Recent from talks
Nothing was collected or created yet.
Α,β-Unsaturated carbonyl compound
View on Wikipedia
α,β-Unsaturated carbonyl compounds are organic compounds with the general structure (O=CR)−Cα=Cβ−R.[1][2] Such compounds include enones and enals, but also carboxylic acids and the corresponding esters and amides. In these compounds, the carbonyl group is conjugated with an alkene (hence the adjective unsaturated). Unlike the case for carbonyls without a flanking alkene group, α,β-unsaturated carbonyl compounds are susceptible to attack by nucleophiles at the β-carbon. This pattern of reactivity is called vinylogous. Examples of unsaturated carbonyls are acrolein (propenal), mesityl oxide, acrylic acid, and maleic acid. Unsaturated carbonyls can be prepared in the laboratory in an aldol reaction and in the Perkin reaction.
Classifications
[edit]α,β-Unsaturated carbonyl compounds can be subclassified according to the nature of the carbonyl and alkene groups.
- Parent α,β-unsaturated carbonyls
-
Methyl vinyl ketone, the simplest α,β-unsaturated ketone
-
Acrolein, the simplest α,β-unsaturated aldehyde
-
Methyl acrylate, an α,β-unsaturated ester
-
Acrylamide, precursor to polyacrylamide
-
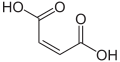
Maleic acid, an α,β-unsaturated dicarbonyl
-
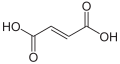
Fumaric acid, isomeric with maleic acid
Acryloyl group
[edit]
α,β-Unsaturated carbonyl compounds featuring a carbonyl conjugated to an alkene that is terminal, or vinylic, contain the acryloyl group (H2C=CH−C(=O)−); it is the acyl group derived from acrylic acid. The preferred IUPAC name for the group is prop-2-enoyl, and it is also known as acrylyl or simply (and incorrectly) as acryl. Compounds containing an acryloyl group can be referred to as "acrylic compounds".
α,β-Unsaturated acids, esters, and amides
[edit]An α,β-unsaturated acid is a type of α,β-unsaturated carbonyl compound that consists of an alkene conjugated to a carboxylic acid.[3] The simplest example is acrylic acid (CH2=CHCO2H). These compounds are prone to polymerization, giving rise to the large area of polyacrylate plastics. Acrylate polymers are derived from but do not contain the acrylate group.[4] The carboxyl group of acrylic acid can react with ammonia to form acrylamide, or with an alcohol to form an acrylate ester. Acrylamide and methyl acrylate are commercially important examples of α,β-unsaturated amides and α,β-unsaturated esters, respectively. They also polymerize readily. Acrylic acid, its esters, and its amide derivatives feature the acryloyl group.
α,β-Unsaturated dicarbonyls are also common. The parent compounds are maleic acid and the isomeric fumaric acid. Maleic acid forms esters, an imide, and an anhydride, i.e. diethyl maleate, maleimide, and maleic anhydride. Fumaric acid, as fumarate, is an intermediate in the Krebs citric acid cycle, which is of great importance in bioenergy.
Enones
[edit]An enone (or alkenone) is an organic compound containing both alkene and ketone functional groups. In an α,β-unsaturated enone, the alkene is conjugated to the carbonyl group of the ketone.[3] The simplest enone is methyl vinyl ketone (butenone, CH2=CHCOCH3). Enones are typically produced using an aldol condensation or Knoevenagel condensation. Some commercially significant enones produced by condensations of acetone are mesityl oxide (dimer of acetone) and phorone and isophorone (trimers).[5] In the Meyer–Schuster rearrangement, the starting compound is a propargyl alcohol. Another method to access α,β-unsaturated carbonyls is via selenoxide elimination. Cyclic enones can be prepared via the Pauson–Khand reaction.

Cyclic enones
[edit]The cyclic enones include cyclopropenone, cyclobutenone,[6] cyclopentenone, cyclohexenone, and cycloheptenone.[7]
Enals
[edit]An enal (or alkenal) is an organic compound containing both alkene and aldehyde functional groups. In an α,β-unsaturated enal, the alkene is conjugated to the carbonyl group of the aldehyde (formyl group).[3] The simplest enal is acrolein (CH2=CHCHO). Other examples include cis-3-hexenal (essence of mowed lawns) and cinnamaldehyde (essence of cinnamon).
- Other α,β-unsaturated carbonyls
-
E-Crotonaldehyde, an enal that exists as an isomer
-
Cyclohexenone, common cyclic enone
-
testosterone, the male sex hormone
-
Cinnamaldehyde, essence of cinnamon
-
Paraquinone, a particularly electrophilic α,β-unsaturated carbonyl
-
Enone complex of iron tricarbonyl
Reactions of α,β-unsaturated carbonyls
[edit]α,β-Unsaturated carbonyls are electrophilic at both the carbonyl carbon as well as the β-carbon. Depending on conditions, either site is attacked by nucleophiles. Additions to the alkene are called conjugate additions. One type of conjugate addition is the Michael addition, which is used commercially in the conversion of mesityl oxide into isophorone. Owing to their extended conjugation, α,β-unsaturated carbonyls are prone to polymerization. In terms of industrial scale, polymerization dominates the use of α,β-unsaturated carbonyls. Again because of their electrophilic character, the alkene portion of α,β-unsaturated carbonyls is good dienophiles in Diels–Alder reactions. They can be further activated by Lewis acids, which bind to the carbonyl oxygen. α,β-Unsaturated carbonyls are good ligands for low-valent metal complexes, examples being (bda)Fe(CO)3 and tris(dibenzylideneacetone)dipalladium(0).
α,β-Unsaturated carbonyls are readily hydrogenated. Hydrogenation can target the carbonyl or the alkene (conjugate reduction) selectively, or both functional groups.
Enones undergo the Nazarov cyclization reaction and in the Rauhut–Currier reaction (dimerization).
When appropriately irradiated, they undergo enone–alkene cycloadditions.
α,β-Unsaturated thioesters
[edit]α,β-Unsaturated thioesters are intermediates in several enzymatic processes. Two prominent examples are coumaroyl-coenzyme A and crotonyl-coenzyme A. They arise by the action of acyl-CoA dehydrogenases.[8] Flavin adenine dinucleotide (FAD) is a required co-factor.

Safety
[edit]Since α,β-unsaturated compounds are electrophiles and alkylating agents, many α,β-unsaturated carbonyl compounds are toxic. The endogenous scavenger compound glutathione naturally protects from toxic electrophiles in the body. Some drugs (amifostine, N-acetylcysteine) containing thiol groups may protect from such harmful alkylation.
See also
[edit]References
[edit]- ^ Patai, Saul; Rappoport, Zvi, eds. (1989). Enones. Patai's Chemistry of Functional Groups. Vol. 1. doi:10.1002/9780470772218. ISBN 9780470772218.
- ^ Patai, Saul; Rappoport, Zvi, eds. (1989). Enones. Patai's Chemistry of Functional Groups. Vol. 2. doi:10.1002/9780470772225. ISBN 9780470772225.
- ^ a b c Smith, Michael B.; March, Jerry (2007), Advanced Organic Chemistry: Reactions, Mechanisms, and Structure (6th ed.), New York: Wiley-Interscience, ISBN 978-0-471-72091-1
- ^ Ohara, Takashi; Sato, Takahisa; Shimizu, Noboru; Prescher, Günter; Schwind, Helmut; Weiberg, Otto; Marten, Klaus; Greim, Helmut (2003). "Acrylic Acid and Derivatives". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a01_161.pub2. ISBN 3527306730.
- ^ Siegel, Hardo; Eggersdorfer, Manfred (2000). "Ketones". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a15_077. ISBN 9783527306732.
- ^ Ross, A. G.; Li, X.; Danishefsky, S. J. (2012). "Preparation of Cyclobutenone". Organic Syntheses. 89: 491. doi:10.15227/orgsyn.089.0491.
- ^ Ito, Y.; Fujii, S.; Nakatuska, M.; Kawamoto, F.; Saegusa, T. (1979). "One-Carbon Ring Expansion of Cycloalkanones to Conjugated Cycloalkenones: 2-Cyclohepten-1-One". Organic Syntheses. 59: 113. doi:10.15227/orgsyn.059.0113.
- ^ Thorpe, Colin; Kim, Jujng-Ja P. (1 June 1995). "Structure and mechanism of action of the Acyl-CoA dehydrogenases". The FASEB Journal. 9 (9): 718–725. doi:10.1096/fasebj.9.9.7601336. ISSN 0892-6638. PMID 7601336. S2CID 42549744.
Α,β-Unsaturated carbonyl compound
View on GrokipediaDefinition and Nomenclature
Structural Features
α,β-Unsaturated carbonyl compounds are organic molecules that contain a carbonyl group (C=O) conjugated with a carbon-carbon double bond (C=C), positioned such that the double bond lies between the α-carbon (directly adjacent to the carbonyl carbon) and the β-carbon.[7] This structural arrangement defines the class, encompassing aldehydes, ketones, and carboxylic acid derivatives where the conjugation extends the functional group's reactivity.[8] The general formula for acyclic α,β-unsaturated carbonyl compounds is R-CH=CH-C(=O)-R', where R and R' represent hydrogen or organic substituents, allowing for a range of structural variations including cyclic forms and additional substitutions on the α- or β-carbons.[7] In these systems, the separation of the C=C and C=O by a single bond enables conjugation, forming an extended π-system that facilitates electron delocalization across the three atoms involved (β-carbon, α-carbon, and carbonyl carbon).[8] This delocalization influences the molecule's electronic properties, lowering the energy of the system and altering bond lengths and strengths compared to isolated functional groups.[7] The conjugation is vividly illustrated by the resonance structures of these compounds, which demonstrate the partial double bond character between the α-carbon and the carbonyl carbon. For the simplest case, acrolein (CH₂=CH-CHO), the resonance forms are: In the second contributor, the β-carbon bears a partial positive charge due to the electron-withdrawing effect of the oxygen, while the negative charge resides on oxygen, stabilizing the overall structure through π-overlap.[8] This resonance delocalization extends the π-system, affecting reactivity by making the β-carbon more electrophilic.[7] These structural features were first recognized in the 19th century during investigations into acrolein, the prototypical α,β-unsaturated aldehyde, which was named and characterized as an aldehyde by Jöns Jacob Berzelius in 1839 and first prepared via dry distillation by Joseph Redtenbacher in 1843.[9][10]Naming Conventions
α,β-Unsaturated carbonyl compounds are named according to the substitutive nomenclature rules outlined in the IUPAC Recommendations 2013, where the principal characteristic group (e.g., the carbonyl) determines the suffix, and the conjugated double bond is indicated by the infix "-en-" with appropriate locants.[11] For aldehydes, the suffix "-enal" is used, with the chain numbered to give the carbonyl carbon position 1 and the lowest possible locant to the double bond; for example, the compound with the formula CH₃CH=CHCHO is named but-2-enal.[11] Ketones employ the suffix "-enone", with locants specifying both the carbonyl and the double bond positions to ensure the lowest set of locants overall, as in pent-3-en-2-one for CH₃COCH=CHCH₃.[11] Carboxylic acid derivatives follow similar patterns, using suffixes such as "-enoic acid" for acids (e.g., but-2-enoic acid for CH₃CH=CHCOOH) and "-enoate" for esters (e.g., methyl but-2-enoate).[11] Trivial names persist for some common α,β-unsaturated carbonyls, often retained in IUPAC for simplicity despite the preference for systematic names. Acrolein, the trivial name for prop-2-enal (CH₂=CHCHO), derives from its acrid odor and is widely used in industrial contexts.[12] Crotonaldehyde, referring to (E)-but-2-enal (CH₃CH=CHCHO), originates from its relation to crotonic acid and is common in synthetic chemistry.[13] Another example is mesityl oxide, the retained name for 4-methylpent-3-en-2-one ((CH₃)₂C=CHCOCH₃), linked to its derivation from acetone.[14] Stereochemistry of the C=C double bond is specified using the E/Z designation based on the Cahn-Ingold-Prelog priority rules, placed in parentheses before the name with the locant of the double bond.[11] For instance, the trans isomer of but-2-enal is (E)-but-2-enal, where the higher-priority groups (the aldehyde and methyl) are on opposite sides.[11] This descriptor is essential for distinguishing configurational isomers in conjugated systems. The nomenclature of α,β-unsaturated carbonyl compounds evolved from descriptive terms like "α,β-unsaturated aldehyde" in early 20th-century literature to the precise IUPAC systematic approach, formalized in the 1971 revisions of Sections A, B, and C, which emphasized chain-based naming over Greek-letter locants for broader applicability in complex molecules.[15] This shift, driven by IUPAC's Organic Nomenclature Commission, promoted uniformity post-1970s, though trivial names remain accepted for well-known compounds.[15]Classifications
Enals
Enals, or α,β-unsaturated aldehydes, are organic compounds featuring an aldehyde functional group (-CHO) conjugated to a carbon-carbon double bond between the α and β positions relative to the carbonyl carbon.[16][17] This conjugation imparts distinctive reactivity and properties to the molecule, distinguishing enals from saturated aldehydes.[18] The simplest enal is acrolein, with the structure , a volatile compound produced industrially on a large scale.[12] Other prominent examples include crotonaldehyde, , which occurs as a (E)-isomer in natural sources and serves as a chemical intermediate, and cinnamaldehyde, , the primary flavor component derived from cinnamon bark.[13][19] Enals exhibit high volatility, particularly in lower homologs like acrolein and crotonaldehyde, which are colorless to pale yellow liquids with boiling points around 53°C and 102°C, respectively.[12][13] They possess pungent, irritating odors—acrolein described as acrid and choking, crotonaldehyde suffocating, and cinnamaldehyde cinnamon-like—that arise from their conjugated systems.[20][13][19] A notable property is their tendency to polymerize exothermically, especially acrolein, which forms polymers upon exposure to acids, bases, or oxygen, necessitating stabilizers in storage.[12][21] Acrolein holds historical significance as the first identified enal, named and characterized by Swedish chemist Jöns Jacob Berzelius in 1839 through the thermal decomposition of glycerol, highlighting early observations of unsaturated carbonyl chemistry.[9] In synthesis, enals serve as versatile intermediates for producing fragrances, such as cinnamaldehyde in cinnamon-based scents, and polymers, where compounds like acrolein contribute to the formation of specialty resins and coatings via controlled polymerization or as precursors in fine chemical routes.[19][22][23]Enones
Enones represent a subclass of α,β-unsaturated carbonyl compounds where the carbonyl functionality is a ketone (>C=O) directly conjugated with a carbon-carbon double bond (C=C), typically in the α,β-position. This conjugation extends the π-system, influencing reactivity and electronic properties.[24] Acyclic enones are common synthetic building blocks, exemplified by methyl vinyl ketone (CH=CHC(O)CH), a versatile electrophile in conjugate addition reactions due to its unsubstituted vinyl group. Another prominent example is chalcone (PhCH=CHC(O)Ph), a trans-1,3-diphenyl-2-propen-1-one featuring two aromatic rings linked by the enone moiety, often synthesized via Claisen-Schmidt condensation and valued for its biological activities.[25][26] Cyclic enones incorporate the conjugated system within a ring structure, such as 2-cyclohexen-1-one, where the carbonyl is at position 1 and the double bond spans positions 2 and 3 in a six-membered ring. This compound serves as a key intermediate in steroid synthesis, enabling the construction of polycyclic frameworks through reactions like Robinson annulation.[27][28] In cyclic enones, the ring geometry enforces planarity across the conjugated π-system, enhancing overlap of p-orbitals and thereby stabilizing the enone functionality compared to flexible acyclic analogs. Early enones, such as mesityl oxide ((CH)C=CHC(O)CH), were identified in 19th-century investigations of acetone self-condensation under basic conditions, marking foundational discoveries in aldol chemistry.[29][30]Carboxylic Acid Derivatives
α,β-Unsaturated carboxylic acid derivatives are organic compounds featuring a carbonyl group in the form of a carboxylic acid (-COOH), ester (-COOR), or amide (-CONR₂) conjugated to an α,β-carbon-carbon double bond, which imparts unique electrophilic properties due to resonance between the π-systems.[1] Representative examples include acrylic acid (), the simplest α,β-unsaturated carboxylic acid, and crotonic acid (), a β-substituted analog. Among the esters, methyl acrylate () is widely employed as a monomer in radical polymerization reactions to form polyacrylates.[31] For amides, acrylamide () exemplifies the class, with studies from 2002 highlighting its neurotoxic effects through nerve cell damage in animal models.[32] A key distinction in reactivity for the acid derivatives arises from the acidic proton of the -COOH group, which has a pKa around 4.5 and readily forms water-soluble salts with bases, enhancing their handling and solubility in aqueous media compared to non-acidic counterparts.Properties
Physical Properties
α,β-Unsaturated carbonyl compounds are typically polar molecules due to the carbonyl group, resulting in physical properties such as moderate to high boiling points and solubility in polar solvents. Low molecular weight representatives, like acrolein (CH₂=CHCHO), exist as volatile, colorless liquids at room temperature with a boiling point of 53°C and density of 0.839 g/mL.[12] Higher homologs, such as crotonaldehyde (CH₃CH=CHCHO), are also liquids but less volatile, boiling at 104°C with a density of 0.853 g/mL.[13] In contrast, larger or more substituted compounds often appear as solids or viscous liquids. The conjugation between the carbonyl and alkene enhances molecular polarity, leading to slightly elevated boiling points compared to their saturated analogs. For example, crotonaldehyde boils at 104°C, while the saturated butanal has a boiling point of 75°C; similarly, methyl crotonate boils at 119–121°C versus 80°C for methyl propionate.[33] This trend arises from increased dipole moments rather than significant changes in molecular weight.[34] Solubility in water is generally good for small, low molecular weight α,β-unsaturated carbonyls owing to hydrogen bonding with the carbonyl oxygen. Acrolein dissolves at 20 g/100 mL, crotonaldehyde at 15–18 g/100 mL, and acrylic acid (CH₂=CHCOOH) is fully miscible.[35][33][36] Solubility decreases with increasing chain length or nonpolar substituents, favoring organic solvents like ethanol or ether. Many α,β-unsaturated carbonyls possess pungent, acrid odors and can be lachrymatory, irritating the eyes and mucous membranes. Acrolein, for instance, is a colorless to pale yellow liquid with a strong, choking smell that causes tearing.[12] Crotonaldehyde similarly exhibits a penetrating, irritating odor as a straw-colored liquid.[13] Acrylic acid appears as a clear, colorless liquid with an acrid scent.[37] The following table summarizes key physical properties for representative examples:| Compound | Boiling Point (°C) | Density (g/mL at 20–25°C) | Water Solubility | Appearance and Odor |
|---|---|---|---|---|
| Acrolein | 53 | 0.839 | 20 g/100 mL | Colorless liquid; pungent, lacrimatory |
| Crotonaldehyde | 104 | 0.853 | 15–18 g/100 mL | Straw-colored liquid; penetrating, pungent |
| Acrylic acid | 141 | 1.051 | Miscible | Colorless liquid; acrid |