparisons of cyanobacterial genes involved in photosystem assembly and oxygen evolution. Geological proxies, such as banded iron formations and sulfur isotope excursions, suggest localized oxygen production predated the Great Oxidation Event (GOE) by hundreds of millions of years, though atmospheric accumulation only occurred around 2.4 billion years ago due to sinks like reduced iron and methane. Fossil evidence, including microbially induced sedimentary structures and thiopurine biomarkers from 2.7 Ga rocks, corroborates early cyanobacterial activity, but direct morphological fossils of cyanobacteria date to about 1.75 billion years ago, reflecting preservation biases rather than origination timing.[38][70] Debates persist on whether oxygenic photosynthesis arose de novo or via lateral gene transfer from anoxygenic bacteria, with comparative genomics favoring an endogenous evolution within a proto-cyanobacterial lineage adapting to low-sulfide environments. The GOE, triggered by cyanobacterial proliferation and the oxidation of oceanic reductants, marked a causal shift from an anoxic to oxygenated world, enabling aerobic respiration but initially devastating anaerobic ecosystems. These origins underscore cyanobacteria's pivotal role in transforming Earth's geochemistry, with empirical constraints from independent isotopic and phylogenetic datasets converging on a pre-2.7 Ga timeline despite uncertainties in early rock records.[71][72][73]Endosymbiotic Events
The primary endosymbiotic event established oxygenic photosynthesis in eukaryotes through the engulfment of a free-living cyanobacterium by a heterotrophic protist host, leading to the endosymbiont's integration as the progenitor of chloroplasts. This singular occurrence produced primary plastids—bounded by two membranes—in the Archaeplastida clade, which includes glaucophytes, rhodophytes (red algae), and chlorophytes (green algae, ancestral to land plants). Molecular divergence estimates place the origin of photosynthetic eukaryotes before 1,558 million years ago (MYA), with the divergence of red and green algal lineages around 1,500 MYA.[74] Supporting evidence encompasses the double-membrane structure of primary plastids (inner cyanobacterial-derived, outer phagosomal), circular plastid DNA akin to bacterial chromosomes, 70S ribosomes, and nuclear genes of cyanobacterial phylogenetic affinity encoding organelle-targeted proteins.[75] Secondary endosymbioses ensued when eukaryotic predators engulfed primary plastid-bearing algae, retaining the endosymbiont and yielding complex plastids typically enclosed by three or four membranes, often with vestigial nucleomorphs in lineages like cryptophytes and chlorarachniophytes. These events diversified algal groups beyond Archaeplastida; red algal-derived secondary plastids characterize chromalveolates (e.g., diatoms, oomycetes, haptophytes, and some dinoflagellates), while green algal-derived ones appear in euglenozoans and chlorarachniophytes, with independent acquisitions inferred from phylogenetic incongruences.[76] [77] Gene transfers from endosymbiont nuclei to the host genome accompanied these integrations, shrinking plastid genomes to 0.1–1% of cyanobacterial sizes and necessitating sophisticated protein import via endoplasmic reticulum-derived membranes.[78] Tertiary and higher-order endosymbioses, rarer and lineage-specific, involved engulfment of secondary or tertiary algae, as in peridinin-lacking dinoflagellates acquiring haptophyte or green algal plastids, evidenced by chimeric membrane topologies and gene phylogenies. These serial events, spanning from the Proterozoic to Phanerozoic, underscore endosymbiosis's role in algal diversification, though host-endosymbiont compatibility constraints limited primary events to one major success, with secondary occurrences numbering at least four to six independently.[79] [80] A parallel, recent primary endosymbiosis (~120 MYA) in the amoeboid Paulinella chromatophora exemplifies ongoing potential, featuring chromorelicts with reduced but functional photosynthetic genes.[81]Connections to Land Plants and Anoxic Events
Land plants, or embryophytes, evolved from a lineage of streptophyte green algae within the Charophyceae clade, sharing derived traits such as phragmoplast-mediated cell division, rosette-shaped cellulose-synthesizing complexes, and similar cell wall compositions including fucosylated xyloglucans.[82][83] This ancestral alga was likely a freshwater-dwelling, filamentous or branched multicellular form capable of zygote retention, a precursor to the embryophyte embryo.[84] Molecular clock estimates place the divergence of streptophytes from chlorophyte green algae around 700–1,000 million years ago, with the embryophyte lineage emerging approximately 500–470 million years ago during the mid-Ordovician period.[85]01028-9) The transition to terrestrial life involved adaptations for desiccation resistance, such as cuticle-like secretions and upright growth, building on algal precursors like hormone signaling pathways (e.g., auxin responses) present in streptophytes prior to land colonization.[86] Fossil evidence, including sporangia from the Ordovician (~450 million years ago), supports this timeline, indicating that early embryophytes radiated in freshwater habitats before fully exploiting subaerial environments.[87] Phylogenetic analyses consistently position land plants as a monophyletic group nested within streptophytes, with closest algal relatives including orders like Charales and Zygnematales, though the precise sister group remains debated due to incomplete sampling of extant diversity.[88][89] The proliferation of early vascular land plants during the Devonian period (~419–359 million years ago) indirectly influenced marine algal dynamics and contributed to oceanic anoxic events through enhanced nutrient delivery. Root systems and forest formation increased weathering of continental silicates, elevating fluxes of phosphorus and other bioavailable nutrients into coastal oceans, which fueled eutrophication and explosive algal productivity.[90] This nutrient pulse promoted widespread phytoplankton blooms, particularly of cyanobacteria and eukaryotic algae, leading to high organic matter export to seafloors, where microbial respiration depleted bottom-water oxygen and expanded anoxic zones.[90] Multiple Late Devonian anoxic episodes, coinciding with Kellwasser and Hangenberg events (~372 and 359 million years ago), correlate with peaks in terrestrial plant biomass and associated biotic crises, including marine extinctions.[90] Enhanced carbon burial during these events, driven by algal-derived organic sediments, drew down atmospheric CO2 and may have amplified global cooling, though primary drivers included volcanism and sea-level changes.[90] In later Mesozoic oceanic anoxic events (e.g., OAE2 ~94 million years ago), diversified eukaryotic phytoplankton, such as coccolithophores and dinoflagellates descended from algal lineages, sustained high primary production under warm, stratified oceans, further linking algal evolution to anoxia through carbon cycling feedbacks.[91][92] These events selected for resilient algal groups adapted to low-oxygen niches, shaping modern phytoplankton communities.[93]Habitats and Distribution
Primary Aquatic Environments
![Kelp forest in Monterey][float-right] The oceans constitute the primary aquatic environment for algae, hosting the majority of global algal primary production through phytoplankton communities. These microscopic algae, including diatoms, dinoflagellates, and coccolithophores, dominate the open ocean's photic zone, where they account for approximately half of Earth's total primary production despite comprising less than 1% of global photosynthetic biomass.[94] Annually, oceanic phytoplankton fix between 30 and 50 billion metric tons of carbon, underscoring their outsized role in marine ecosystems and global biogeochemical cycles.[95] Macroalgae, or seaweeds, primarily inhabit shallow coastal marine waters, typically less than 100 meters deep, where light penetration supports their growth on rocky substrata or sediments. Species such as kelp (e.g., in the order Laminariales) form extensive underwater forests in temperate and polar regions, while tropical reefs feature coralline red algae and turf-forming species that stabilize substrates and contribute to habitat complexity.[96] These benthic macroalgal communities represent the largest vegetated habitats in the sea, with productivity concentrated in nutrient-rich upwelling zones and estuaries.[97] Freshwater environments, including lakes, rivers, and ponds, support significant algal populations, particularly of green algae (Chlorophyta) and cyanobacteria, though they encompass a smaller fraction of global algal biomass compared to marine systems. Approximately 80% of green algal species occur in freshwater habitats, thriving in planktonic, periphytic, or benthic forms adapted to varying nutrient levels and flow regimes.[98] In lotic systems like streams, periphyton-dominated algae drive food webs, while lentic waters such as lakes host phytoplankton blooms influenced by seasonal stratification.[99] Brackish waters in estuaries bridge marine and freshwater realms, fostering transitional algal assemblages resilient to salinity fluctuations.[7]Extreme and Symbiotic Habitats
Algae inhabit a range of extreme environments characterized by conditions lethal to most eukaryotic life, including high temperatures, low pH, hypersalinity, and subzero temperatures. Thermophilic algae, such as the red alga Cyanidioschyzon merolae from the class Cyanidiophyceae, thrive in acidic volcanic hot springs with temperatures exceeding 50°C and pH values below 2, where they dominate microbial biomass through adaptations like heat-stable enzymes and unique metabolic pathways for carbon fixation.[100][101] In geothermal sites like Yellowstone National Park, cyanobacteria form colorful mats in waters from 40°C to 70°C, contributing to primary production despite thermal stress.[102][103] Acidophilic algae persist in acid mine drainage (AMD) sites, where pH drops below 3 and heavy metals like iron and aluminum abound; species such as the green alga Ulothrix and euglenoids proliferate by tolerating dissolved metals and utilizing them for growth, often forming biofilms that influence metal cycling.[104][105] Hypersaline environments, including salt lakes and evaporation ponds, host halophilic green algae like Dunaliella salina, which accumulate compatible solutes such as glycerol to counter osmotic stress at NaCl concentrations up to 3.5 mol/L, enabling survival where water activity is low.[106] Cryophilic algae, including snow algae like Chlamydomonas nivalis and diatoms in Antarctic sea ice, endure temperatures near 0°C with high salinity and limited light by producing antifreeze proteins and pigments that enhance light harvesting under snow cover, forming visible red or green patches that accelerate ice melt through albedo reduction.[107][108] These adaptations underscore algae's physiological resilience, often involving membrane modifications and osmoprotectant synthesis verified through genomic and proteomic studies.[109] In symbiotic habitats, algae form mutualistic associations that expand their ecological niches beyond free-living states. Lichens represent a terrestrial symbiosis where fungal partners (typically ascomycetes) provide structural support and mineral access, while algal photobionts—often green algae like Trebouxia or cyanobacteria—supply fixed carbon via photosynthesis, enabling colonization of nutrient-poor substrates such as rocks and bark in arid or cold regions.[110][111] Marine symbioses include dinoflagellate algae (zooxanthellae, e.g., Symbiodinium spp.) within scleractinian corals, where algae translocate up to 90% of photosynthetic products to the host for calcification and growth, in exchange for inorganic nutrients and a protected environment; this partnership, originating in the Triassic period around 240 million years ago, underpins reef ecosystems but is vulnerable to thermal stress causing bleaching.[112] Similar associations occur with cnidarians like sea anemones and jellyfish, where algae enhance host nutrition in oligotrophic waters.[113] These symbioses demonstrate causal dependencies, with algal photosynthesis driving host metabolism, as evidenced by stable isotope tracing and controlled exclusion experiments.[114]Ecological Roles
Primary Production and Oxygen Dynamics
Phytoplankton, dominated by eukaryotic and prokaryotic algae, account for approximately 50% of global net primary production despite comprising less than 1% of photosynthetic biomass.[94] Annual marine primary production by phytoplankton is estimated at 45-50 gigatons of carbon.[115] This productivity, driven by oxygenic photosynthesis in sunlit surface waters, forms the base of oceanic food webs and influences carbon sequestration through the biological pump.[116] Algae fix carbon dioxide using the enzyme ribulose-1,5-bisphosphate carboxylase/oxygenase (Rubisco), which catalyzes the incorporation of CO₂ into organic compounds. Microalgae benefit from high surface area-to-volume ratios that enable rapid CO₂ uptake during photosynthesis.[45][117] Temperature influences these processes, with low temperatures inhibiting Rubisco activity and reducing carbon fixation rates, while optimal ranges enhance enzymatic efficiency and overall productivity.[118] Marine algae contribute roughly 50% of Earth's atmospheric oxygen through photosynthesis, with the remainder primarily from terrestrial plants.[5] Cyanobacteria and eukaryotic phytoplankton such as diatoms and dinoflagellates release oxygen as a byproduct while fixing carbon dioxide, maintaining long-term atmospheric levels via burial of organic matter that prevents re-oxidation.[119] Estimates attributing over 70% to algae exceed empirical measurements and overlook net balances from respiration and decomposition.[120] Oxygen dynamics in algal-dominated systems exhibit diurnal fluctuations: supersaturation during daylight from photosynthetic release contrasts with depletion at night due to community respiration.[121] Intense blooms can elevate dissolved oxygen to 190% of saturation midday but foster hypoxia upon senescence as bacterial decomposition consumes oxygen faster than replenishment, contributing to dead zones in eutrophic waters.[122] Such events underscore causal links between nutrient enrichment, algal proliferation, and localized anoxia, independent of broader atmospheric trends.[123]Nutrient Cycling and Symbioses
Algae, particularly phytoplankton, drive nutrient cycling in aquatic ecosystems by assimilating dissolved inorganic nutrients such as nitrogen (N) and phosphorus (P) into biomass via photosynthesis, with subsequent remineralization by heterotrophic microbes recycling these elements to support ongoing primary production. In ocean surface waters, nitrogen recycling alone sustains roughly 80% of phytoplankton productivity, equivalent to approximately 6,800 teragrams of nitrogen per year, primarily through the microbial loop where bacteria decompose algal exudates and detritus, releasing bioavailable forms like ammonium.[124][125] Phosphorus cycles similarly, though often more constrained by slower regeneration rates and sinking export, limiting algal growth in vast oligotrophic regions and influencing community composition toward species with efficient uptake strategies.[126] This internal recycling minimizes reliance on external inputs from upwelling or rivers, stabilizing productivity in stratified waters but rendering systems vulnerable to disruptions like stratification intensification under climate change.[127] Symbiotic associations amplify algal contributions to nutrient cycling by facilitating localized retention and exchange in nutrient-scarce habitats. In lichens, fungi partner with green algae (e.g., Trebouxia spp.) or cyanobacteria (e.g., Nostoc spp.), where photobionts supply fixed carbon and the mycobiont absorbs atmospheric or soil-derived minerals, enabling these composites to thrive on barren rocks and recycle nutrients at microscales unavailable to free-living algae.[128] This symbiosis, ancient and widespread, covers about 8% of Earth's land surface and enhances soil formation by weathering substrates, indirectly bolstering terrestrial nutrient inputs to aquatic systems.[129] In marine settings, dinoflagellate algae (Symbiodinium clade) symbiose with cnidarians like corals and sea anemones, translocating up to 90% of photosynthates to hosts while receiving ammonium and phosphate waste products for reuse, which sustains high productivity in phosphorus-limited reefs despite low ambient concentrations.[130] Sponges similarly host algal or cyanobacterial symbionts that fix carbon and nitrogen, filtering and retaining nutrients from seawater currents to support sponge growth and export-resistant cycling in benthic environments.[131] These partnerships exemplify causal efficiency: algal autotrophy subsidizes heterotrophic hosts, reducing nutrient leakage and buffering ecosystems against scarcity, though bleaching events from thermal stress disrupt this balance, releasing unused nutrients and altering local cycles.[113]Harmful Algal Blooms and Disruptions
Harmful algal blooms (HABs) consist of rapid proliferations of certain phytoplankton species, often cyanobacteria or dinoflagellates, that produce toxins or cause oxygen depletion in aquatic environments.[7] These events disrupt ecosystems by releasing potent cyanotoxins such as microcystins, anatoxins, and saxitoxins, which inhibit photosynthesis in other organisms and bioaccumulate in food webs.[132] HABs form when nutrient imbalances—primarily excess phosphorus and nitrogen from agricultural runoff, sewage discharge, and urban wastewater—fuel eutrophication, enabling algae to outcompete other species under favorable conditions like warm temperatures and calm waters.[122] [133] The primary driver of HABs is anthropogenic nutrient pollution rather than natural variability alone, as evidenced by correlations between fertilizer application rates and bloom intensity in watersheds.[134] For instance, phosphorus loading from nonpoint agricultural sources has historically triggered blooms in systems like Lake Erie, where 1960s-1970s eutrophication led to widespread hypoxia and nuisance growths covering up to 20% of the lake's surface area.[135] Decomposition of algal biomass following blooms consumes dissolved oxygen, creating hypoxic "dead zones" that suffocate fish and invertebrates, collapsing local biodiversity and altering trophic structures by favoring toxin-tolerant species.[136] In marine settings, dinoflagellate HABs like those from Karenia brevis produce brevetoxins that cause mass marine mammal strandings and shellfish contamination, disrupting coastal food chains.[137] Human health risks from HABs include acute neurotoxic and hepatotoxic effects from cyanotoxins and saxitoxins, manifesting as nausea, vomiting, bloody diarrhea, muscle paralysis, respiratory failure, and liver damage upon ingestion or inhalation.[138] [139] In the 2014 Toledo, Ohio water crisis, microcystin levels from a Lake Erie bloom exceeded safe thresholds, forcing a two-day shutdown of drinking water for 500,000 residents and highlighting vulnerabilities in municipal supplies.[140] Economically, HABs impose costs through fishery closures, tourism declines, and water treatment expenses; the same Lake Erie event resulted in $43 million in lost recreational revenue.[141] In the Gulf of Mexico, seasonal dead zones—hypoxic areas exceeding 6,705 square miles in 2024—reduce shrimp and fish yields by displacing commercial stocks, with five-year averages surpassing 4,298 square miles due to Mississippi River nutrient flux.[142] [143] These disruptions extend to long-term ecological shifts, such as persistent anoxia fostering invasive species dominance and eroding habitat for native fisheries, with global HAB frequency rising alongside intensified agriculture since the mid-20th century.[144] While warmer stratification may prolong blooms, empirical data underscore nutrient reduction as the causal leverage point, as reductions in point-source phosphorus in the 1980s temporarily curbed Lake Erie events before nonpoint sources reversed gains.[145] Monitoring via satellite imagery and toxin assays has documented over 300 HAB events annually in U.S. waters, emphasizing the need for watershed management to mitigate recurrent hypoxic expansions.[146]Human Uses and Cultivation
Traditional and Cultural Applications
Algae have been utilized by various cultures for food and medicinal purposes over millennia, with evidence from archaeological dental remains indicating consumption in Europe as early as 6400 BCE, where seaweed provided essential vitamins, nutrients, and protein before widespread animal agriculture.[147] In prehistoric coastal sites from Spain to Lithuania, starch granules and molecular biomarkers in human dental calculus confirm regular intake of species like Pyropia and Palmaria, suggesting it served as a staple rather than occasional foraging.[148] In Mesoamerica, the Aztecs harvested spirulina (Arthrospira platensis) from Lake Texcoco starting around the 16th century, drying it into tecuitlatl cakes consumed by warriors for endurance and traded in markets, as documented in Spanish colonial accounts by chroniclers like Bernardino de Sahagún.[149] This cyanobacterium, rich in protein, supported populations in nutrient-scarce regions. In Asia, seaweed consumption dates to the 4th century in Japan and 6th century in China, where species like nori (Porphyra) and kombu (Saccharina japonica) became dietary staples, often prepared as delicacies or everyday foods for their iodine and mineral content.[150] Traditional Chinese medicine employed seaweeds such as Sargassum for treating edema, goiter, and inflammation, attributing efficacy to their diuretic and anti-inflammatory properties observed empirically.[151] European coastal communities, particularly in Ireland and Scotland, traditionally gathered dulse (Palmaria palmata) for direct consumption or as a famine food, with records from the 12th century onward noting its role in sustaining populations during shortages like the Irish Potato Famine of the 1840s, where it provided caloric relief amid crop failure.[152] In Hawaii, limu (various algae) held cultural importance in indigenous diets and rituals, used for flavoring, medicine against infections, and as offerings, with over 100 species identified in traditional knowledge systems predating European contact.[153] These applications reflect algae's accessibility in aquatic environments and their nutritional density, though efficacy claims in medicine often rely on anecdotal tradition rather than controlled historical trials.Modern Cultivation Techniques
Modern algae cultivation techniques distinguish between microalgae, often grown in controlled terrestrial systems for biofuels, nutraceuticals, and wastewater treatment, and macroalgae (seaweeds), primarily farmed in open marine environments for food and industrial uses. Microalgal cultivation relies on optimizing light, nutrients, CO2 supply, and mixing to achieve high biomass densities, with systems scaled from laboratory flasks to commercial hectares. Macroalgal farming emphasizes vegetative propagation and spatial arrangement to maximize growth in nutrient-rich coastal or offshore waters, with global production reaching over 35 million metric tons wet weight annually as of 2020, dominated by species like Eucheuma and Kappaphycus in tropical regions.[154] For microalgae, open raceway ponds represent the most economical large-scale method, featuring shallow (0.2-0.3 m deep) channels with paddlewheels for circulation, covering areas up to several hectares and achieving areal productivities of 10-30 g/m²/day under optimal conditions like 25-30°C temperatures and continuous nutrient dosing. These systems, operational since the 1950s and refined through engineering improvements in liners and aeration, incur capital costs of approximately $50,000-100,000 per hectare but face challenges from evaporation, predation, and contamination by unwanted species, limiting yields to 0.1-0.3 g/L/day.[155][156] Closed photobioreactors (PBRs), such as tubular or flat-panel designs, offer superior control over parameters like pH (7-9) and dissolved oxygen, enabling higher densities (1-5 g/L) and productivities up to 1-2 g/L/day, particularly for strains like Chlorella or Nannochloropsis, but at 2-10 times the capital expense ($300,000-1,000,000 per hectare equivalent) due to materials like borosilicate glass or plastics and energy demands for pumping and cooling.[157][158]| Cultivation System | Key Advantages | Key Disadvantages | Typical Productivity | Estimated Production Cost (Lipids, $/gal) |
|---|---|---|---|---|
| Open Raceway Ponds | Low construction and operational costs; scalable land use | High contamination risk; weather dependence; lower densities | 10-50 g/m²/day | 9-13[158][159] |
| Closed PBRs | Contamination resistance; precise environmental control; higher yields | High capital and energy costs; biofouling; maintenance intensive | 0.5-2 g/L/day | 20-32[158][159] |