Recent from talks
Nothing was collected or created yet.
Biobank
View on Wikipedia
A biobank is a type of biorepository that stores biological samples (usually human) for use in research. Biobanks have become an important resource in medical research, supporting many types of contemporary research like genomics and personalized medicine.
Biobanks can give researchers access to data representing a large number of people. Samples in biobanks and the data derived from those samples can often be used by multiple researchers for cross purpose research studies. For example, many diseases are associated with single-nucleotide polymorphisms. Genome-wide association studies using data from tens or hundreds of thousands of individuals can identify these genetic associations as potential disease biomarkers. Many researchers struggled to acquire sufficient samples prior to the advent of biobanks.
Biobanks have provoked questions on privacy, research ethics, and medical ethics. Viewpoints on what constitutes appropriate biobank ethics diverge. However, a consensus has been reached that operating biobanks without establishing carefully considered governing principles and policies could be detrimental to communities that participate in biobank programs.
Background
[edit]The term "biobank" first appeared in the late 1990s and is a broad term that has evolved in recent years.[1][2] One definition is "an organized collection of human biological material and associated information stored for one or more research purposes."[3][4] Collections of plant, animal, microbe, and other nonhuman materials may also be described as biobanks[5][6] but in some discussions the term is reserved for human specimens.[3]
Biobanks usually incorporate cryogenic storage facilities for the samples.[7] They may range in size from individual refrigerators to warehouses, and are maintained by institutions such as hospitals, universities, nonprofit organizations, and pharmaceutical companies.[7]
Biobanks may be classified by purpose or design. Disease-oriented biobanks usually have a hospital affiliation through which they collect samples representing a variety of diseases, perhaps to look for biomarkers affiliated with disease.[8][9] Population-based biobanks need no particular hospital affiliation because they take samples from large numbers of all kinds of people, perhaps to look for biomarkers for disease susceptibility in a general population.[10]
- Virtual biobanks integrate epidemiological cohorts into a common pool.[11] Virtual biobanks allow for sample collection to meet national regulations.[12]
- Tissue banks harvest and store human tissues for transplantation and research. As biobanks become more established, it is expected that tissue banks will merge with biobanks.[12]
- Population banks store biomaterial as well as associated characteristics such as lifestyle, clinical, and environmental data.[12]
In 2008, United States researchers stored 270 million specimens in biobanks, and the rate of new sample collection was 20 million per year.[13] These numbers represent a fundamental worldwide change in the nature of research between the time when such numbers of samples could not be used and the time when researchers began demanding them.[13] Collectively, researchers began to progress beyond single-center research centers to a next-generation qualitatively different research infrastructure.[14] Some of the challenges raised by the advent of biobanks are ethical, legal, and social issues pertaining to their existence, including the fairness of collecting donations from vulnerable populations, providing informed consent to donors, the logistics of data disclosure to participants, the right to ownership of intellectual property, and the privacy and security of donors who participate.[13] Because of these new problems, researchers and policymakers began to require new systems of research governance.[14]
Many researchers have identified biobanking as a key area for infrastructure development in order to promote drug discovery and drug development.[3]
Types and applications
[edit]Human genetics research
[edit]By the late 1990s, scientists realized that although many diseases are caused at least in part by a genetic component, few diseases originate from a single defective gene; most genetic diseases are caused by multiple genetic factors on multiple genes.[15] Because the strategy of looking only at single genes was ineffective for finding the genetic components of many diseases, and because new technology made the cost of examining a single gene versus doing a genome-wide scan about the same, scientists began collecting much larger amounts of genetic information when any was to be collected at all.[15] At the same time technological advances also made it possible for wide sharing of information, so when data was collected, many scientists doing genetics work found that access to data from genome-wide scans collected for any one reason would actually be useful in many other types of genetic research.[15] Whereas before data usually stayed in one laboratory, now scientists began to store large amounts of genetic data in single places for community use and sharing.[15]
An immediate result of doing genome-wide scans and sharing data was the discovery of many single-nucleotide polymorphisms, with an early success being an improvement from the identification of about 10,000 of these with single-gene scanning and before biobanks versus 500,000 by 2007 after the genome-wide scanning practice had been in place for some years.[15] A problem remained; this changing practice allowed the collection of genotype data, but it did not simultaneously come with a system to gather the related phenotype data.[15] Whereas genotype data comes from a biological specimen like a blood sample, phenotype data has to come from examining a specimen donor with an interview, physical assessment, review of medical history, or some other process which could be difficult to arrange.[15] Even when this data was available, there were ethical uncertainties about the extent to which and the ways in which patient rights could be preserved by connecting it to genotypic data.[15] The institution of the biobank began to be developed to store genotypic data, associate it with phenotypic data, and make it more widely available to researchers who needed it.[15]
Biobanks including genetic testing samples have historically been composed of a majority of samples from individuals from European ancestry.[16] Diversification of biobank samples is needed and researchers should consider the factors effecting the underrepresented populations.[17][18]
Conservation, ecosystem restoration and geoengineering
[edit]This section needs expansion. You can help by adding to it. (December 2020) |
In November 2020 scientists began collecting living fragments, tissue and DNA samples of the endangered corals from the Great Barrier Reef for a precautionary biobank for potential future restoration and rehabilitation activities.[19] A few months earlier another Australian team of researchers reported that they evolved such corals to be more heat-resistant.[20]
Biological specimens
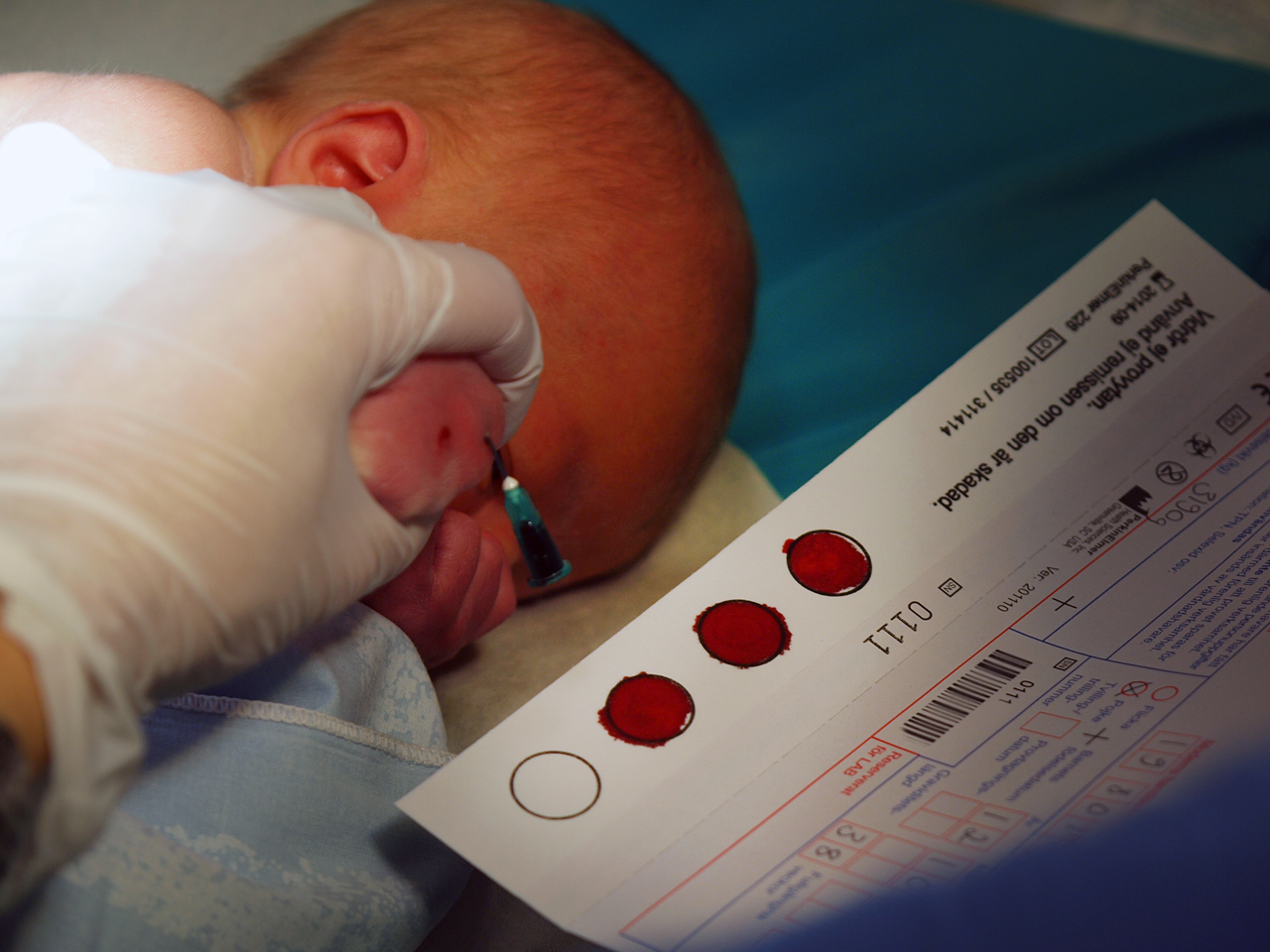
[edit]The specimens stored by a biobank and made available to researchers are taken by sampling. Specimen types include blood, urine, skin cells, organ tissue, and other materials. Increasingly, methods for sampling tissue specimens are becoming more targeted, sometimes involving the use of MRI to determine which specific areas of tissue should be sampled.[21][22] The biobank keeps these specimens in good condition until a researcher needs them to conduct a test, do an experiment, or perform an analysis.[citation needed]
Storage
[edit]Biobanks, like other DNA databases, must carefully store and document access to samples and donor information.[23] The samples must be maintained reliably with minimal deterioration over time, and they must be protected from physical damage, both accidental and intentional. The registration of each sample entering and exiting the system is centrally stored, usually on a computer-based system that can be backed up frequently.[23] The physical location of each sample is noted to allow the rapid location of specimens. Archival systems de-identify samples to respect the privacy of donors and allow blinding of researchers to analysis.[23] The database, including clinical data, is kept separately with a secure method to link clinical information to tissue samples.[23] Room temperature storage of samples is sometimes used, and was developed in response to perceived disadvantages of low-temperature storage, such as costs and potential for freezer failure.[23] Current systems are small and are capable of storing nearly 40,000 samples in about one tenth of the space required by a −80 °C (−112 °F) freezer. Replicates or split samples are often stored in separate locations for security.[23]
Ownership
[edit]One controversy of large databases of genetic material is the question of ownership of samples. As of 2007, Iceland had three different laws on ownership of the physical samples and the information they contain.[24] Icelandic law holds that the Icelandic government has custodial rights of the physical samples themselves while the donors retain ownership rights.[24] In contrast, Tonga and Estonia give ownership of biobank samples to the government, but their laws include strong protections of donor rights.[24]
Ethics
[edit]The key event which arises in biobanking is when a researcher wants to collect a human specimen for research. When this happens, some issues which arise include the following: right to privacy for research participants, ownership of the specimen and its derived data, the extent to which the donor can share in the return of the research results, and the extent to which a donor is able to consent to be in a research study.[25]
With respect to consent, the main issue is that biobanks usually collect samples and data for multiple future research purposes and it is not feasible to obtain specific consent for all possible future research. It has been discussed that one-off consent or a broad consent for various research purposes may not suffice ethical and legal requirements.[26][27] Dynamic consent is an approach to consent that may be better suited to biobanking, because it enables ongoing engagement and communication between the researchers and sample/data donors over time.
Governance
[edit]There is no internationally accepted set of governance guidelines that are designed to work with biobanks. Biobanks typically try to adapt to the broader recommendations that are internationally accepted for human subject research and change guidelines as they become updated. For many types of research and particularly medical research, oversight comes at the local level from an institutional review board. Institutional review boards typically enforce standards set by their country's government. To different extents, the law used by different countries is often modeled on biobank governance recommendations that have been internationally proposed.
Key organizations
[edit]Some examples of organizations that participated in creating written biobanking guidelines are the following:[2] World Medical Association, Council for International Organizations of Medical Sciences, Council of Europe, Human Genome Organisation, World Health Organization, and UNESCO. The International Society for Biological and Environmental Repositories (ISBER) is a global biobanking organization which creates opportunities for networking, education, and innovations and harmonizes approaches to evolving challenges in biological and environmental repositories. ISBER connects repositories globally through best practices. The ISBER Best Practices, Fourth Edition was launched on January 31, 2018 with a LN2 addendum that was launched early May 2019.[28]
History
[edit]In 1998, the Icelandic Parliament passed the Act on Health Sector Database. This act allowed for the creation of a national biobank in that country. In 1999, the United States National Bioethics Advisory Commission issued a report containing policy recommendations about handling human biological specimens.[13] In 2005, the United States National Cancer Institute founded the Office of Biorepositories and Biospecimen Research so that it could have a division to establish a common database and standard operating procedures for its partner organizations with biospecimen collections.[13] In 2006, the Council of the European Union adopted a policy on human biological specimens, which was novel for discussing issues unique to biobanks.[13]
Economics
[edit]Researchers have called for a greater critical examination of the economic aspects of Biobanks, particularly those facilitated by the state.[29] National biobanks are often funded by public/private partnerships, with finance provided by any combination of national research councils, medical charities, pharmaceutical company investment, and biotech venture capital.[30] In this way, national biobanks enable an economic relationship mediated between states, national populations, and commercial entities. It has been illustrated that there is a strong commercial incentive underlying the systematic collection of tissue material. This can be seen particularly in the field of genomic research where population sized study lends itself more easily toward diagnostic technologies rather than basic etiological studies.[31] Considering the potential for substantial profit, researchers Robert Mitchell and Catherine Waldby[29] argue that because biobanks enroll large numbers of the national population as productive participants, who allow their bodies and prospective medical histories to create a resource with commercial potential, their contribution should be seen as a form of "clinical labor" and therefore participants should also benefit economically.
Legal cases
[edit]There have been cases when the ownership of stored human specimens have been disputed and taken to court. Some cases include:
See also
[edit]References
[edit]- ^ Hewitt R, Watson P (October 2013). "Defining biobank". Biopreservation and Biobanking. 11 (5): 309–15. doi:10.1089/bio.2013.0042. PMID 24835262.
- ^ a b Cambon-Thomsen A, Rial-Sebbag E, Knoppers BM (August 2007). "Trends in ethical and legal frameworks for the use of human biobanks". The European Respiratory Journal. 30 (2): 373–82. doi:10.1183/09031936.00165006. PMID 17666560. S2CID 15217640.
- ^ a b c Hewitt RE (January 2011). "Biobanking: the foundation of personalized medicine". Current Opinion in Oncology. 23 (1): 112–9. doi:10.1097/CCO.0b013e32834161b8. PMID 21076300. S2CID 205547716.
- ^ Kauffmann F, Cambon-Thomsen A (May 2008). "Tracing biological collections: between books and clinical trials". JAMA. 299 (19): 2316–8. doi:10.1001/jama.299.19.2316. PMID 18492973.
- ^ ISO 20387: "Biotechnology — Biobanking — General requirements for biobanking" (2018)
- ^ ISO/TR 22758: "Biotechnology — Biobanking — Implementation guide for ISO 20387" (2020)
- ^ a b Silberman S (June 2010). "The Flesh Files". Wired. Vol. 18, no. 6. pp. 157–161, 182, 184, 188, 190.
- ^ Bevilacqua G, Bosman F, Dassesse T, Höfler H, Janin A, Langer R, et al. (April 2010). "The role of the pathologist in tissue banking: European Consensus Expert Group Report". Virchows Archiv. 456 (4): 449–54. doi:10.1007/s00428-010-0887-7. PMC 2852521. PMID 20157825.
- ^ Paskal W, Paskal AM, Dębski T, Gryziak M, Jaworowski J (October 2018). "Aspects of Modern Biobank Activity - Comprehensive Review". Pathology & Oncology Research. 24 (4): 771–785. doi:10.1007/s12253-018-0418-4. PMC 6132819. PMID 29728978.
- ^ Riegman PH, Morente MM, Betsou F, de Blasio P, Geary P, et al. (Marble Arch International Working Group on Biobanking for Biomedical Research) (October 2008). "Biobanking for better healthcare". Molecular Oncology. 2 (3): 213–22. doi:10.1016/j.molonc.2008.07.004. PMC 5527804. PMID 19383342.
- ^ Gottweis H, Petersen AR (20 June 2008). Biobanks: governance in comparative perspective. Taylor & Francis. p. 92. ISBN 978-0-415-42737-1. Retrieved 1 February 2012.
- ^ a b c Labant MA (Jan 15, 2012). "Biobank Diversity Facilitates Drug & Diagnostic Development". Genetic Engineering & Biotechnology News. 32 (2): 42,44. doi:10.1089/gen.32.2.21. ISSN 1935-472X. Retrieved 1 February 2012.
- ^ a b c d e f Haga S, Beskow L (2008). "Ethical, Legal, and Social Implications of Biobanks for Genetics Research". Genetic Dissection of Complex Traits. Advances in Genetics. Vol. 60. pp. 505–544. doi:10.1016/S0065-2660(07)00418-X. ISBN 978-0-12-373883-7. PMID 18358331.
- ^ a b Fullerton SM, Anderson NR, Guzauskas G, Freeman D, Fryer-Edwards K (January 2010). "Meeting the governance challenges of next-generation biorepository research". Science Translational Medicine. 2 (15): 15cm3. doi:10.1126/scitranslmed.3000361. PMC 3038212. PMID 20371468.
- ^ a b c d e f g h i Greely HT (2007). "The uneasy ethical and legal underpinnings of large-scale genomic biobanks". Annual Review of Genomics and Human Genetics. 8: 343–64. doi:10.1146/annurev.genom.7.080505.115721. PMID 17550341.
- ^ Fisher ER, Pratt R, Esch R, Kocher M, Wilson K, Lee W, Zierhut HA (February 2020). "The role of race and ethnicity in views toward and participation in genetic studies and precision medicine research in the United States: A systematic review of qualitative and quantitative studies". Molecular Genetics & Genomic Medicine. 8 (2) e1099. doi:10.1002/mgg3.1099. PMC 7005620. PMID 31867882.
- ^ Ewing AT, Turner AD, Sakyi KS, Elmi A, Towson M, Slade JL, et al. (September 2020). "Amplifying Their Voices: Advice, Guidance, and Perceived Value of Cancer Biobanking Research Among an Older, Diverse Cohort". Journal of Cancer Education. 37 (3): 683–693. doi:10.1007/s13187-020-01869-0. PMC 10286728. PMID 32975747. S2CID 221917485.
- ^ Ewing AT, Kalu N, Cain G, Erby LH, Ricks-Santi LJ, Tetteyfio-Kidd Telemaque E, Scott DM (October 2019). "Factors associated with willingness to provide biospecimens for genetics research among African American cancer survivors". Journal of Community Genetics. 10 (4): 471–480. doi:10.1007/s12687-019-00411-0. PMC 6754482. PMID 30877487.
- ^ Lu D. "Biobank to house 800 coral species so we can restore reefs in future". New Scientist. Retrieved 9 December 2020.
- ^ "Scientists successfully develop 'heat resistant' coral to fight bleaching". phys.org. Retrieved 29 December 2020.
- ^ Heavey S, Haider A, Sridhar A, Pye H, Shaw G, Freeman A, Whitaker H (October 2019). "Use of Magnetic Resonance Imaging and Biopsy Data to Guide Sampling Procedures for Prostate Cancer Biobanking". Journal of Visualized Experiments (152). doi:10.3791/60216. PMID 31657791.
- ^ Heavey S, Costa H, Pye H, Burt EC, Jenkinson S, Lewis GR, et al. (May 2019). "PEOPLE: PatiEnt prOstate samPLes for rEsearch, a tissue collection pathway utilizing magnetic resonance imaging data to target tumor and benign tissue in fresh radical prostatectomy specimens". The Prostate. 79 (7): 768–777. doi:10.1002/pros.23782. PMC 6618051. PMID 30807665.
- ^ a b c d e f Macleod AK, Liewald DC, McGilchrist MM, Morris AD, Kerr SM, Porteous DJ (February 2009). "Some principles and practices of genetic biobanking studies". The European Respiratory Journal. 33 (2): 419–25. doi:10.1183/09031936.00043508. PMID 19181915. S2CID 6669308.
- ^ a b c Nwabueze RN (2007-09-30). Biotechnology and the Challenge of Property: Property Rights in Dead Bodies. Aldershot, England: Ashgate Press. pp. 169–170. ISBN 978-0-7546-7168-8.
- ^ Hawkins AK, O'Doherty KC (October 2011). ""Who owns your poop?": insights regarding the intersection of human microbiome research and the ELSI aspects of biobanking and related studies". BMC Medical Genomics. 4: 72. doi:10.1186/1755-8794-4-72. PMC 3199231. PMID 21982589.
- ^ Hoeyer K (2012). "Trading in Cold Blood?". Trust in Biobanking. pp. 21–41.
- ^ Ewing AT, Erby LA, Bollinger J, Tetteyfio E, Ricks-Santi LJ, Kaufman D (April 2015). "Demographic differences in willingness to provide broad and narrow consent for biobank research". Biopreservation and Biobanking. 13 (2): 98–106. doi:10.1089/bio.2014.0032. PMC 4574731. PMID 25825819.
- ^ "ISBER Best Practices For Repositories - ISBER". www.isber.org. Retrieved 2020-02-11.
- ^ a b Mitchell R, Waldby C (May 2010). "National Biobanks: Clinical Labor, Risk Production, and the Creation of Biovalue". Science, Technology, & Human Values. 35 (3): 330–355. doi:10.1177/0162243909340267. PMC 2879701. PMID 20526462.
- ^ Lewis G (2004). "Tissue collection and the pharmaceutical industry: investigating corporate biobanks.". In Tutton R, Corrigan O (eds.). Genetic databases: Socio-ethical issues in the collection and use of DNA. London and New York: Routledge. pp. 181–201.
- ^ Rajan KS (2006). Biocapital: The constitution of postgenomic life. Durham: Duke University Press.
Further reading
[edit]- Kaye J, Stranger M (2009). Principles and Practice in Biobank Governance. Ashgate Publishing, Ltd. ISBN 978-0-7546-7825-0..
- Solbakk JH (2009). The Ethics of Research Biobanking. Springer. ISBN 978-0-387-93871-4..
- European Commission. Joint Research Centre. Institute for Prospective Technological Studies (2010), "Biobanks in Europe: Prospects for harmonisation and networking", Annual Report / Tuar, Publications Office, doi:10.2791/41701, ISBN 978-92-79-15783-7, ISSN 1018-5593
- Biobanking for Medicine: Technology and Market 2012-2022, Visiongain, Dec 13, 2011, archived from the original on March 20, 2016, retrieved February 2, 2012
- Chinese biobank: Share information and resources, China National GeneBank (Shenzhen), Jan 31, 2011
- ISO 20387:2018 "Biotechnology — Biobanking — General requirements for biobanking".
- ISO/TR 22758:2020 "Biotechnology — Biobanking — Implementation guide for ISO 20387".
External links
[edit]- Specimen Central biorepository list, A worldwide listing of active biobanks and biorepositories
- Harvested Organs Revolutionize Medicine - 2007 PBS/Wired Science report
- 8 minute biobank video made by Genetic Alliance
- International Society for Biological and Environmental Repositories (ISBER) Archived 2018-05-10 at the Wayback Machine
Biobank
View on GrokipediaFundamentals
Definition and Core Purposes
A biobank is a type of biorepository consisting of systematically organized collections of biological specimens—such as blood, tissue, DNA, or other biospecimens—linked to donor-associated data including health records, demographics, and lifestyle information, primarily for use in biomedical research.[1] These repositories emphasize long-term preservation under controlled conditions to maintain sample integrity, distinguishing them from ad hoc collections by their structured governance, ethical protocols, and infrastructure for retrieval and distribution.[9] While the term can encompass non-human samples from animals, plants, or microbes, biobanks most commonly focus on human materials to enable studies relevant to human health.[10] The core purposes of biobanks center on facilitating large-scale, longitudinal research that would otherwise be infeasible due to the rarity, volume, or time required to acquire fresh samples. They support investigations into disease mechanisms, genetic variations, environmental influences on health, and biomarker identification for diagnostics and therapeutics.[11] By providing annotated specimens, biobanks enable personalized medicine initiatives, such as pharmacogenomics, where genetic data correlates with drug responses, and population-level analyses to identify risk factors for conditions like cancer or cardiovascular disease.[12] This infrastructure accelerates scientific discovery by allowing reuse of samples across multiple studies, reducing redundancy in sample acquisition, and promoting collaborative research while adhering to consent and privacy standards.[13]Essential Components and Operations
Biobanks require robust physical infrastructure to maintain sample viability, including controlled-temperature storage systems such as ultra-low temperature freezers operating at -80°C or liquid nitrogen tanks at -196°C, along with backup power supplies and environmental monitoring to prevent degradation from temperature fluctuations or power failures.[14] These facilities must incorporate biosafety measures, such as clean rooms for processing and secure access controls to mitigate contamination risks and unauthorized entry.[15] Information technology systems form a core component, encompassing laboratory information management systems (LIMS) for tracking specimen inventory, metadata annotation, and linkage to electronic health records or clinical databases, ensuring traceability from donor to end-use.[16] These systems facilitate standardized data entry, audit trails for compliance with regulations like HIPAA or GDPR, and integration with electronic data capture tools to associate phenotypic and genotypic information without compromising donor privacy.[17] Quality management protocols, often aligned with ISO 20387 standards, include regular audits, risk assessments, and validation of storage conditions to guarantee sample integrity over long-term preservation.[18] Human resources and governance structures are indispensable, comprising specialized personnel such as biobank managers, technicians trained in biospecimen handling, and ethicists to oversee informed consent processes and access policies.[19] A management committee typically establishes operational policies, ensuring ethical compliance, equitable resource allocation, and collaboration with research institutions while addressing potential conflicts of interest in sample distribution.[20] Operations commence with specimen collection under standardized protocols to minimize pre-analytical variables, followed by processing steps like centrifugation for plasma separation or fixation for tissues, all documented to preserve biospecimen utility.[21] Accessioning assigns unique identifiers, enabling cataloging in the LIMS alongside donor demographics and clinical annotations, while ongoing quality control involves periodic viability testing and inventory reconciliation.[14] Distribution to researchers occurs via request-review processes that verify scientific merit, ethical approvals, and material transfer agreements, with return of derivative data to enrich the biobank's repository.[22] Data management integrates de-identified clinical and genomic datasets, employing encryption and access tiers to support longitudinal studies while mitigating re-identification risks.[23]Historical Development
Pre-2000 Origins
The origins of biobanking trace back to 19th-century pathological collections in medical institutions, where preserved human tissues from autopsies were stored primarily for diagnostic review, teaching, and rudimentary research into disease mechanisms. These early archives, often housed in hospital pathology departments or museums, laid the groundwork for systematic specimen preservation, with the U.S. Army Medical Museum—established in 1862—representing one of the first organized efforts to collect and catalog pathological specimens for military health studies.[24] By the late 19th century, similar microbial collections emerged, such as the service-oriented repository founded by Frantisek Kral in 1896 for preserving bacterial strains.[25] In the mid-20th century, advances in cryopreservation and cell culture techniques enabled more structured biobanking. The establishment of the HeLa cell line in 1951 from Henrietta Lacks' cervical tumor cells at Johns Hopkins Hospital marked a pivotal development in cell line biobanking, providing an immortalized human cell resource that facilitated virology and cancer research worldwide.[11] Concurrently, longitudinal cohort studies began incorporating sample storage; the Framingham Heart Study, launched in 1948 by the U.S. Public Health Service, systematically collected and archived blood sera and other biospecimens from participants to investigate cardiovascular risk factors, serving as a prototype for population-based repositories.[26] By the 1980s, dedicated disease-oriented biobanks proliferated in response to emerging epidemics and targeted research needs. The University of California, San Francisco AIDS Specimen Bank, initiated in December 1982, exemplified this shift by storing plasma and peripheral blood mononuclear cells from HIV patients to accelerate virological and immunological studies amid the AIDS crisis.[27] Pre-1990s biobanks were predominantly ad-hoc, project-specific collections in academic or clinical settings utilizing surplus diagnostic materials, with limited standardization in storage or consent protocols; a 1999 Rand Corporation analysis estimated over 307 million such U.S. biospecimens derived from 178 million individuals.[27] These efforts underscored biobanking's evolution from incidental preservation to intentional resource-building, though ethical frameworks for donor consent and data linkage remained underdeveloped until genomic advancements in the late 1990s.[28]2000s Expansion and Institutionalization
The 2000s witnessed accelerated expansion of biobanks, driven by post-genomic research demands after the Human Genome Project's completion in 2003, which highlighted the need for large-scale sample repositories to link genetic variants with health outcomes.[27] This period saw the proliferation of population-based biobanks, with over two-thirds of U.S. biobanks established in the decade preceding 2013 surveys, often tied to academic, hospital, or research institute operations focused on disease-specific or general biomedical studies.[29] Globally, initiatives multiplied in Europe, North America, and Asia, emphasizing longitudinal data collection from hundreds of thousands of participants to enable epidemiological and genetic analyses.[27] Prominent examples included the UK Biobank, formally established in the early 2000s by the Medical Research Council, Wellcome Trust, Department of Health, and Scottish Government, with recruitment of 500,000 volunteers aged 40-69 occurring from 2006 to 2010 across 22 assessment centers.[30][31] The Estonian Genome Project Biobank, launched in 2000, enrolled over 52,000 participants by 2010, integrating genetic, clinical, and lifestyle data for personalized medicine research.[32] Other key developments encompassed expansions in Iceland's deCODE Genetics repository (initiated late 1990s but scaled in the 2000s with national genotyping efforts) and national cohorts in Sweden, Denmark, Latvia, Canada (Cartagene, approved 2009), South Korea, and Japan, often recruiting 100,000-500,000 donors to support genome-wide association studies.[27] Institutionalization progressed through formalized governance, ethical protocols, and networking to address sustainability challenges like high operational costs—estimated at millions annually per large biobank—and data privacy under frameworks such as the U.S. Health Insurance Portability and Accountability Act (HIPAA, effective 2003 for many provisions).[33][27] European efforts focused on harmonization, with the 2008 launch of the Biobanking and Biomolecular Resources Research Infrastructure (BBMRI) initiative promoting standards for sample quality, consent, and interoperability across fragmented national systems lacking uniform regulation as of 2005.[34][35] Ethical guidelines evolved toward broad, tiered consent models allowing secondary research uses while mandating oversight by institutional review boards, reflecting a shift from restrictive to facilitative policies amid growing scientific output, evidenced by a 6% annual mean increase in biobanking-related journal publications since 2000.[7][36] These structures mitigated early "bubble" risks of over-expansion without infrastructure, fostering long-term viability through public-private funding and international collaborations.[33]2020s Advances and Global Scaling
The COVID-19 pandemic catalyzed significant operational expansions in biobanks worldwide, as they supplied biological samples essential for viral characterization, vaccine development, and longitudinal studies of immune responses. Biobanks facilitated rapid access to diverse specimens, enabling researchers to process and distribute materials under heightened biosafety protocols while bridging clinical data gaps. For instance, global biobanks supported multi-ancestry analyses that revealed genetic factors influencing disease severity, underscoring their role in real-time pandemic response. This period highlighted challenges like harmonizing sample standardization across institutions but also accelerated infrastructure investments for future scalability.[37][38][39] Technological integrations advanced biobank capabilities, particularly through whole-genome sequencing (WGS) and AI-driven analytics. In 2025, the UK Biobank released nearly 500,000 whole genomes, enhancing noncoding variant studies linked to diseases and supporting high-performance computing for complex models. Similarly, the U.S. All of Us Research Program expanded its genomic dataset by nearly 70% that year, incorporating whole genome sequences from over 245,000 participants to bolster precision medicine in underrepresented populations. These updates, alongside initiatives like the Global Biobank Meta-analysis Initiative, enabled cross-biobank harmonization for polygenic risk scoring and multi-ancestry genetic discovery, reducing biases from European-centric data.[40][41][42] Global scaling efforts emphasized diversification and international collaboration, with national projects incorporating WGS for population-specific insights. By 2023, Mexico's Biobank advanced medical genomics for indigenous ancestries, contributing to broader Latin American representation. Emerging frameworks integrated semantic computing and AI for generative data reasoning, transitioning biobanks toward intelligent medicine platforms. Infrastructure growth reflected this, with population biobanks incorporating diverse ancestries to address genetic research gaps, though challenges persist in equitable access for low-resource regions.[43][44][45]Classification
By Research Scope and Focus
Biobanks are classified by research scope and focus into population-based and disease-oriented categories, reflecting their primary objectives in investigating broad health determinants versus targeted pathological mechanisms.[21][1] Population-based biobanks assemble samples from large, representative cohorts without initial disease stratification, emphasizing epidemiological patterns, genetic variants, and gene-environment interactions across healthy and at-risk individuals.[11] These repositories support longitudinal studies on disease incidence, prevalence, and multifactorial etiologies, such as the interplay of genetics and lifestyle in common conditions like cardiovascular disease or diabetes.[46] By design, they prioritize scale, with collections often exceeding hundreds of thousands of participants, to generate reference data for population-level inferences.[47] Disease-oriented biobanks concentrate on biospecimens from individuals diagnosed with particular ailments, facilitating in-depth analyses of disease-specific biology, including biomarker discovery, therapeutic response prediction, and progression modeling.[1] Subtypes include those dedicated to oncology, where tumor tissues, matched normal samples, and clinical metadata are preserved to probe carcinogenesis and personalized treatment efficacy; neurology-focused collections for neurodegenerative disorders; or rare disease repositories targeting understudied genetic anomalies.[1][48] These biobanks typically originate from hospital or clinical settings, yielding smaller but highly annotated datasets optimized for hypothesis-driven research into causal pathways and intervention outcomes.[47] Hybrid or mixed-scope biobanks integrate elements of both approaches, often evolving from disease-centric origins to incorporate population controls for comparative validity, thereby enabling versatile applications from precision diagnostics to public health surveillance.[49] This classification influences sample selection criteria, data linkage strategies, and analytical power, with population-based designs excelling in rarity detection through sheer volume, while disease-oriented ones provide granular phenotypic depth essential for mechanistic insights.[2] Credible sources, such as peer-reviewed analyses from biobanking consortia, underscore that these foci determine downstream utility, with disease-specific collections showing higher specialization but potential ascertainment bias from clinical recruitment.[1]By Ownership and Operational Model
Public biobanks are typically owned and operated by government entities, academic institutions, or non-profit organizations, with funding derived from public grants, charities, and institutional resources to support unrestricted research access for public benefit.[1] These biobanks emphasize long-term sample preservation for population-scale studies, such as the UK Biobank, which collected genetic and health data from 500,000 UK participants between 2006 and 2010 under oversight from the Medical Research Council and Department of Health.[50] Similarly, the U.S. National Cancer Institute's NCTN Biobanks store annotated cancer biospecimens from clinical trials, distributing them to researchers via federal governance protocols.[51] Academic biobanks, a subset of public models, are affiliated with universities or hospitals and prioritize hypothesis-driven research, often integrating samples with electronic health records for disease-specific inquiries.[1] They rely on institutional budgets and competitive grants, with operational focus on quality control standards like ISO 20387:2018 for sample handling and ethical compliance.[52] Private or commercial biobanks, owned by for-profit companies, operate as service providers supplying biospecimens to pharmaceutical and biotech firms for product development, generating revenue through sample sales and customized procurement.[1] Examples include BioIVT, which maintains a global biorepository of human tissues for drug testing, and REPROCELL's Bioserve, offering diseased and normal samples with associated clinical data under proprietary access terms.[53] These entities often partner with public sources but retain ownership rights, differing from public models by emphasizing commercial viability over open dissemination.[54] Hybrid operational models blend public and private elements, such as public-private partnerships where government-funded biobanks license samples to industry, as seen in collaborations facilitating biotech access to rare specimens while maintaining non-profit core governance.[55] Networked models, like the European BBMRI-ERIC consortium coordinating 515 biobanks with over 60 million samples, enable cross-ownership data sharing under standardized protocols to enhance research efficiency without transferring ownership.[56]Specimens and Preservation Techniques
Types of Biological Materials
Biobanks primarily store human-derived biological materials that enable longitudinal research into genetics, disease mechanisms, and biomarkers. These include bodily fluids, solid tissues, and extracted biomolecules, selected for their stability, yield of genetic material, and relevance to clinical phenotypes. Bodily fluids such as blood, urine, and saliva predominate due to minimally invasive collection methods; for instance, blood samples yield plasma, serum, and buffy coats for proteomic and genomic extraction, while UK Biobank maintains over 17 million containers of such fluids from 500,000 participants as of July 2025.[57] [58] [1] Tissues constitute another core category, encompassing fresh-frozen specimens for preserving cellular integrity and formalin-fixed paraffin-embedded (FFPE) blocks for archival durability and histopathological analysis; these are often sourced from surgical resections or biopsies, with tumor tissues prioritized in disease-specific biobanks.[1] [11] Vital cellular materials, including viable cells from blood, bone marrow, or fetal tissues, support functional assays but require cryogenic preservation to maintain viability.[1] Molecular derivatives like isolated DNA, RNA, proteins, and cultured cell lines extend sample utility for high-throughput sequencing and functional studies; DNA from buffy coats or saliva, for example, facilitates genome-wide association studies, while RNA preserves transcriptomic data.[59] [11] Less common excretions or specialized samples—such as cerebrospinal fluid, adipose tissue, or umbilical cord blood—target niche applications like neurological or developmental research, though their inclusion varies by biobank mandate.[60][59]Storage Methods and Technological Standards
Biobanks primarily utilize cryopreservation for long-term storage of biological specimens, rapidly cooling samples to temperatures between -160°C and -190°C, often in liquid nitrogen vapor phase, to inhibit enzymatic degradation, prevent ice crystal formation, and maintain cellular viability.[61] This method contrasts with mechanical ultra-low temperature freezers operating at -80°C, which serve as a cost-effective option for stable materials like extracted DNA but require more frequent monitoring due to potential mechanical failures.[62] For short-term preservation, refrigeration at 4°C or -20°C freezers suffice for nucleic acids, though these risk gradual degradation from residual enzymatic activity.[63] Technological standards emphasize redundancy and monitoring, including backup power systems, automated temperature alarms, and liquid nitrogen refill protocols to avert sample loss during outages, with ISBER recommending at least 10% excess backup capacity for mechanical systems versus less for vapor-phase storage due to inherent safety advantages.[63] Sample tracking employs cryogenic-resistant barcodes or RFID tags, integrated with laboratory information management systems (LIMS) for real-time inventory and audit trails documenting processing, aliquoting, and quality metrics like viability post-thaw.[64] International standards such as ISO 20387:2018 outline requirements for controlled environments, including humidity regulation below 50% and minimal light exposure to curb oxidative damage, alongside validation of storage vessels for leak-proof seals under thermal stress.[13]| Storage Method | Temperature Range | Typical Samples | Key Advantages | Key Risks |
|---|---|---|---|---|
| Mechanical Freezer | -80°C | DNA, proteins | Cost-effective, accessible | Power failure, compressor wear |
| Liquid Nitrogen Vapor | -150°C to -196°C | Cells, tissues, viable biospecimens | Stable without power, ultra-low degradation | Asphyxiation hazard, refill logistics |
| Refrigerated (-20°C) | -20°C | Short-term nucleic acids | Energy-efficient for interim | Enzymatic breakdown over time |