Recent from talks
Nothing was collected or created yet.
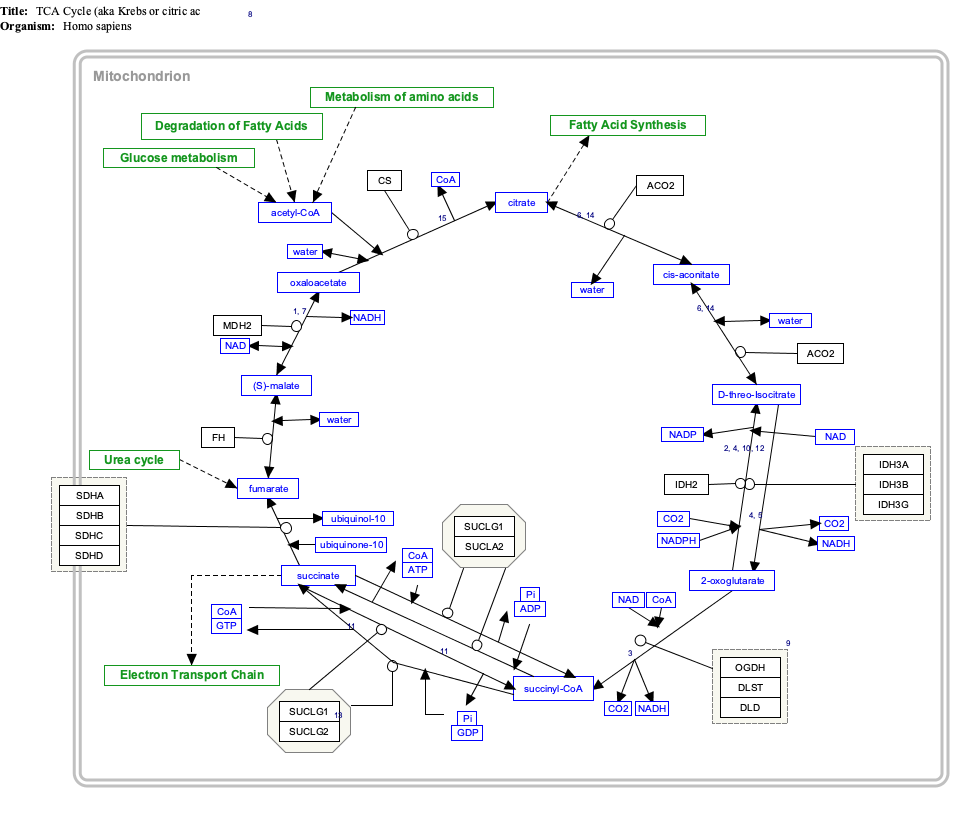
Fumaric acid
View on Wikipedia | |
 | |
| Names | |
|---|---|
| Preferred IUPAC name
(2E)-But-2-enedioic acid | |
Other names
| |
| Identifiers | |
3D model (JSmol)
|
|
| 605763 | |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| DrugBank | |
| ECHA InfoCard | 100.003.404 |
| EC Number |
|
| E number | E297 (preservatives) |
| 49855 | |
| KEGG | |
PubChem CID
|
|
| RTECS number |
|
| UNII | |
| UN number | 9126 |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C4H4O4 | |
| Molar mass | 116.072 g·mol−1 |
| Appearance | White solid |
| Density | 1.635 g/cm3 |
| Melting point | 287 °C (549 °F; 560 K) (decomposes)[2] |
| 6.3 g/L at 25 °C[1] | |
| Acidity (pKa) | pka1 = 3.03, pka2 = 4.44 (15 °C, cis isomer) |
| −49.11·10−6 cm3/mol | |
| non zero | |
| Pharmacology | |
| D05AX01 (WHO) | |
| Hazards | |
| GHS labelling: | |

| |
| Warning | |
| H319 | |
| P264, P280, P305+P351+P338, P313 | |
| NFPA 704 (fire diamond) | |
| 375 °C (707 °F; 648 K) | |
| Related compounds | |
Related carboxylic acids
|
|
Related compounds
|
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
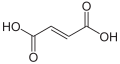
Fumaric acid or trans-butenedioic acid is an organic compound with the formula HO2CCH=CHCO2H. A white solid, fumaric acid occurs widely in nature. It has a fruit-like taste and has been used as a food additive. Its E number is E297.[3] The salts and esters are known as fumarates. Fumarate can also refer to the C
4H
2O2−
4 ion (in solution). Fumaric acid is the trans isomer of butenedioic acid, while maleic acid is the cis isomer.
Biosynthesis and occurrence
[edit]It is produced in eukaryotic organisms from succinate in complex 2 of the electron transport chain via the enzyme succinate dehydrogenase.
Fumaric acid is found in fumitory (Fumaria officinalis), bolete mushrooms (specifically Boletus fomentarius var. pseudo-igniarius), lichen, and Iceland moss.
Fumarate is an intermediate in the citric acid cycle used by cells to produce energy in the form of adenosine triphosphate (ATP) from food. It is formed by the oxidation of succinate by the enzyme succinate dehydrogenase. Fumarate is then converted by the enzyme fumarase to malate.
Human skin naturally produces fumaric acid when exposed to sunlight.[4][5]
Fumarate is also a product of the urea cycle.
Click on genes, proteins and metabolites below to link to respective articles. [§ 1]
- ^ The interactive pathway map can be edited at WikiPathways: "TCACycle_WP78".
Uses
[edit]Food
[edit]Fumaric acid has been used as a food acidulant since 1946. It is approved for use as a food additive in the EU,[6] USA[7] and Australia and New Zealand.[8] As a food additive, it is used as an acidity regulator and can be denoted by the E number E297. It is generally used in beverages and baking powders for which requirements are placed on purity. Fumaric acid is used in the making of wheat tortillas as a food preservative and as the acid in leavening.[9] It is generally used as a substitute for tartaric acid and occasionally in place of citric acid, at a rate of 1 g of fumaric acid to every ~1.5 g of citric acid, in order to add sourness, similarly to the way malic acid is used. As well as being a component of some artificial vinegar flavors, such as "Salt and Vinegar" flavored potato chips,[10] it is also used as a coagulant in stove-top pudding mixes.
The European Commission Scientific Committee on Animal Nutrition, part of DG Health, found in 2014 that fumaric acid is "practically non-toxic" but high doses are probably nephrotoxic after long-term use.[11]
Medicine
[edit]Fumaric acid was developed as a medicine to treat the autoimmune condition psoriasis in the 1950s in Germany as a tablet containing 3 esters, primarily dimethyl fumarate, and marketed as Fumaderm by Biogen Idec in Europe. Biogen would later go on to develop the main ester, dimethyl fumarate, as a treatment for multiple sclerosis.
In patients with relapsing-remitting multiple sclerosis, the ester dimethyl fumarate (BG-12, Biogen) significantly reduced relapse and disability progression in a phase 3 trial. It activates the Nrf2 antioxidant response pathway, the primary cellular defense against the cytotoxic effects of oxidative stress.[12]
Other uses
[edit]Fumaric acid is used in the manufacture of polyester resins and polyhydric alcohols and as a mordant for dyes.
Fumaric acid can be used to make 6-methylcoumarin.[13]
When fumaric acid is added to their feed, lambs produce up to 70% less methane during digestion.[14]
Synthesis
[edit]Fumaric acid is produced based on catalytic isomerisation of maleic acid in aqueous solutions at low pH. It precipitates from the reaction solution. Maleic acid is accessible in large volumes as a hydrolysis product of maleic anhydride, produced by catalytic oxidation of benzene or butane.[3]
Historic and laboratory routes
[edit]Fumaric acid was first prepared from succinic acid.[15] A traditional synthesis involves oxidation of furfural (from the processing of maize) using chlorate in the presence of a vanadium-based catalyst.[16]
Reactions
[edit]The chemical properties of fumaric acid can be anticipated from its component functional groups. This weak acid forms a diester, it undergoes bromination across the double bond,[17] and it is a good dienophile.
Safety
[edit]See also
[edit]- Citric acid cycle (TCA cycle)
- Fumarate reductase
- Photosynthesis
- Maleic acid, the cis isomer of fumaric acid
- Succinic acid
References
[edit]- ^ Record in the GESTIS Substance Database of the Institute for Occupational Safety and Health
- ^ Pubchem. "Fumaric acid". pubchem.ncbi.nlm.nih.gov.
- ^ a b c Lohbeck, Kurt; Haferkorn, Herbert; Fuhrmann, Werner; Fedtke, Norbert (2000). "Maleic and Fumaric Acids". Ullmann's Encyclopedia of Industrial Chemistry. doi:10.1002/14356007.a16_053. ISBN 3-527-30673-0.
- ^ Active Ingredients Used in Cosmetics: Safety Survey, Council of Europe. Committee of Experts on Cosmetic Products
- ^ "Fumaric Acid Foods". Archived from the original on October 4, 2014. Retrieved 2018-04-22.
- ^ UK Food Standards Agency: "Current EU approved additives and their E Numbers". Retrieved 2011-10-27.
- ^ US Food and Drug Administration: "Listing of Food Additives Status Part II". Food and Drug Administration. Archived from the original on January 8, 2010. Retrieved 2011-10-27.
- ^ Australia New Zealand Food Standards Code"Standard 1.2.4 - Labelling of ingredients". 8 September 2011. Retrieved 2011-10-27.
- ^ "Fumaric Acid - The Chemical Company". The Chemical Company. Retrieved 2018-04-22.
- ^ Eats, Serious. "The Science Behind Salt and Vinegar Chips". www.seriouseats.com.
- ^ European Commission: "European Commission Report of the Scientific Committee on Animal Nutrition on the Safety of Fumaric Acid" (PDF). Retrieved 2014-03-07.
- ^ Gold R.; Kappos L.; Arnold D.L.; et al. (September 20, 2012). "Placebo-Controlled Phase 3 Study of Oral BG-12 for Relapsing Multiple Sclerosis". N Engl J Med. 367 (12): 1098–1107. doi:10.1056/NEJMoa1114287. hdl:2078.1/124401. PMID 22992073. S2CID 6614191.
- ^ Thompson, T. J.; Edee, R. Herbert (1925). "Preparation of 6-Methylcoumarin and ITS Derivatives". Journal of the American Chemical Society. 47 (10): 2556–2559. Bibcode:1925JAChS..47.2556T. doi:10.1021/ja01687a019. ISSN 0002-7863.
- ^ "Scientists look to cut cow flatulence". phys.org. March 21, 2008.
- ^ Volhard, J. "Darstellung von Maleïnsäureanhydrid" Justus Liebig's Annalen der Chemie 1892, volume 268, page 255-6. doi:10.1002/jlac.18922680108
- ^ Nicholas A. Milas (1931). "Fumaric Acid". Organic Syntheses. 11: 46. doi:10.15227/orgsyn.011.0046.
- ^ Herbert S. Rhinesmith (1938). "α,β-Dibromosuccinic Acid". Organic Syntheses. 18: 17. doi:10.15227/orgsyn.018.0017.
External links
[edit]Fumaric acid
View on GrokipediaIntroduction and Properties
Chemical Structure and Nomenclature
Fumaric acid is an organic compound with the molecular formula C₄H₄O₄ (molecular weight 116.07 g/mol) and the structural formula HOOC-CH=CH-COOH, featuring a carbon-carbon double bond in the trans (E) configuration.[9] This trans arrangement positions the two carboxylic acid groups on opposite sides of the double bond, contributing to its distinct geometric isomerism.[10] The IUPAC name for fumaric acid is (E)-but-2-enedioic acid, reflecting its systematic nomenclature as a derivative of butenedioic acid with specified stereochemistry.[11] It is commonly known as fumaric acid, a name derived from its historical isolation from the fumitory plant (Fumaria officinalis) in the 19th century.[12] Other synonyms include trans-butenedioic acid and allomaleic acid, emphasizing its relation to the broader class of dicarboxylic acids.[9] Fumaric acid exists as the trans isomer of butenedioic acid, in contrast to its cis counterpart, maleic acid, which has the carboxylic groups on the same side of the double bond.[10] The trans configuration of fumaric acid imparts greater thermodynamic stability compared to maleic acid, primarily due to a lower dipole moment that minimizes molecular polarity and intramolecular repulsion.[9] This stability difference influences their respective reactivities and applications, with fumaric acid being the preferred form in many industrial and biological contexts.[9]Physical and Chemical Properties
Fumaric acid appears as a white crystalline solid, often in the form of odorless powder or granules.[9] It has a melting point of 287 °C, at which it sublimes without fully liquefying.[9] The density of the solid is 1.635 g/cm³ at 20 °C.[9] Fumaric acid exhibits low solubility in water, approximately 6.3 g/L at 25 °C, but shows higher solubility in alcohols such as ethanol (5.76 g/100 g at 30 °C).[9]| Property | Value | Conditions |
|---|---|---|
| Appearance | White crystalline solid | Room temperature |
| Melting point | 287 °C (sublimes) | - |
| Density | 1.635 g/cm³ | 20 °C |
| Solubility in water | 6.3 g/L | 25 °C |
| Solubility in ethanol | 5.76 g/100 g | 30 °C |