Recent from talks
All channels
Be the first to start a discussion here.
Be the first to start a discussion here.
Be the first to start a discussion here.
Be the first to start a discussion here.
Welcome to the community hub built to collect knowledge and have discussions related to Aneurysmal bone cyst.
Nothing was collected or created yet.
Aneurysmal bone cyst
View on Wikipediafrom Wikipedia
Not found
Aneurysmal bone cyst
View on Grokipediafrom Grokipedia
An aneurysmal bone cyst (ABC) is a benign, non-malignant, tumor-like vascular lesion of bone characterized by multiple blood-filled cystic spaces separated by fibrous septa containing osteoid tissue and osteoclast-like giant cells, which can expand rapidly, cause local bone destruction, and lead to pathologic fractures.[1] These lesions are rare, accounting for 1% to 2% of all primary bone tumors, with an incidence of approximately 1 in 100,000 individuals annually, and they predominantly affect children and adolescents under 20 years of age, showing a slight female predominance (female-to-male ratio of 1:1 to 1.3:1).[2][1]
ABCs most commonly arise in the metaphysis of long bones such as the femur and tibia (about 67% of cases), followed by the spine (15%) and pelvis (9%), though they can occur in any bone.[1] The etiology remains unclear, but evidence suggests they may develop as primary lesions due to genetic abnormalities like the t(16;17)(q22;p13) translocation involving the USP6 gene in up to 69% of cases, or as secondary reactive processes to trauma, other benign bone tumors (e.g., chondroblastoma or giant cell tumor), or vascular malformations.[1] Clinically, patients often present with insidious onset of localized pain over weeks to months, swelling, or deformity; acute severe pain may signal a pathologic fracture, while spinal involvement can cause neurologic symptoms such as radiculopathy or myelopathy due to compression.[3][2]
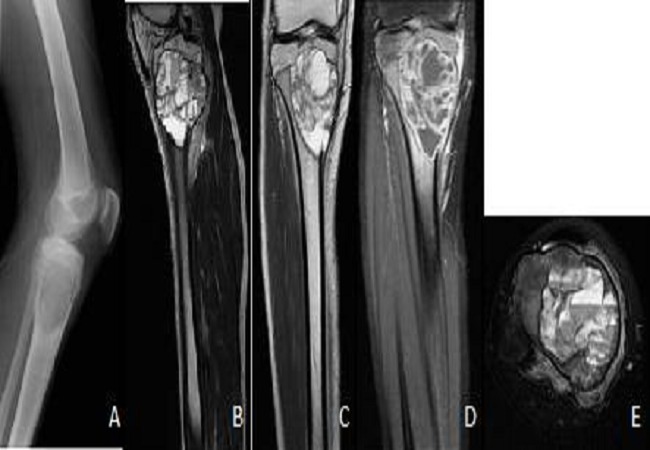
Diagnosis typically involves radiographic imaging, where ABCs appear as expansile, lytic lesions with thinned "eggshell" cortical borders and internal septations; advanced modalities like MRI reveal characteristic fluid-fluid levels from blood sedimentation, aiding differentiation from malignant mimics such as telangiectatic osteosarcoma.[1] Histopathologic confirmation via biopsy is often required, showing cavernous blood-filled spaces without endothelial lining and the presence of spindle cells, multinucleated giant cells, and reactive bone formation.[2] Treatment is primarily surgical, with curettage and bone grafting as the standard approach to decompress the lesion and restore stability, though adjuncts like phenol or cryotherapy reduce recurrence rates (which approach 25%); alternatives include percutaneous sclerotherapy, selective arterial embolization, or denosumab for unresectable cases, particularly in the spine.[3][1]