Recent from talks
Nothing was collected or created yet.
Planarian
View on WikipediaThis article's lead section may need to be rewritten. The reason given is: it does not summarise the body. (December 2023) |
| Planarian | |
|---|---|

| |
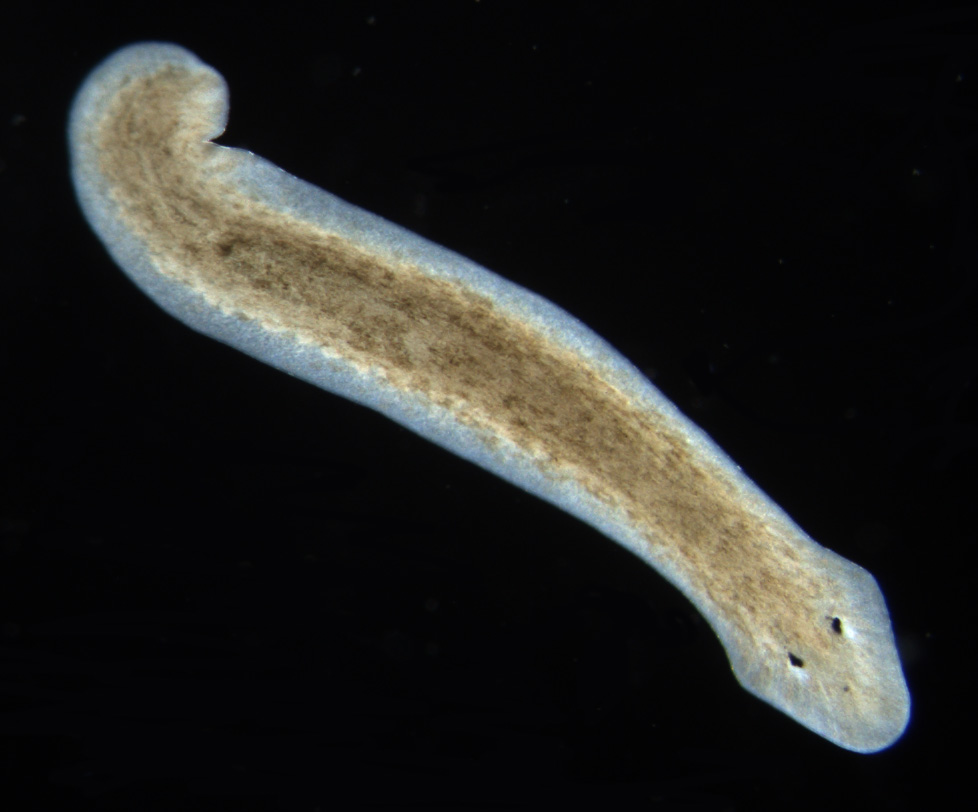
| Dugesia subtentaculata, a dugesiid. | |
| Scientific classification | |
| Kingdom: | Animalia |
| Phylum: | Platyhelminthes |
| Subphylum: | Rhabditophora |
| Order: | Tricladida Lang, 1884 |
| Subdivis[1] | |
| |
Planarians (triclads) are free-living flatworms of the class Turbellaria,[2][3] order Tricladida,[4] which includes hundreds of species, found in freshwater, marine, and terrestrial habitats.[5] Planarians are characterized by a three-branched intestine, including a single anterior and two posterior branches.[5] Their body is populated by adult stem cells called neoblasts, which planarians use for regenerating missing body parts.[6] Many species are able to regenerate any missing organ, which has made planarians a popular model in research of regeneration and stem cell biology.[7] The genome sequences of several species are available, as are tools for molecular biology analysis.[7][8]
The order Tricladida is split into three suborders, according to their phylogenetic relationships: Maricola, Cavernicola and Continenticola. Formerly, the Tricladida was split according to their habitat: Maricola (marine planarians); Paludicola (freshwater planarian); and Terricola (land planarians).[9]
Planarians move by beating cilia on the ventral dermis, allowing them to glide along on a film of mucus. Some also can move by undulations of the whole body by the contractions of muscles built into the body membrane.[10]
Triclads play an important role in watercourse ecosystems and are often very important as bio-indicators.[11]
Phylogeny and taxonomy
[edit]Phylogeny
[edit]Phylogenetic supertree after Sluys et al., 2009:[1]
| Tricladida | |
Taxonomy
[edit]


Linnaean ranks after Sluys et al., 2009:[1]
- Order Tricladida
- Suborder Maricola
- Superfamily Cercyroidea
- Family Centrovarioplanidae
- Family Cercyridae
- Family Meixnerididae
- Superfamily Bdellouroidea
- Family Uteriporidae
- Family Bdellouridae
- Superfamily Procerodoidea
- Family Procerodidae
- Superfamily Cercyroidea
- Suborder Cavernicola[12]
- Family Dimarcusidae
- Suborder Continenticola
- Superfamily Planarioidea
- Family Planariidae
- Family Dendrocoelidae
- Family Kenkiidae
- Superfamily Geoplanoidea
- Family Dugesiidae
- Family Geoplanidae
- Superfamily Planarioidea
- Suborder Maricola
Anatomy and physiology
[edit]Planarians are bilaterian flatworms that lack a fluid-filled body cavity, and the space between their organ systems is filled with parenchyma.[5][13] Planarians lack a circulatory system, and absorb oxygen through their body wall. They uptake food to their gut using a muscular pharynx, and nutrients diffuse to internal tissues. A three-branched intestine runs across almost the entire body, and includes a single anterior and two posterior branches. The planarian intestine is a blind sac, having no exit cavity, and therefore planarians uptake food and egest waste through the same orifice, located near the middle of the ventral body surface.[5]
The excretory system is made of many tubes with many flame cells and excretory pores on them. Also, flame cells remove unwanted liquids from the body by passing them through ducts which lead to excretory pores, where waste is released on the dorsal surface of the planarian.
The triclads have an anterior end or head where sense organs, such as eyes and chemoreceptors, are usually found. Some species have auricles that protrude from the margins of the head. The auricles can contain chemical and mechanical sensory receptors.[14]
The number of eyes in the triclads is variable depending on the species. While many species have two eyes (e.g. Dugesia or Microplana), others have many more distributed along the body (e.g. most Geoplaninae). Sometimes, those species with two eyes may present smaller accessory or supernumerary eyes. The subterranean triclads are often eyeless or blind.[14]
The body of the triclads is covered by a ciliated epidermis that contains rhabdites. Between the epidermis and the gastrodermis there is a parenchymatous tissue or mesenchyme.[14]
Nervous system
[edit]
The planarian nervous systems consists of a bilobed shaped cerebral ganglion that is referred to as the planarian brain.[15] Longitudinal ventral nerve chords extend from the brain to the tail. Transverse nerves, commissure, connect the ventral nerve chords forming ladder-like nerve system.[5] The brain has been shown to exhibit spontaneous electrophysiological oscillations,[16] similar to the electroencephalographic (EEG) activity of other animals.
The planarian has a soft, flat, wedge-shaped body that may be black, brown, blue, gray, or white. The blunt, triangular head has two ocelli (eyespots), pigmented areas that are sensitive to light. There are two auricles (earlike projections) at the base of the head, which are sensitive to touch and the presence of certain chemicals. The mouth is located in the middle of the underside of the body, which is covered with hairlike projections (cilia). There are no circulatory or respiratory systems; oxygen enters and carbon dioxide leaves the planarian's body by diffusing through the body wall.
Reproduction
[edit]This article needs additional citations for verification. (November 2016) |

Triclads reproduce sexually and asexually, and different species may be able to reproduce by one or both modes.[5] Planarians are hermaphrodites. In sexual reproduction, the mating generally involves mutual insemination.
Thus, one of their gametes will combine with the gamete of another planarian. Each planarian transports its secretion to the other planarian, giving and receiving sperm. Eggs develop inside the body and are shed in capsules. Weeks later, the eggs hatch and grow into adults. In asexual reproduction, the planarian fissions and each fragment regenerates its missing tissues, generating complete anatomy and restoring functions.[17] Asexual reproduction, similar to regeneration following injury, requires neoblasts, adult stem cells, which proliferate and produce differentiated cells.[17] Some researchers claim that the products derived from bisecting a planarian are similar to the products of planarian asexual reproduction; however, debates about the nature of asexual reproduction in planarians and its effect on the population are ongoing.[18] Some species of planarian are exclusively asexual, whereas some can reproduce both sexually and asexually.[19] In most of the cases the sexual reproduction involve two individuals; auto fecundation has been rarely reported (e.g. in Cura foremanii).[14]
Neoblasts
[edit]Neoblasts are abundant adult stem cells that are found in the planarian parenchyma across the planarian body.[20] They are small and round cells, 5 to 10 μm, and characterized by a large nucleus, which is surrounded by little cytoplasm.[20] Neoblasts are required for regenerating missing tissues and organs, and they continuously replenish tissues by producing new cells.[17] Neoblasts can self-renew and generate progenitors for different cell types. In contrast to adult vertebrate stem cells (e.g., hematopoietic stem cell), neoblasts are pluripotent (i.e., producing all somatic cell types).[21] Moreover, they give rise to differentiating, post-mitotic, cells directly,[22] and not by producing rapidly-dividing transit amplifying cells.[20] Consequently, neoblasts divide frequently, and apparently lack a large sub-population of dormant or slow-cycling cells.[23]
As a model system in biological and biomedical research
[edit]The life history of planarians make them a model system for investigating a number of biological processes, many of which may have implications for human health and disease. Advances in molecular genetic technologies has made the study of gene function possible in these animals and scientists are studying them worldwide. Like other invertebrate model organisms, for example C. elegans and D. melanogaster, the relative simplicity of planarians facilitates experimental study.
Planarians have a number of cell types, tissues and simple organs that are homologous to human cells, tissues and organs. However, regeneration has attracted the most attention. Thomas Hunt Morgan was responsible for some of the first systematic studies (that still underpin modern research) before the advent of molecular biology as a discipline.
Planarians are also an emerging model organism for aging research. These animals have an apparently limitless regenerative capacity, and asexual Schmidtea mediterranea has been shown to maintain its telomere length through regeneration.[24]
Live planarians are increasingly used in toxicological research due to their regenerative capabilities, simple anatomy, and sensitivity to environmental changes. Their ability to regenerate lost body parts provides a unique model to study the effects of chemical exposures on cellular processes, while their rapid response to toxins makes them an efficient tool for screening potential environmental and pharmaceutical hazards. An example of this application is a fluorescence-based skin irritability assay, where planaria are exposed to various chemicals, and fluorescence dye is used to evaluate their epithelial damage in response to irritation, providing an effective screening method.[25]
Regeneration
[edit]Planarian regeneration combines new tissue production with reorganization to the existing anatomy, morphallaxis.[17] The rate of tissue regrowth varies between species, but in frequently used lab species, functional regenerated tissues are available already 7–10 days following tissue amputation.[17] Regeneration starts following an injury that require the growth of a new tissue.[26] Neoblasts localized near the injury site proliferate to generate a structure of differentiating cells called blastema. Neoblasts are required for new cell production, and they therefore provide the cellular basis for planarian regeneration.[27] Cell signaling mechanisms provide positional information that regulates the cell types and tissues that are produced from the neoblasts in regeneration.[28] Many signaling molecules that provide positional information to neoblasts, in regeneration and homeostasis, are expressed in muscle cells.[29] Following injury, muscle cells throughout the body can alter the expression of genes that encode molecules that provide positional information.[29] Therefore, the activities of neoblasts and muscle cells following injuries are essential for successful regeneration.[30]
Historically, planarians have been considered "immortal under the edge of a knife."[31] Very small pieces of the planarian, estimated to be as little as 1/279th of the organism it is cut from, can regenerate back into a complete organism over the course of a few weeks.[32] New tissues can grow due to pluripotent stem cells that have the ability to create all the various cell types.[33] These adult stem cells are called neoblasts, and comprise 20% or more of the cells in the adult animal.[34] They are the only proliferating cells in the worm, and they differentiate into progeny that replace older cells. In addition, existing tissue is remodeled to restore symmetry and proportion of the new planaria that forms from a piece of a cut up organism.[34][17]
The organism itself does not have to be completely cut into separate pieces for the regeneration phenomenon to be witnessed. In fact, if the head of a planarian is cut in half down its center, and each side retained on the organism, it is possible for the planarian to regenerate two heads and continue to live.[35] Researchers, including those from Tufts University in the U.S., sought to determine how microgravity and micro-geomagnetic fields would affect the growth and regeneration of planarian flatworms, Dugesia japonica. They discovered that one of the amputated fragments sent to space regenerated into a double-headed worm. The majority of such amputated worms (95%) did not do so, however. An amputated worm regenerated into a double-head worm after spending five weeks aboard the International Space Station (ISS) – though regeneration of amputated worms as double-headed heteromorphosis is not a rare phenomenon unique to a microgravity environment.[36] Double-headed planaria regenerates can be induced by exposing amputated fragments to electrical fields. Electrical field exposure with opposite polarity can induce a planarian with two tails. Double-headed planaria regenerates can also be induced by treating amputated fragments with pharmacological agents that alter levels of calcium, cyclic AMP, and protein kinase C activity in cells,[37] as well as by genetic expression blocks (interference RNA) to the canonical Wnt/β-Catenin signalling pathway.[28]
Biochemical memory experiments
[edit]In 1955, Robert Thompson and James V. McConnell conditioned planarian flatworms by pairing a bright light with an electric shock. After repeating this several times they took away the electric shock, and only exposed them to the bright light. The flatworms would react to the bright light as if they had been shocked. Thompson and McConnell found that if they cut the worm in two, and allowed both worms to regenerate, each half would develop the light-shock reaction. In 1963, McConnell repeated the experiment, but instead of cutting the trained flatworms in two he ground them into small pieces and fed them to other flatworms. He reported that the flatworms learned to associate the bright light with a shock much faster than flatworms who had not been fed trained worms.
This experiment intended to test whether memory could be transferred chemically. The experiment was repeated with mice, fish, and rats, but it always failed to produce the same results. The perceived explanation was that rather than memory being transferred to the other animals, it was the hormones in the ingested ground animals that changed the behavior.[38] McConnell believed that this was evidence of a chemical basis for memory, which he identified as memory RNA. McConnell's results are now attributed to observer bias.[39][40] No blinded experiment has ever reproduced his results of planarians scrunching when exposed to light. Subsequent explanations of this scrunching behaviour associated with cannibalism of trained planarian worms were that the untrained flatworms were only following tracks left on the dirty glassware rather than absorbing the memory of their fodder.
In 2012, Tal Shomrat and Michael Levin have shown that planarians exhibit evidence of long-term memory retrieval after regenerating a new head.[41]
Planarian species used for research and education
[edit]Several planarian species are commonly used for biological research. Popular experimental species are Schmidtea mediterranea, Schmidtea polychroa, and Dugesia japonica,[5] which in addition to excellent regenerative abilities, are easy to culture in the lab. In recent decades, S. mediterranea has emerged as the species of choice for modern molecular biology research, due to its diploid chromosomes and the availability of both asexual and sexual strains.[7]
The most frequently used planarian in high school and first-year college laboratories is the brownish Girardia tigrina. Other common species used are the blackish Planaria maculata and Girardia dorotocephala.
See also
[edit]- Worm Runner's Digest – Satirical (and serious) science journal (1959–1979)
References
[edit]- ^ a b c Sluys, R.; Kawakatsu, M.; Riutort, M.; Baguñà, J. (2009). "A new higher classification of planarian flatworms (Platyhelminthes, Tricladida)". Journal of Natural History. 43 (29–30): 1763–1777. Bibcode:2009JNatH..43.1763S. doi:10.1080/00222930902741669. S2CID 85174457.
- ^ "Planarian (flatworm) – Britannica Online Encyclopedia". Encyclopædia Britannica, Inc. Retrieved 2010-05-01.
- ^ Campbell NA, Reece JB (2019). Biology. Benjamin Cummings. pp. 1230 pp. ISBN 978-0-8053-7146-8.
- ^ "Tricladida". Integrated Taxonomic Information System. Retrieved July 23, 2007.
- ^ a b c d e f g Sluys, Ronald; Riutort, Marta (2018), Rink, Jochen C. (ed.), "Planarian Diversity and Phylogeny", Planarian Regeneration: Methods and Protocols, Methods in Molecular Biology, vol. 1774, New York, NY: Springer, pp. 1–56, doi:10.1007/978-1-4939-7802-1_1, ISBN 978-1-4939-7802-1, PMID 29916154pp 3., "Planarians (the popular name for the group as a whole), or triclad flatworms (the more scientific designation of the same group), are acoelomate bilaterians".
- ^ Vila-Farré, Miquel; Rozanski, Andrei; Ivanković, Mario; Cleland, James; Brand, Jeremias N.; Thalen, Felix; Grohme, Markus A.; von Kannen, Stephanie; Grosbusch, Alexandra L.; Vu, Hanh T.-K.; Prieto, Carlos E.; Carbayo, Fernando; Egger, Bernhard; Bleidorn, Christoph; Rasko, John E. J. (2023-10-19). "Evolutionary dynamics of whole-body regeneration across planarian flatworms". Nature Ecology & Evolution. 7 (12): 2108–2124. Bibcode:2023NatEE...7.2108V. doi:10.1038/s41559-023-02221-7. ISSN 2397-334X. PMC 10697840. PMID 37857891. S2CID 264347538.
- ^ a b c Newmark PA, Sánchez Alvarado A (March 2002). "Not your father's planarian: a classic model enters the era of functional genomics". Nature Reviews. Genetics. 3 (3): 210–9. doi:10.1038/nrg759. PMID 11972158. S2CID 28379017.
- ^ Rozanski, Andrei; Moon, HongKee; Brandl, Holger; Martín-Durán, José M; Grohme, Markus A; Hüttner, Katja; Bartscherer, Kerstin; Henry, Ian; Rink, Jochen C (2019-01-08). "PlanMine 3.0—improvements to a mineable resource of flatworm biology and biodiversity". Nucleic Acids Research. 47 (D1): D812 – D820. doi:10.1093/nar/gky1070. ISSN 0305-1048. PMC 6324014. PMID 30496475.
- ^ Hallez P. (1892). Classification des Ticlades, Bulletin de la Société Zoologique de France.
- ^ Rompolas P, Patel-King RS, King SM (2009). Schmidtea mediterranea: a model system for analysis of motile cilia. Methods in Cell Biology. Vol. 93. pp. 81–98. doi:10.1016/S0091-679X(08)93004-1. ISBN 978-0-12-381377-0. PMID 20409812.
- ^ Manenti R (2010). "– Effect of landscape features and water quality on Triclads inhabiting head waters: the example of Polycelis felina" (PDF). Revue Écologie Terre et Vie. 65 (2): 279–285. doi:10.3406/revec.2010.1533. hdl:2434/147984. S2CID 54499235.
- ^ Sluys, R. (1990). "A monograph of the Dimarcusidae (Platyhelminthes, Seriata, Tricladida)". Zoologica Scripta. 19 (1): 13–29. doi:10.1111/j.1463-6409.1990.tb00237.x. S2CID 84915439.
- ^ Riutort, Marta; Álvarez-Presas, Marta; Lázaro, Eva; Sol, Eduard; Paps, Jordi (2012). "Evolutionary history of the Tricladida and the Platyhelminthes: an up-to-date phylogenetic and systematic account". The International Journal of Developmental Biology. 56 (1–2–3): 5–17. doi:10.1387/ijdb.113441mr. ISSN 0214-6282. PMID 22450992.
- ^ a b c d Kenk, R., 1972. Freshwater planarians (Turbellarians) of North America.
- ^ Cebrià, Francesc; Nakazawa, Masumi; Mineta, Katsuhiko; Ikeo, Kazuho; Gojobori, Takashi; Agata, Kiyokazu (2002-04-03). "Dissecting planarian central nervous system regeneration by the expression of neural-specific genes". Development, Growth & Differentiation. 44 (2): 135–146. doi:10.1046/j.1440-169x.2002.00629.x. ISSN 0012-1592. PMID 11940100.
- ^ Aoki R, Wake H, Sasaki H, Agata K (March 2009). "Recording and spectrum analysis of the planarian electroencephalogram". Neuroscience. 159 (2): 908–914. doi:10.1016/j.neuroscience.2008.11.011. PMID 19063945. S2CID 207244874.
- ^ a b c d e f Reddien, Peter W.; Alvarado, Alejandro Sánchez (2004-11-01). "Fundamentals of Planarian Regeneration". Annual Review of Cell and Developmental Biology. 20 (1): 725–757. doi:10.1146/annurev.cellbio.20.010403.095114. ISSN 1081-0706. PMID 15473858.
- ^ Neuhof M, Levin M, Rechavi O (September 2016). "Vertically- and horizontally-transmitted memories - the fading boundaries between regeneration and inheritance in planaria". Biology Open. 5 (9): 1177–88. doi:10.1242/bio.020149. PMC 5051648. PMID 27565761.
- ^ Newmark, Phillip A.; Alvarado, Alejandro Sánchez (2002-03-01). "Not your father's planarian: a classic model enters the era of functional genomics". Nature Reviews Genetics. 3 (3): 210–219. doi:10.1038/nrg759. ISSN 1471-0056. PMID 11972158. S2CID 28379017.
- ^ a b c Rink, Jochen C. (2018), Rink, Jochen C. (ed.), "Stem Cells, Patterning and Regeneration in Planarians: Self-Organization at the Organismal Scale", Planarian Regeneration: Methods and Protocols, Methods in Molecular Biology, vol. 1774, New York, NY: Springer, pp. 57–172, doi:10.1007/978-1-4939-7802-1_2, ISBN 978-1-4939-7802-1, PMID 29916155
- ^ Wagner, Daniel E.; Wang, Irving E.; Reddien, Peter W. (2011-05-13). "Clonogenic Neoblasts Are Pluripotent Adult Stem Cells That Underlie Planarian Regeneration". Science. 332 (6031): 811–816. Bibcode:2011Sci...332..811W. doi:10.1126/science.1203983. ISSN 0036-8075. PMC 3338249. PMID 21566185.
- ^ Raz, Amelie A.; Wurtzel, Omri; Reddien, Peter W. (2021-04-20). "Planarian stem cells specify fate yet retain potency during the cell cycle". Cell Stem Cell. 28 (7): 1307–1322.e5. doi:10.1016/j.stem.2021.03.021. PMC 8254784. PMID 33882291.
- ^ Newmark, Phillip A.; Sánchez Alvarado, Alejandro (2000-04-15). "Bromodeoxyuridine Specifically Labels the Regenerative Stem Cells of Planarians". Developmental Biology. 220 (2): 142–153. doi:10.1006/dbio.2000.9645. ISSN 0012-1606. PMID 10753506.
- ^ Tan TC, Rahman R, Jaber-Hijazi F, Felix DA, Chen C, Louis EJ, Aboobaker A (March 2012). "Telomere maintenance and telomerase activity are differentially regulated in asexual and sexual worms". Proceedings of the National Academy of Sciences of the United States of America. 109 (11): 4209–14. Bibcode:2012PNAS..109.4209T. doi:10.1073/pnas.1118885109. PMC 3306686. PMID 22371573.
- ^ Shah, Syed Ibrahim; Williams, Adrian C.; Lau, Wing Man; Khutoryanskiy, Vitaliy V. (2020-12-01). "Planarian toxicity fluorescent assay: A rapid and cheap pre-screening tool for potential skin irritants". Toxicology in Vitro. 69 105004. Bibcode:2020ToxVi..6905004S. doi:10.1016/j.tiv.2020.105004. ISSN 0887-2333. PMID 33010358.
- ^ Wenemoser, Danielle; Reddien, Peter W. (2010-08-15). "Planarian regeneration involves distinct stem cell responses to wounds and tissue absence". Developmental Biology. 344 (2): 979–991. doi:10.1016/j.ydbio.2010.06.017. PMC 2950745. PMID 20599901.
- ^ Hayashi, Tetsutaro; Asami, Maki; Higuchi, Sayaka; Shibata, Norito; Agata, Kiyokazu (2006-07-13). "Isolation of planarian X-ray-sensitive stem cells by fluorescence-activated cell sorting". Development, Growth & Differentiation. 48 (6): 371–380. doi:10.1111/j.1440-169X.2006.00876.x. ISSN 0012-1592. PMID 16872450. S2CID 10048289.
- ^ a b Gurley KA, Rink JC, Sánchez Alvarado A (January 2008). "Beta-catenin defines head versus tail identity during planarian regeneration and homeostasis". Science. 319 (5861): 323–7. Bibcode:2008Sci...319..323G. doi:10.1126/science.1150029. PMC 2755502. PMID 18063757.
- ^ a b Witchley, Jessica N.; Mayer, Mirjam; Wagner, Daniel E.; Owen, Jared H.; Reddien, Peter W. (2013-08-29). "Muscle cells provide instructions for planarian regeneration". Cell Reports. 4 (4): 633–641. doi:10.1016/j.celrep.2013.07.022. ISSN 2211-1247. PMC 4101538. PMID 23954785.
- ^ Reddien, Peter W. (2018-10-04). "The cellular and molecular basis for planarian regeneration". Cell. 175 (2): 327–345. doi:10.1016/j.cell.2018.09.021. ISSN 0092-8674. PMC 7706840. PMID 30290140.
- ^ Dalyell JG (1814). Observations on some interesting phenomena in animal physiology, exhibited by several species of planariae. Edinburgh.
- ^ Handberg-Thorsager M, Fernandez E, Salo E (2008). "Stem cells and regeneration in planarians". Frontiers in Bioscience. 13 (13): 6374–94. doi:10.2741/3160. PMID 18508666.
- ^ Saló E, Abril JF, Adell T, Cebrià F, Eckelt K, Fernandez-Taboada E, Handberg-Thorsager M, Iglesias M, Molina MD, Rodríguez-Esteban G (2009). "Planarian regeneration: achievements and future directions after 20 years of research". The International Journal of Developmental Biology. 53 (8–10): 1317–27. doi:10.1387/ijdb.072414es. hdl:2445/192658. PMID 19247944.
- ^ a b Aboobaker AA (May 2011). "Planarian stem cells: a simple paradigm for regeneration". Trends in Cell Biology. 21 (5): 304–11. doi:10.1016/j.tcb.2011.01.005. PMID 21353778.
- ^ "Do it again. Round up of regenerating animals". New Scientist. New Scientist. Retrieved 2012-10-21.
- ^ Morokuma J, Durant F, Williams KB, Finkelstein JM, Blackiston DJ, Clements T, Reed DW, Roberts M, Jain M, Kimel K, Trauger SA, Wolfe BE, Levin M (April 2017). "Planarian regeneration in space: Persistent anatomical, behavioral, and bacteriological changes induced by space travel". Regeneration. 4 (2): 85–102. doi:10.1002/reg2.79. PMC 5469732. PMID 28616247.
- ^ Chan JD, Agbedanu PN, Zamanian M, Gruba SM, Haynes CL, Day TA, Marchant JS (February 2014). "'Death and axes': unexpected Ca²⁺ entry phenologs predict new anti-schistosomal agents". PLOS Pathogens. 10 (2) e1003942. doi:10.1371/journal.ppat.1003942. PMC 3930560. PMID 24586156.
- ^ Kentridge B. "Investigations of the cellular bases of memory". University of Durham. Archived from the original on 2012-10-15. Retrieved 2007-02-08.
- ^ Rilling M (1996). "The mystery of the vanished citations: James McConnell's forgotten 1960s quest for planarian learning, a biochemical engram, and celebrity". American Psychologist. 51 (6): 589–598. doi:10.1037/0003-066X.51.6.589.
- ^ For a general review, see also Chapouthier G (1973). "Chapter 1: Behavioral studies of the molecular basis of memory". In Deutsch JA (ed.). The Physiological Basis of Memory. New York and London: Academic Press. pp. l–25.
- ^ Shomrat T, Levin M (October 2013). "An automated training paradigm reveals long-term memory in planarians and its persistence through head regeneration". The Journal of Experimental Biology. 216 (Pt 20): 3799–810. doi:10.1242/jeb.087809. PMID 23821717.
External links
[edit]- More information on freshwater planarians and their biology
- More information on the genetic screen to identify regeneration genes
- YouTube videos: Planaria eating worm segment, Planarian
- Schmidtea mediterranea, facts, anatomy, image Archived 2010-12-30 at the Wayback Machine at GeoChemBio.com
- Alejandro Sanchez-Alvarado's Seminar: Regeneration in Planarians
- Link to an article discussing some work on planarian immortality
- A user-friendly visualization tool and database of planarian regeneration experiments
- Aboobaker, Aziz (27 February 2008). "Immortal Worms". Test Tube. Brady Haran for the University of Nottingham.
- Tricladida on the Encyclopedia of Life (EOL)
- Land planarians on the UF / IFAS Featured Creatures Web site