Recent from talks
Nothing was collected or created yet.
Fertility testing
View on WikipediaThis article needs more reliable medical references for verification or relies too heavily on primary sources. (June 2018) |
| Fertility testing | |
|---|---|
 | |
| Purpose | assess fertility |
Fertility testing is the process by which fertility is assessed, both generally and also to find the "fertile window" in the menstrual cycle. General health affects fertility, and STI testing is an important related field.
Women
[edit]
Healthy women are fertile from puberty until menopause, although fertility is typically much reduced towards the extremes of this period. The onset of puberty is typically identified by menarche and the presence of secondary sexual characteristics such as breast development, the appearance of pubic hair and changes to body fat distribution. The end of fertility typically comes somewhat before menopause, as fertility declines to a point where establishing a viable pregnancy is very unlikely.
Ovulation testing
[edit]Various methods of predicting the timing of ovulation exist, some of which may be performed at home or in a clinical setting. Knowing the timing of ovulation can help a woman to determine the days of the menstrual cycle that are most likely to result in conception.
Stretch test
[edit]
The cervix is a structure between the vaginal canal and the uterus. The cervical cells secrete mucus that changes its consistency over different parts of the menstrual cycle. During the fertile window, the mucus increases in quantity and becomes clear and stretchy and is known as "egg-white cervical mucus." This mucus allows sperm to survive in and travel through it. In contrast, when outside of the fertile window, the mucus does not stretch, is sticky, and is not clear.
The stretch test can be performed prior to and immediately after urination. Mucus can be sampled with by either wiping with toilet paper or inserting a clean finger into the vagina. The mucus quality can then be observed by stretching the mucus between the finger and thumb as shown in the image.[2]
Ovulation prediction kit
[edit]Urinary ovulation prediction kits are typically found over-the-counter and there are many brands to choose from. This test measures the amount of luteinizing hormone, a hormone that increases just before ovulation, that is in the urine. Before ovulation, the luteinizing hormone levels dramatically increase; this is known as the "LH surge". This test can recognize the LH surge about 1-1.5 days prior to ovulation. Additionally, some ovulation prediction kits detect estrone-3-glucuronide. This is a breakdown product of estrogen and will have increased levels in the urine around the time of ovulation. This test is able to detect luteinizing hormone and estrone-3-glucuronide 90% of the time.
This test can be used in multiple ways. A few drops of urine can be added to the test device tip. Alternatively, the test device tip can be held in the urine stream. Finally, the test device tip can be dipped into a cup of urine. The test will indicate positive or negative results in about five minutes.[3]
Electronic fertility monitors
[edit]
A fertility monitor is an electronic device which may use various methods to assist the user with fertility awareness. A fertility monitor may analyze changes in hormone levels in urine, basal body temperature, electrical resistance of saliva and vaginal fluids, or a combination of these methods. These devices may assist in pregnancy achievement. An updated 2023 Cochrane review has found that the use of urine ovulation test probably improves life births in women under 40 but that further study on risk and benefits is needed on timed intercourse via the use of these test.[4]
Daily ultrasound
[edit]Daily ultrasounds are used to follow the development of follicles which can help predict ovulation. The ultrasounds can predict ovulation with a 24-hour overlap to actual ovulation.[5]
Serum progesterone
[edit]Serum progesterone level is measured during the mid-luteal phase of the menstrual cycle. In women who are experiencing infertility, this test is only somewhat helpful for predicting ovulation.[6]
Cervical position
[edit]The cervix becomes soft, high, open and wet during the fertile window.
Basal body temperature charting
[edit]Basal body temperature changes during the menstrual cycle. Higher levels of progesterone released during the menstrual cycle causes an abrupt increase in basal body temperature by 0.5 °C to 1 °C at the time of ovulation.[7] This enables identification of the fertile window through the use of commercial thermometers. This test can also indicate if there are issues with ovulation.[8]
Calendar methods
[edit]In women who have regular menstrual cycles, the fertile window occurs at approximately the same time every month. If the first day of menses is considered day 1, then ovulation occurs around day 14. In regular cycles that are 26–32 days long, the fertile window occurs on days 8–19.[9]
Diagnostic testing for infertility
[edit]Women who are of fertile age may be infertile for a number of reasons. Various diagnostic tests are available to establish reasons. Several diagnostic procedures and clinical instruments are used for to evaluate anatomical causes of infertility. Some use a combination of imaging such as an X-ray or ultrasound with a contrast agent to visualize anatomic structures within the uterus and fallopian tubes. An electronic, flexible scope with a camera can also be inserted through the cervix to display live images. A variety of hormones can be tested at different times in the menstrual cycle to determine the likelihood of different responses to stimulation for In vitro fertilization (IVF).
| Test | Method | Invasiveness |
|---|---|---|
| Anti-Müllerian hormone testing | Lab test | Blood draw |
| Cycle-day-three follicle-stimulating hormone (FSH) testing | Lab test | Blood draw |
| Clomiphene citrate challenge test (CCCT) | Lab test | Blood draw |
| Antral follicle count | Ultrasound imaging | Non-invasive |
| X-ray hysterosalpingography | X-ray imaging | Minimally invasive |
| Hystero contrast sonography (HyCoSy) | Ultrasound with contrast dye | Minimally invasive |
| Saline infusion sonohysterography (SHG) | Ultrasound with saline | Minimally invasive |
| Hystero foam sonography (HyFoSy) | Ultrasound with foam | Minimally invasive |
| Ovarian ultrasound | Ultrasound | Minimally invasive |
| Three-dimensional sonography | Ultrasound with 3D imaging | Minimally invasive |
| Hysteroscopy | Transvaginal endoscope | Invasive |
| Laparoscopy with chromotubation | Abdominal laparoscope | Invasive |
Anti-Müllerian hormone testing
[edit]Anti-Müllerian hormone (AMH) is a glycoprotein hormone produced by granulosa cells in preantral and small antral follicles of the ovaries.[11] Testing for plasma levels of AMH allows physicians to estimate ovarian reserve. Estimations of ovarian reserve help to determine the likelihood of pregnancy by In vitro fertilization (IVF). AMH testing is considered to be one of the most accurate estimates of ovarian reserve, can be used for assessment at any point in the menstrual cycle, and is non-invasive.[12]
Cycle-day-three follicle-stimulating hormone (FSH) testing
[edit]Follicle-stimulating hormone (FSH) is a peptide hormone which causes the primordial follicles in the ovaries to develop and to produce estrogen.[13] FSH levels are elevated early in the cycle of women who have lower ovarian reserve, because their follicles do not produce enough estrogen to inhibit FSH production,[14] therefore high levels early on in a woman's menstrual cycle can indicate lower ovarian reserve and lower likelihood of retrieving eggs for IVF. To test for ovarian reserve in women with infertility, FSH levels are measured from blood samples taken on day three of the menstrual cycle and compared to standards to determine the likelihood of pregnancy after IVF treatment.
Clomiphene citrate challenge test (CCCT)
[edit]The clomifene citrate challenge test is similar to cycle-day-three FSH testing. To perform this test blood samples are taken on day three of the menstrual cycle to obtain FSH and estradiol levels, then 100 mg of clomiphene citrate are given orally once a day on days 5 through 9 of the menstrual cycle, and finally on day 10 of the menstrual cycle a second blood sample is taken to measure FSH levels. CCCT is not better at predicting ovarian response in IVF patients than baseline FSH on day 3.[15]
Antral follicle count
[edit]Antral follicles are cells early in the process of developing from an oogonium into a mature oocyte. A physician may use a transvaginal ultrasound to visualize and count the number of antral follicles in each of a woman's ovaries in order to determine her ovarian reserve; however AFC is not predictive of embryo quality.[12] A higher number of antral follicles indicates a higher likelihood of pregnancy by IVF.
X-ray hysterosalpingography
[edit]Hysterosalpingography (HSG) is an invasive x-ray imaging technique used to evaluate the shape and size of the uterus and openness of the fallopian tubes. It is a diagnostic test used in the investigation of infertility from genetic or infectious causes such as uterine fibroids, uterine polyps, uterine anomalies, scarring or tumors.[16]
A HSG is performed after menses and before ovulation during the first half of a menstrual cycle. It is not performed if the patient is pregnant, has a pelvic infection, or heavy bleeding at the time of the test.[17]
The procedure usually takes 30 minutes and often takes place in an outpatient setting such as a hospital or clinic. The patient is draped and positioned on her back as if for a pelvic exam with feet elevated. A speculum is used to visualize the cervix. The cervix is cleaned with an antiseptic and injected with a local anesthetic to minimize discomfort and pain. A small catheter is used to fill the uterus with an iodinated contrast dye. X-ray images are taken as the contrast dye makes its way through the uterus and fallopian tubes. After images have been captured, the catheter is removed and contrast dye may either spill outside of the vagina or become absorbed.[16][17]
Risks associated with HSG are rare and include exposure to radiation, infection, allergic reactions to the contrast dye or antiseptic. It is normal for patients to experience mild to moderate abdominal cramping, pain and vaginal spotting for a few days after the procedure.[16]
Hystero contrast sonography (HyCoSy)
[edit]
Hystero contrast sonography (HyCoSy) is a transvaginal ultrasound imaging technique used to evaluate the uterus, fallopian tubes and ovaries. It is a screening test used to determine the need for a diagnostic laparoscopy.[18]
A HyCoSy is typically performed after menses and before ovulation during the first half of a menstrual cycle. Unlike a HSG, a HyCoSy can be used to investigate causes of heavy bleeding.[19]
The procedure usually takes 15–20 minutes and often takes place in an outpatient setting such as a hospital or clinic. The patient is draped and positioned on her back as if for a pelvic exam with feet elevate. A speculum is used to visualize the cervix. The cervix is cleaned with an antiseptic such as iodine and injected with a local anesthetic to minimize discomfort and pain. A small catheter is used to fill the uterus and fallopian tubes with a contrast agent consisting of a galactose solution called Echovist to enhance visibility. A transvaginal ultrasound is inserted into the vagina and manually positioned to visualize the uterus, fallopian tubes, and ovaries. Once images have been captured, the ultrasound probe and catheter are removed. The contrast agent used during the study may either spill outside of the vagina or become absorbed.[18]
HyCoSy does not carry the same risks as X-ray hysterosalpingography because it does not use radiation or iodinated contrast dye.
Saline infusion sonohysterography (SHG)
[edit]Saline infusion sonohysterography is identical in procedure to hystero contrast sonography (HyCoSy) but uses saline instead of a contrast agent.[18]
Hystero foam sonography (HyFoSy)
[edit]An alternative to saline and Echovist, the galactose solution used to enhance visualization of anatomic features via ultrasound in HyCoSy, was needed because of limitations and high costs.[18] A sterile gel foam designed for gynecological use paved the entry for a new technique called hystero foam sonography (HyFoSy). The gel offers more stability than saline and patients may experience less discomfort and fluid leakage.[18]
Ovarian ultrasound
[edit]Ultrasound scans of the ovaries (optimally by transvaginal ultrasonography) may be conducted to establish the development of ovarian follicles. This can be useful particularly in the diagnosis of polycystic ovary syndrome.
Three-dimensional sonography
[edit]Three-dimension sonography is a 3D ultrasound technique that uses a series of 2D images to render 3D images of the uterus and fallopian tubes.
Hysteroscopy
[edit]Hysteroscopy is used to visualize the inside of the uterus using a thin, lighted, flexible camera that is inserted vaginally and through the cervix. The camera projects live images on an external screen. It is used to evaluate intrauterine causes of infertility.
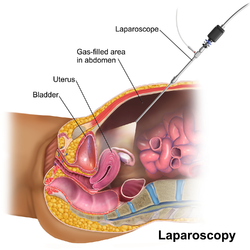
Laparoscopy with chromotubation
[edit]Laparoscopy is a minimally-invasive surgical procedure in which a camera is inserted into the abdominal cavity via a small (0.5 - 1.5 cm) incision. It is often used to diagnose endometriosis. Chromopertubation is a combined laparoscopic procedure commonly referred to as a "laparoscopy and dye" test. It uses the injection of a blue dye solution (methylene blue or indigo carmine) into the uterus to help determine the openness of the fallopian tubes. Though considered to be a "gold standard" for diagnosing disorders of fallopian tube patency, it is an invasive procedure requiring general anesthesia.[20]
Men
[edit]Men who have gone through puberty should be fertile throughout life. The semen in ejaculate contains sex cells called sperm. After intercourse, sperm travel to the egg through the female reproductive tract, typically causing fertilisation to occur in the fallopian tubes.
Fertility testing for men involves semen testing and genetic testing, as other factors such as impotence are obvious. Semen can be tested for sperm count, sperm motility, sperm morphology, pH, volume, fructose content, oxidative stress, maturation assessment, DNA fragmentation and acrosome activity. Checks are also made to identify undescended testicles and retrograde ejaculation, along with medical history, such as cancer treatment, radiation, drug use, etc. In some cases the hamster zona-free ovum test may also be used to diagnose fertility. Genetic testing and chromosomal analysis can rule out some other causes of male infertility, such as Klinefelter syndrome.
A recent study identified epigenetic patterns in male sperm that may contribute to infertility.[21]
Male Fertility Testing
[edit]Several diagnostic tests are used to evaluate male fertility by assessing sperm quality, function, hormone levels, and genetic integrity. These tests help determine the underlying causes of infertility and guide treatment strategies in assisted reproduction.
Semen Analysis (Spermiogram) is the standard test for evaluating male fertility. It measures key parameters such as sperm concentration (count), motility (movement), morphology (shape), volume, pH, and the presence of white blood cells or debris. The World Health Organization (WHO)[22] provides reference ranges for normal semen values. This test is often the first step in male fertility assessment.
DNA Fragmentation Test evaluates the integrity of the sperm's genetic material. High levels of DNA fragmentation are associated with reduced fertilization rates, impaired embryo development, and increased risk of miscarriage. Tests such as the TUNEL assay,[23] SCSA (Sperm Chromatin Structure Assay),[24] and Comet assay[25] are commonly used to measure DNA fragmentation.
Sperm Oxidative Stress Test assesses the presence of reactive oxygen species (ROS) that can damage sperm membranes and DNA. Excessive oxidative stress is a known contributor to male infertility. Tests like MiOXSYS[26] and chemiluminescence assays[27] are used to quantify oxidative stress levels and guide antioxidant treatment strategies.
Sperm Maturation Assessment evaluates the functional maturity of spermatozoa. Immature sperm may exhibit abnormalities in chromatin packaging, membrane composition, or the presence of residual cytoplasm. Techniques such as chromomycin A3 staining (CMA3),[28] hyaluronic acid binding assayst,[29] or nuclear protein transition studies[30] are used to assess sperm maturity, which is important for successful fertilization and embryo development.
Hormonal Blood Tests are performed to evaluate endocrine function, which plays a critical role in spermatogenesis. Commonly measured hormones include:
- Follicle-stimulating hormone (FSH) – regulates sperm production.
- Luteinizing hormone (LH) – stimulates testosterone production.
- Testosterone – essential for sperm development and libido.
- Prolactin – elevated levels can indicate pituitary disorders.
- Estradiol and sex hormone-binding globulin (SHBG) – help assess hormone balance.
These tests can help diagnose hormonal imbalances, hypogonadism, or underlying endocrine disorders that may affect male fertility.
See also
[edit]References
[edit]- ^ Dunson DB, Baird DD, Wilcox AJ, Weinberg CR (July 1999). "Day-specific probabilities of clinical pregnancy based on two studies with imperfect measures of ovulation". Human Reproduction. 14 (7): 1835–9. doi:10.1093/humrep/14.7.1835. PMID 10402400.
- ^ "Cervical mucus testing – Pregnancy Info". www.pregnancyinfo.ca. Retrieved 2021-09-20.
- ^ Health, Center for Devices and Radiological (2018-11-03). "Ovulation (Urine Test)". FDA. Archived from the original on April 29, 2019.
- ^ Gibbons, Tatjana; Reavey, Jane; Georgiou, Ektoras X.; Becker, Christian M. (2023-09-15). "Timed intercourse for couples trying to conceive". The Cochrane Database of Systematic Reviews. 2023 (9) CD011345. doi:10.1002/14651858.CD011345.pub3. ISSN 1469-493X. PMC 10501857. PMID 37709293.
- ^ Queenan, J. T.; O'Brien, G. D.; Bains, L. M.; Simpson, J.; Collins, W. P.; Campbell, S. (August 1980). "Ultrasound scanning of ovaries to detect ovulation in women". Fertility and Sterility. 34 (2): 99–105. doi:10.1016/S0015-0282(16)44889-2. ISSN 0015-0282. PMID 7409241.
- ^ Miller, Laura (April 2019). "Is a mid-luteal phase serum progesterone level accurate for predicting the ovulatory status of women?". Evidence-Based Practice. 22 (4): 24–25. doi:10.1097/ebp.0000000000000232. ISSN 1095-4120. S2CID 212964389.
- ^ Hall J (2010). Guyton and Hall textbook of medical physiology (12th ed.). Philadelphia, Pa.: Saunders/Elsevier. ISBN 978-1-4160-4574-8.
- ^ "Infertility Diagnosis". ARC Fertility. Retrieved 4 November 2016.
- ^ "Rhythm method for natural family planning - Mayo Clinic". www.mayoclinic.org. Retrieved 2021-09-20.
- ^ Palatnik A, Strawn E, Szabo A, Robb P (May 2012). "What is the optimal follicular size before triggering ovulation in intrauterine insemination cycles with clomiphene citrate or letrozole? An analysis of 988 cycles". Fertility and Sterility. 97 (5): 1089–94.e1–3. doi:10.1016/j.fertnstert.2012.02.018. PMID 22459633.
- ^ Moolhuijsen, Loes M. E.; Visser, Jenny A. (2020-11-01). "Anti-Müllerian Hormone and Ovarian Reserve: Update on Assessing Ovarian Function". The Journal of Clinical Endocrinology and Metabolism. 105 (11): 3361–3373. doi:10.1210/clinem/dgaa513. ISSN 1945-7197. PMC 7486884. PMID 32770239.
- ^ a b Fleming, Richard; Seifer, David B.; Frattarelli, John L.; Ruman, Jane (October 2015). "Assessing ovarian response: antral follicle count versus anti-Müllerian hormone". Reproductive Biomedicine Online. 31 (4): 486–496. doi:10.1016/j.rbmo.2015.06.015. ISSN 1472-6491. PMID 26283017.
- ^ Orlowski, Michelle; Sarao, Manbeer S. (2021), "Physiology, Follicle Stimulating Hormone", StatPearls, Treasure Island (FL): StatPearls Publishing, PMID 30571063, retrieved 2021-09-13
- ^ Abdalla, H.; Thum, M. Y. (April 2004). "An elevated basal FSH reflects a quantitative rather than qualitative decline of the ovarian reserve". Human Reproduction. 19 (4): 893–898. doi:10.1093/humrep/deh141. ISSN 0268-1161. PMID 15016786.
- ^ Hendriks, Dave J.; Mol, Ben-Willem J.; Bancsi, Laszlo F. J. M. M.; te Velde, Egbert R.; Broekmans, Frank J. M. (October 2006). "The clomiphene citrate challenge test for the prediction of poor ovarian response and nonpregnancy in patients undergoing in vitro fertilization: a systematic review". Fertility and Sterility. 86 (4): 807–818. doi:10.1016/j.fertnstert.2006.03.033. ISSN 1556-5653. PMID 16962116.
- ^ a b c Radiology (ACR), Radiological Society of North America (RSNA) and American College of. "Hysterosalpingography". Radiologyinfo.org. Retrieved 2021-09-08.
- ^ a b Chalazonitis A, Tzovara I, Laspas F, Porfyridis P, Ptohis N, Tsimitselis G (September 2009). "Hysterosalpingography: technique and applications". Current Problems in Diagnostic Radiology. 38 (5): 199–205. doi:10.1067/j.cpradiol.2008.02.003. PMID 19632497.
- ^ a b c d e Lo Monte G, Capobianco G, Piva I, Caserta D, Dessole S, Marci R (January 2015). "Hysterosalpingo contrast sonography (HyCoSy): let's make the point!". Archives of Gynecology and Obstetrics. 291 (1): 19–30. doi:10.1007/s00404-014-3465-4. PMID 25234517. S2CID 25799624.
- ^ Dessole S, Capobianco G, Ambrosini G (2000). "Timing of sonohysterography in menstruating women". Gynecologic and Obstetric Investigation. 50 (2): 144. doi:10.1159/000010302. PMID 10965203. S2CID 1156706.
- ^ Panchal S, Nagori C (January 2014). "Imaging techniques for assessment of tubal status". Journal of Human Reproductive Sciences. 7 (1): 2–12. doi:10.4103/0974-1208.130797. PMC 4018793. PMID 24829524.
- ^ Aston KI, Uren PJ, Jenkins TG, Horsager A, Cairns BR, Smith AD, Carrell DT (December 2015). "Aberrant sperm DNA methylation predicts male fertility status and embryo quality". Fertility and Sterility. 104 (6): 1388–97.e1–5. doi:10.1016/j.fertnstert.2015.08.019. PMID 26361204.
- ^ "WHO laboratory manual for the examination and processing of human semen, 6th ed". www.who.int. Retrieved 2025-07-25.
- ^ "Sperm DNA Integrity Tests | Center for Male Reproductive Medicine & Microsurgery". maleinfertility.org. Retrieved 2025-07-25.
- ^ "Sperm Chromatin Structure Assay (SCSA) | Center for Women's Health | OHSU". www.ohsu.edu. Retrieved 2025-07-25.
- ^ Simon, Luke; Carrell, Douglas T. (2013). "Sperm DNA damage measured by comet assay". Spermatogenesis. Methods in Molecular Biology. Vol. 927. pp. 137–146. doi:10.1007/978-1-62703-038-0_13. ISBN 978-1-62703-037-3. ISSN 1940-6029. PMID 22992910.
- ^ "Male InfertilityOxidative System". MiOXSYS. Retrieved 2025-07-25.
- ^ Dias, Tânia R. (2021). "Measurement of Reactive Oxygen Species in Semen Samples Using Chemiluminescence". Reactive Oxygen Species. Methods in Molecular Biology. Vol. 2202. pp. 103–109. doi:10.1007/978-1-0716-0896-8_9. ISBN 978-1-0716-0895-1. ISSN 1940-6029. PMID 32857350.
- ^ Gómez-Giménez, Belén; Lacalle, Estíbaliz; Martínez-Pastor, Felipe; Soriano-Úbeda, Cristina (2025). "Analysis of the Chromatin Structure by Chromomycin A3 (CMA3) and Flow Cytometry". Spermatology. Methods in Molecular Biology. Vol. 2897. pp. 507–515. doi:10.1007/978-1-0716-4406-5_33. ISBN 978-1-0716-4405-8. ISSN 1940-6029. PMID 40202656.
- ^ Davila-Garza, A.; Stronk, J.; Patrizio, P.; Huszar, G. (2013-09-01). "Role of the sperm-hyaluronic acid binding score (HBA-score) in andrological and fertility assessment: HBA-score values, sperm concentrations and total motile sperm content in semen of 768 men". Fertility and Sterility. 100 (3): S437. doi:10.1016/j.fertnstert.2013.07.548. ISSN 0015-0282.
- ^ Zhao, Ming; Shirley, Cynthia R.; Hayashi, Shotaro; Marcon, Ludovic; Mohapatra, Bhagyalaxmi; Suganuma, Ryota; Behringer, Richard R.; Boissonneault, Guylain; Yanagimachi, Ryuzo; Meistrich, Marvin L. (April 2004). "Transition nuclear proteins are required for normal chromatin condensation and functional sperm development". Genesis (New York, N.Y.: 2000). 38 (4): 200–213. doi:10.1002/gene.20019. ISSN 1526-954X. PMID 15083521.
Fertility testing
View on GrokipediaOverview
Definition and Purpose
Fertility testing encompasses a systematic series of medical evaluations aimed at identifying factors that may impair conception, including assessments of hormonal levels, structural integrity of reproductive organs, and functional aspects of gamete production and transport.[5][6] According to the American Society for Reproductive Medicine (ASRM) 2023 committee opinion, infertility is defined as a disease, condition, or status characterized by: (1) the inability to achieve a successful pregnancy based on a patient's medical, sexual, and reproductive history, age, physical findings, diagnostic testing, or relevant proteins/steroid biochemistry in accordance with evidence-based thresholds; (2) the inability to carry a pregnancy to live birth; or (3) the need for fertility services to achieve a successful pregnancy.[6][1][2] This updated definition promotes inclusivity for diverse populations, including LGBTQ+ individuals, single parents, and those not engaging in heterosexual intercourse, ensuring broader access to evaluation and care. The core purpose of fertility testing is to diagnose underlying reproductive issues, thereby informing personalized treatment strategies such as hormonal therapies, intrauterine insemination, or assisted reproductive technologies (ART) like in vitro fertilization (IVF), while also offering reassurance to individuals with normal fertility and facilitating preconception counseling to optimize future reproductive health.[5][7] For instance, tests like semen analysis or anti-Müllerian hormone (AMH) levels may briefly indicate potential concerns, guiding further intervention without exhaustive procedural details.[5] Over the 20th century, fertility testing evolved from rudimentary techniques, such as basal body temperature charting for ovulation tracking and early semen microscopy first introduced in 1677 by Antonie van Leeuwenhoek, to the advent of IVF in 1978, which integrated diagnostic insights with laboratory-based reproduction.[8][9] By the early 21st century, advancements in biomarkers, genetic screening, and imaging have further refined these methods, enhancing their role within comprehensive ART frameworks as of 2025.[9] Globally, infertility impacts approximately 1 in 6 people of reproductive age, affecting an estimated 17.5% of the adult population and underscoring the need for accessible testing.[10][2] For individuals or couples under 35 years old, fertility testing is typically recommended after 12 months of unsuccessful attempts at conception through unprotected intercourse.[11][7]Indications for Testing
Fertility testing is primarily indicated for individuals or couples experiencing infertility, which may later be classified as unexplained if no cause is identified after evaluation.[5] This guideline, established by the American Society for Reproductive Medicine (ASRM), aims to facilitate early intervention to optimize outcomes, as fertility declines with age and delays can reduce success rates.[5] For women over 40, evaluation may be initiated sooner than the standard timelines due to the accelerated decline in ovarian reserve and higher risks of chromosomal abnormalities.[5] Similarly, the American Urological Association (AUA) and ASRM advise concurrent evaluation of the male partner in all infertile couples, following the same 12- or 6-month timelines based on the female partner's age.[12] Testing is also warranted earlier in the presence of known risk factors that may impair fertility. In women, these include irregular menstrual cycles (such as oligomenorrhea or amenorrhea), history of pelvic infections, endometriosis, polycystic ovary syndrome (PCOS), or prior pelvic surgeries that could lead to adhesions or tubal damage.[5][1] For men, indications encompass clinical varicocele, genital tract infections (e.g., epididymitis), or occupational exposures to heat, chemicals, or toxins, which can adversely affect spermatogenesis.[12][13] These factors prompt prompt assessment to identify treatable causes and prevent further fertility compromise. Lifestyle and environmental triggers further heighten the need for testing, including smoking, excessive alcohol use, obesity, and advancing paternal age over 40, which is associated with increased risks to offspring health.[1] Trends toward delayed parenthood, driven by socioeconomic factors and improved access to contraception, have amplified demand for fertility evaluations as more individuals pursue conception later in life.[14] Additionally, preconception testing is recommended for high-risk groups, such as carriers of genetic disorders, to screen for conditions that could affect reproductive success or offspring health.[15]Assessment in Women
Ovulation Detection Methods
Ovulation detection methods encompass a range of non-invasive and minimally invasive techniques designed to identify the timing and occurrence of ovulation, which is crucial for optimizing conception efforts or diagnosing conditions like anovulation. These approaches leverage physiological changes in body temperature, hormone levels, cervical characteristics, and ultrasound imaging to pinpoint the fertile window, typically spanning the 24-48 hours around ovulation. Home-based methods empower individuals to track cycles affordably, while clinical options provide higher precision for fertility evaluations.[16] Basal body temperature (BBT) charting involves recording daily morning temperatures immediately upon waking, before any activity, to detect the biphasic shift that occurs post-ovulation due to rising progesterone levels. This shift, usually an increase of 0.5 to 1°F (0.3 to 0.6°C), confirms ovulation has happened but does not predict it in advance, limiting its utility for timing intercourse. Accuracy can be affected by factors such as illness, stress, alcohol consumption, or inconsistent measurement, with studies indicating retrospective identification of the ovulatory period in about 80-90% of cycles when charted properly.[17][18] Ovulation prediction kits (OPK) are urine-based tests that detect the luteinizing hormone (LH) surge, which precedes ovulation by 24-36 hours, allowing prospective identification of the fertile window. These kits, available over-the-counter from brands like First Response or Clearblue, measure LH levels comparable to serum thresholds and achieve detection accuracy of 90-99% when used correctly, though false positives can occur in conditions like PCOS. Sensitivity varies by brand, with thresholds typically around 25-30 mIU/mL for positive results, making them a reliable home tool for most women with regular cycles.[19][20][16] Electronic fertility monitors, such as the Clearblue Fertility Monitor, enhance OPK functionality by analyzing urine samples for both estrogen and LH levels across multiple days, identifying up to six fertile days per cycle with 99% accuracy in hormone detection. These devices provide digital readouts of fertility status (low, high, peak) and integrate with smartphone apps for data tracking and cycle predictions as of 2025 models featuring touch screens and Bluetooth connectivity. Clinical validation supports their use in natural family planning, though they require daily testing during the expected fertile period and may be costlier than basic kits.[21][22] Cervical mucus observation, including the stretch test (also known as the spinnbarkeit test), monitors changes in vaginal discharge throughout the cycle to infer ovulation. Mucus transitions from sticky and opaque in the pre-ovulatory phase to clear, slippery, and stretchy (resembling raw egg whites) during the fertile window, facilitating sperm survival and transport. The Billings Ovulation Method formalizes this by emphasizing vulvar sensations and mucus patterns without internal examination, achieving typical-use effectiveness of 78-97% for fertility awareness when taught properly. This method relies on daily observations after being upright for several hours, with peak fertility indicated by slippery mucus lasting 1-2 days around ovulation.[23][24][25] Serum progesterone testing serves as a biochemical confirmation of ovulation via a blood draw, typically in the mid-luteal phase (around cycle day 21 for a 28-day cycle), where levels exceeding 5 ng/mL indicate corpus luteum formation post-ovulation. This threshold reflects adequate progesterone production to support potential implantation, with values below suggesting anovulation or luteal phase deficiency. While highly specific (positive likelihood ratio of about 2.8 for levels ≥6 ng/mL), timing is critical, and serial measurements may improve accuracy over single tests in irregular cycles.[26][27][28][29] Calendar and sympto-thermal methods combine cycle length tracking with multiple signs, such as BBT, cervical mucus, and sometimes cervical position, to delineate fertile and infertile phases. Apps like Natural Cycles, validated by the FDA in 2018 as a birth control method, use algorithms incorporating BBT data to predict ovulation with 93% typical-use effectiveness, though efficacy drops with irregular cycles or user error. These approaches promote comprehensive fertility awareness but require consistent daily logging for reliability.[30][31][32] Daily transvaginal ultrasound offers precise clinical monitoring of follicular development, where a dominant follicle grows to 18-25 mm before ovulation, confirmed by its rupture or disappearance on subsequent scans. Performed in fertility clinics starting around cycle day 8-10, this method visualizes real-time ovarian changes with near-100% accuracy for timing ovulation, particularly useful in assisted reproduction. It is minimally invasive but involves a probe insertion, limiting it to medical settings.[33][34] Cervical position tracking complements other signs by noting the cervix's changes: it rises, softens (like the tip of a nose), and opens slightly near ovulation due to estrogen influence, contrasting its low, firm, closed state post-menstruation. Observed manually by inserting clean fingers, this sign peaks 1-2 days before ovulation and enhances accuracy when combined with mucus or BBT observations, though individual variability and hygiene concerns may affect ease of use.[35][36][37]Ovarian Reserve Evaluation
Ovarian reserve evaluation assesses the quantity and quality of a woman's remaining oocytes, providing insights into potential response to fertility treatments such as assisted reproductive technologies. This evaluation is essential for women over 35 or those with risk factors like prior ovarian surgery, as diminished reserve correlates with reduced fertility potential. Key methods include hormonal assays and ultrasound imaging, with interpretations guided by age-specific norms that reflect the natural decline in ovarian function beginning around age 30 and accelerating after 35. According to the 2024 ESHRE guideline on premature ovarian insufficiency, ovarian reserve markers like anti-Müllerian hormone (AMH) can predict the risk of early reserve loss, emphasizing the importance of timely testing. Interpretation of AMH levels should account for assay-specific variability, as different platforms may yield differing results.[38][39] Anti-Müllerian hormone (AMH) testing involves a simple blood draw that measures levels produced by granulosa cells in pre-antral and small antral follicles, serving as a proxy for the antral follicle count and overall ovarian pool. Unlike other hormones, AMH levels remain stable across the menstrual cycle, allowing testing on any day, which enhances accessibility. For women aged 25-35, normal AMH ranges from 1-4 ng/mL, with levels below 1 ng/mL indicating diminished reserve and poorer response to ovarian stimulation. The 2025 ESHRE ovarian stimulation guideline highlights AMH's utility in tailoring controlled ovarian hyperstimulation protocols, as low levels predict fewer oocytes retrieved during IVF.[40][39] Cycle day 3 follicle-stimulating hormone (FSH) testing evaluates basal pituitary-ovarian axis function early in the follicular phase, where elevated FSH signals the pituitary's attempt to recruit follicles from a depleted reserve. Levels exceeding 10 IU/L on day 3 suggest diminished ovarian reserve, often combined with estradiol measurement to improve accuracy; ideally, estradiol should be below 80 pg/mL to avoid masking subtle FSH elevations. The American Society for Reproductive Medicine (ASRM) 2020 committee opinion recommends this test for women with suspected low reserve, noting its prognostic value for IVF outcomes despite assay variability across labs.[39] The clomiphene citrate challenge test (CCCT) dynamically assesses ovarian responsiveness by administering 100 mg of clomiphene citrate daily from cycle days 5-9, followed by FSH measurement on day 3 (baseline) and day 10. A day 10 FSH level above 10 IU/L indicates poor reserve, as it reflects inadequate follicular recruitment under stimulation. This provocative test is more sensitive than basal FSH alone for detecting subtle declines, particularly in women aged 35-40, according to a comprehensive review in Endotext.[41] Antral follicle count (AFC) uses transvaginal ultrasound to enumerate follicles measuring 2-10 mm in both ovaries during the early follicular phase (days 2-5), providing a direct visual estimate of recruitable follicles. Counts below 5-7 bilaterally signify low reserve and predict limited oocyte yield in IVF cycles. The ASRM endorses AFC for its low interobserver variability and complementary role with hormonal tests, as outlined in their 2020 opinion on ovarian reserve measures.[39] Inhibin B, a heterodimeric glycoprotein secreted by granulosa cells, serves as a less commonly used biomarker of early follicular development, with day 3 levels below 45 pg/mL associated with diminished reserve and poor ovarian response. While historically promising, its clinical adoption is limited due to greater assay variability compared to AMH or AFC. A 2021 systematic review confirms inhibin B's correlation with other markers in reproductive-age women but notes its secondary role in modern guidelines.[42] The 2025 ESHRE guidelines integrate these tests with age-specific interpretations, recommending combined use (e.g., AMH plus AFC) for robust counseling on fertility preservation options, as reserve decline varies individually but follows a predictable trajectory post-35.[40]Uterine and Fallopian Tube Assessment
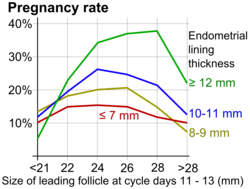
Uterine and fallopian tube assessment is a critical component of fertility evaluation in women, focusing on anatomical integrity to identify blockages, structural anomalies, or abnormalities that may impair sperm transport, egg retrieval, or embryo implantation.[43] These evaluations primarily utilize imaging modalities to visualize the uterine cavity and tubal patency without invasive intervention, helping to guide treatment decisions such as in vitro fertilization (IVF) or surgical correction.[44] Common indications include unexplained infertility, recurrent pregnancy loss, or suspected pelvic inflammatory disease sequelae.[45] Hysterosalpingography (HSG) is a radiographic procedure that involves injecting iodinated contrast dye through the cervix under fluoroscopy to outline the uterine cavity and assess fallopian tube patency.[46] It reveals the shape of the uterus, detecting anomalies like fibroids or adhesions, and determines if tubes are open by observing dye spillage into the peritoneal cavity.[45] Typically performed in the follicular phase post-menses to avoid pregnancy, HSG carries risks such as pelvic infection (occurring in about 1-3% of cases), allergic reactions to the dye, or uterine perforation, though severe complications are rare.[47][44] Hystero-contrast sonography (HyCoSy) employs transvaginal ultrasound with a contrast agent, such as saline mixed with air or foam, to evaluate tubal flow and uterine morphology in real time.[48] The procedure allows visualization of contrast movement through the tubes, confirming patency if it spills beyond the fimbriae, and offers advantages over HSG including no ionizing radiation exposure and immediate results without need for X-ray equipment.[43] Studies indicate HyCoSy has comparable accuracy to HSG for tubal occlusion detection, with sensitivity and specificity exceeding 80-90% in infertile populations.[49] Saline infusion sonohysterography (SHG), also known as sonohysterography, uses transvaginal ultrasound augmented by instilling sterile saline into the uterine cavity to expand and delineate endometrial contours.[50] This enhances detection of intrauterine abnormalities such as polyps, submucosal fibroids, or synechiae that may disrupt implantation, distinguishing intracavitary lesions from myometrial ones with high specificity (up to 95%).[51][52] Performed outpatient with minimal discomfort, SHG is particularly useful in infertility workups for women with irregular bleeding or prior failed implantations.[53] Hystero-foam sonography (HyFoSy) is an ultrasound-based variant using a stable foam contrast (e.g., ExEm Foam) for tubal patency assessment, providing clear visualization of foam transit through the tubes and into the pelvis.[54] It offers similar diagnostic accuracy to HSG and HyCoSy, with meta-analyses reporting sensitivity around 90% and better tolerability due to reduced pain.[55][56] Basic transvaginal ultrasound serves as an initial, non-invasive screening tool to identify gross uterine anomalies, such as bicornuate or septate uterus, which affect up to 5-10% of infertile women and can compromise implantation.[33] It also measures endometrial thickness—ideally 7-14 mm in the luteal phase for optimal receptivity—flagging thin linings associated with poor fertility outcomes.[57] This modality provides a baseline assessment before more specialized tests.[58] Interpretation of these tests distinguishes proximal tubal blockages (near the uterine cornua, often from salpingitis isthmica nodosa) from distal ones (at the fimbriae, commonly due to adhesions), with proximal occlusions accounting for 10-25% of tubal infertility cases.[59] Hydrosalpinx, a dilated tube filled with fluid indicating distal obstruction, prevails in 10-20% of infertile women and negatively impacts IVF success by up to 50% if untreated.[60] While imaging suffices for diagnosis, advanced endoscopy like hysteroscopy may confirm findings in complex cases.[61]Advanced Diagnostic Procedures
Advanced diagnostic procedures in fertility testing encompass invasive techniques that enable direct visualization and intervention within the female reproductive tract, particularly when non-invasive methods such as hysterosalpingography (HSG) yield inconclusive results. These approaches are essential for confirming structural abnormalities, staging conditions like endometriosis, and facilitating immediate treatments to optimize fertility outcomes, often integrating seamlessly with assisted reproductive technology (ART) planning.[5] Hysteroscopy involves the insertion of a thin endoscope through the cervix to inspect the uterine cavity, allowing for the identification of intrauterine pathologies such as polyps, fibroids, or adhesions that may impede implantation. Diagnostic hysteroscopy focuses on visualization, while operative hysteroscopy enables concurrent interventions like polyp removal or adhesiolysis to restore cavity integrity and potentially improve conception rates. This procedure is frequently performed in an office setting using local anesthesia and saline distension, reducing the need for general anesthesia and enabling same-day recovery with minimal discomfort. Complication rates for diagnostic hysteroscopy are low, ranging from 0.012% to 1.65%, with uterine perforation being the most frequent adverse event, though it rarely requires further intervention.[5][62][63] Laparoscopy with chromotubation provides comprehensive surgical assessment of the pelvic cavity and fallopian tubes by introducing a laparoscope through small abdominal incisions under general anesthesia, combined with the injection of a colored dye via the cervix to evaluate tubal patency through observed spillage at the fimbrial ends. This technique serves as the gold standard for staging endometriosis, detecting subtle peritoneal adhesions, or confirming tubal blockages not fully elucidated by prior imaging, thereby guiding decisions on fertility-preserving surgeries. It is particularly valuable for distinguishing treatable causes of infertility, such as mild endometriosis, which may benefit from excision to enhance natural or ART conception. Complication rates for laparoscopic procedures in infertility contexts typically range from 1% to 2%, encompassing risks like infection, bleeding, or visceral injury, with most cases managed conservatively.[5][64] Three-dimensional (3D) sonography enhances transvaginal ultrasound by generating multiplanar reconstructions of the uterus, enabling precise mapping of congenital anomalies such as septate or bicornuate uteri that could compromise embryo implantation. This modality offers diagnostic accuracy comparable to magnetic resonance imaging for differentiating anomaly types, such as measuring indentation depth (1.0-1.5 cm) to distinguish arcuate from septate configurations, and is routinely recommended pre-IVF to inform whether corrective procedures like metroplasty are needed. Beyond basic imaging, advanced ovarian ultrasound employs Doppler to assess vascularity, revealing increased blood flow in pathological lesions, while characteristic features like homogeneous low-level echoes or ground-glass appearance identify endometriomas or functional cysts that may affect ovarian function. These ultrasound refinements provide non-surgical yet detailed insights into adnexal pathology, supporting targeted interventions.[65][66] These procedures are indicated after abnormal HSG findings, such as suspected tubal occlusion, or in persistent infertility cases where non-invasive tests fail to identify a cause, allowing for definitive diagnosis and integration into ART protocols to address barriers like uterine irregularities or pelvic adhesions before cycles commence. Risks generally involve anesthesia-related issues for laparoscopy and minor procedural events for hysteroscopy, with recovery times varying from hours for office-based hysteroscopy to 1-2 days for laparoscopy, often permitting outpatient discharge.[5]Assessment in Men
Semen Analysis
Semen analysis is the cornerstone of male fertility evaluation, providing a direct assessment of sperm production, quality, and function through examination of ejaculated semen. This laboratory test measures various parameters to identify potential issues contributing to infertility, such as low sperm count or poor motility. It is recommended for men experiencing difficulty conceiving after 12 months of unprotected intercourse or in cases of known risk factors like varicocele or prior infections.[67][68] The procedure begins with the patient abstaining from ejaculation for 2-7 days to ensure a representative sample, followed by collection via masturbation into a sterile, wide-mouthed container, ideally at the laboratory to minimize contamination and allow immediate analysis. The sample is then allowed to liquefy for 20-30 minutes at 37°C before evaluation. If collection at home is necessary, the sample must be transported to the lab within 1 hour, kept at body temperature.[67] Key parameters assessed include semen volume, sperm concentration, total sperm count, motility, morphology, vitality, pH, and the presence of white blood cells, with lower reference limits established by the World Health Organization (WHO) based on the 5th centile from fertile men. These values help classify semen quality but are not diagnostic alone, as fertility depends on multiple factors. The following table summarizes the WHO 2021 lower reference limits:| Parameter | Lower Reference Limit |
|---|---|
| Semen volume | ≥1.4 mL |
| Sperm concentration | ≥15 × 10⁶ per mL |
| Total sperm number | ≥39 × 10⁶ per ejaculate |
| Progressive motility | ≥30% |
| Total motility | ≥40% |
| Morphology (normal forms) | ≥4% |
| Vitality (live spermatozoa) | ≥58% |
| pH | ≥7.2 |
| White blood cells | <1 × 10⁶ per mL |

