Recent from talks
Nothing was collected or created yet.
Small intestine cancer
View on Wikipedia| Small intestine cancer | |
|---|---|
| Other names | Small bowel cancer, cancer of the small bowel |
 | |
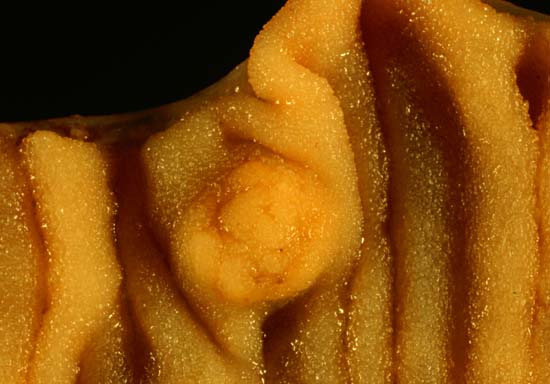
| Picture of a carcinoid tumour that encroaches into lumen of the small bowel. Pathology specimen. The prominent folds are plicae circulares, a characteristic of small bowel. | |
| Specialty | Gastroenterology, general surgery, oncology |
| Symptoms | vomiting blood, blood in the stool |
Small intestine cancer is a cancer of the small intestine. It is relatively rare compared to other gastrointestinal malignancies such as gastric cancer (stomach cancer) and colorectal cancer.[1][2]
Small intestine cancer can be subdivided into duodenal cancer (the first part of the small intestine) and cancer of the jejunum and ileum (the latter two parts of the small intestine). Duodenal cancer has more in common with stomach cancer, while cancer of the jejunum and ileum have more in common with colorectal cancer. Five-year survival rates are 65%.[3]
Experts[which?] believe that small intestine cancer develops much like colorectal cancer. It first begins as a small growth on the inner lining of the intestine (polyp), which over time becomes cancer.[4]
Approximately 50% of adenomas of the small intestine arise in the duodenum even though this location comprises only 4% of the length of the small intestine. These adenomas occur mainly close to the ampulla of Vater, the outlet of the common bile duct from which bile acids are released.[5] This area is also closely associated with the pancreas, so they are treated as pancreatic cancer.
The small intestine works by mixing food and gastric juices into a thick fluid in the stomach and then emptied into the small intestine. It then continues to break down and absorb the nutrients. Although it is referred to as the small intestine, it is the longest section of the GI tract being approximately 20 feet long. The length of the small intestine comprises 75% of the length of the entire gastrointestinal tract[6]
There are three parts of the small intestine. The duodenum is the 1st section of small intestine and only about a foot long. The jejunum and ileum make up most of the small intestine. Most of the nutrients in food are absorbed into the bloodstream in these two parts.[4]
Histopathologic types
[edit]
Subtypes of small intestine cancer include:[4]
- Adenocarcinoma – this cancer begins in the gland cells that line the inside of the intestine. Adenocarcinomas account for about 1 in 3 small intestine cancers.
- Gastrointestinal stromal tumor – this type of cancer starts in connective tissues. The most common sarcoma in the intestine are gastrointestinal stromal tumors (GISTs)
- Lymphoma – these cancers start in lymphocytes.
- Carcinoid tumors of the midgut – this is a type of neuroendocrine tumor (NET). They tend to be slow growing and are the most common type of small intestine tumor.
Most small intestine cancers (especially adenocarcinomas) develop in the duodenum. Cancers developed in the duodenum are often found at the ampulla of Vater.
-
Adenocarcinoma
-
Carcinoid
Risk factors
[edit]
Risk factors for small intestine cancer include:[8][9]
- Age: Cancer risk increases with age. The average age diagnosis is 65.(Cleveland Clinic, 2022)
- Race: In the U.S., small intestine cancer is slightly more common among people who identify as African Americans. Although lymphoma is more common among people who identify as white.(Cleveland Clinic, 2022)
- HIV/AIDS: treatments like radiation therapy may weaken your immune system and make you more susceptible to small intestine cancer. Drugs that suppress your immune system may also increase your risk.(Cleveland Clinic, 2022)
- Sex: Males are 25% more likely to develop the disease.(Cleveland Clinic, 2022)
- Diet: Various research has suggested that diets high in red meat and salted or smoked foods may raise the risk of small intestine cancer.(Cleveland Clinic, 2022). A human prospective study observed a markedly elevated risk for carcinoid tumors of the small intestine associated with dietary intake of saturated fat[10]
- Crohn's disease
- Celiac disease
- Radiation exposure
- Hereditary gastrointestinal cancer syndromes: familial adenomatous polyposis, hereditary nonpolyposis colorectal cancer, Peutz–Jeghers syndrome
- Cholecystectomy, which alters the flow of bile to the small intestine, increases the risk of small intestinal adenocarcinomas,[11] and this risk declines with increasing distance from the common bile duct.
Additional images
[edit]References
[edit]- ^ "Gastric Cancer". The Lecturio Medical Concept Library. Retrieved 22 July 2021.
- ^ "Colon Cancer Treatment (PDQ®)". NCI. May 12, 2014. Archived from the original on July 5, 2014. Retrieved June 29, 2014.
- ^ "SEER Stat Fact Sheets: Small Intestine Cancer". NCI. Retrieved 18 June 2014.
- ^ a b c "What Is a Small Intestine Cancer?". American Cancer Society. Retrieved 13 February 2025.
- ^ Ross RK; Hartnett NM; Bernstein L; Henderson BE. (1991). "Epidemiology of adenocarcinomas of the small intestine: is bile a small bowel carcinogen". British Journal of Cancer. 63 (1): 143–5. doi:10.1038/bjc.1991.29. PMC 1971637. PMID 1989654.
- ^ Maguire, A; Sheahan, K (2018). "Primary small bowel adenomas and adenocarcinomas-recent advances". Virchows Archiv. 473 (3): 265–73. doi:10.1007/s00428-018-2400-7. PMID 29998424.
- ^ Qubaiah, O.; Devesa, S. S.; Platz, C. E.; Huycke, M. M.; Dores, G. M. (2010). "Small Intestinal Cancer: a Population-Based Study of Incidence and Survival Patterns in the United States, 1992 to 2006". Cancer Epidemiology, Biomarkers & Prevention. 19 (8): 1908–1918. doi:10.1158/1055-9965.EPI-10-0328. ISSN 1055-9965. PMC 2919612. PMID 20647399.
- ^ Delaunoit T, Neczyporenko F, Limburg PJ, Erlichman C (March 2005). "Pathogenesis and risk factors of small bowel adenocarcinoma: a colorectal cancer sibling?". Am. J. Gastroenterol. 100 (3): 703–10. doi:10.1111/j.1572-0241.2005.40605.x. PMID 15743371.
- ^ Chen AC, Neugut AI. Malignant Neoplasms of the Small Intestine. eMedicine.com. URL: http://www.emedicine.com/MED/topic2651.htm. Accessed on: June 2, 2006.
- ^ Cross AJ, Leitzmann MF, Subar AF, Thompson FE, Hollenbeck AR, Schatzkin A. A prospective study of meat and fat intake in relation to small intestinal cancer. Cancer Res 2008;68:9274–9
- ^ Lagergren J, Ye W, Ekbom A. Intestinal cancer after cholecystectomy: is bile involved in carcinogenesis. Gastroenterology 2001;121:542–7
Cleveland Clinic. (2022). Small intestine cancer: Symptoms, causes, Prognosis & Treatment. Cleveland Clinic. Retrieved April 4, 2023, from https://my.clevelandclinic.org/health/articles/6225-small-intestine-cancer
Markman, M. (2022, July 20). Intestinal cancer: Causes, symptoms & treatments. Cancer Treatment Centers of America. Retrieved April 4, 2023, from https://www.cancercenter.com/cancer-types/intestinal-cancer
Radhakrishnan, R. (2021, May 6). Is small intestine cancer curable? MedicineNet. Retrieved April 4, 2023, from https://www.medicinenet.com/is_small_intestine_cancer_curable/article.htm
External links
[edit]Small intestine cancer
View on GrokipediaClinical presentation
Symptoms
Small intestine cancer often presents with nonspecific symptoms that can mimic other gastrointestinal disorders, leading to delayed diagnosis. The most common patient-reported complaints include abdominal pain, unexplained weight loss, fatigue, nausea, and vomiting.[2][4][6] Abdominal pain is frequently the initial symptom, typically described as crampy and intermittent, often worsening after eating and located in the mid-abdomen.[6][7] For tumors in the duodenum, pain may be centered in the upper mid-abdomen, while ileal involvement can cause discomfort in the lower abdomen.[7] Unexplained weight loss and fatigue are also prevalent, with the latter sometimes associated with anemia from occult bleeding.[2][6] Nausea and vomiting occur commonly, and changes in bowel habits—such as diarrhea or constipation—may develop as the tumor progresses, along with dark-colored stools due to bleeding.[2][4][6] In late stages, symptoms often intensify due to intestinal obstruction, manifesting as bloating, abdominal distension, and severe cramping pain.[6] The onset of these symptoms is typically insidious, developing gradually over several months, with an average delay of 6 to 12 months from initial presentation to diagnosis owing to their vague nature.[2][6][7]Signs
Small intestine cancer often manifests with objective clinical signs detectable during physical examination, reflecting tumor location, size, and complications such as obstruction or bleeding. Abdominal tenderness upon palpation may be elicited, particularly in the presence of local inflammation or partial bowel obstruction caused by the tumor. Jaundice, presenting as yellowing of the skin and sclerae, arises from biliary obstruction in periampullary tumors located near the ampulla of Vater.[6][2] In advanced disease with peritoneal dissemination, ascites may accumulate, leading to abdominal distension and fluid wave on examination.[7] Cachexia, evident as profound involuntary weight loss accompanied by muscle wasting and general debility, commonly appears in progressive or metastatic cases.[2][6] Chronic blood loss from tumor ulceration frequently results in anemia, manifesting as pallor of the skin and mucous membranes along with tachycardia due to compensatory increased heart rate.[6][8] Bowel obstruction, a potential complication, yields hyperactive bowel sounds in early or partial stages due to proximal peristalsis, progressing to diminished or absent sounds in complete obstruction with paralytic ileus.[9][10] A palpable abdominal mass is identified in approximately 25% of cases overall, occurring more commonly with sarcomas such as gastrointestinal stromal tumors that tend to grow exophytically and attain larger sizes.[11][12]Risk factors and etiology
Genetic and inherited factors
Small intestine cancer arises from a combination of inherited and sporadic genetic alterations. Familial adenomatous polyposis (FAP), an autosomal dominant disorder caused by germline mutations in the APC gene on chromosome 5q21-22, significantly elevates the risk of small bowel adenocarcinoma, particularly in the duodenum.[13] Individuals with FAP face a lifetime risk of 3-5% for small bowel adenocarcinoma, representing a relative risk increase of over 300-fold compared to the general population.[14] These APC mutations lead to truncated proteins that disrupt the regulation of β-catenin, promoting uncontrolled cell proliferation through aberrant activation of the Wnt signaling pathway, which drives polyp formation and progression to malignancy.[14] Peutz-Jeghers syndrome (PJS), an autosomal dominant condition due to germline mutations in the STK11 gene on chromosome 19p13.3, is associated with hamartomatous polyps throughout the gastrointestinal tract and a markedly increased risk of small bowel adenocarcinoma, with a lifetime risk estimated at 13% (relative risk >500-fold).[15] These mutations impair the LKB1 protein's role in regulating cell growth and polarity, leading to polyp formation and heightened malignant transformation. Lynch syndrome, another autosomal dominant condition resulting from germline defects in DNA mismatch repair genes such as MLH1, MSH2, MSH6, and PMS2, is associated with increased incidence of small bowel adenocarcinoma and, to a lesser extent, lymphomas.[16] The lifetime risk of small bowel adenocarcinoma in Lynch syndrome carriers is estimated at 1-4%, conferring a relative risk of over 100-fold relative to the general population.[17] These mutations impair DNA repair, leading to microsatellite instability and accumulation of somatic mutations that contribute to tumorigenesis, often presenting at younger ages and with a higher frequency of multiple primary cancers.[16] In sporadic cases, which comprise the majority of small intestine cancers, somatic mutations play a central role. Adenocarcinomas frequently harbor alterations in KRAS (approximately 42% of cases), TP53 (54%), and APC (11%), which activate oncogenic signaling and disrupt tumor suppression, facilitating tumor initiation and progression.[18] For neuroendocrine tumors of the small intestine, common genetic changes include chromosomal losses, notably of chromosome 18 (observed in up to two-thirds of cases); these alterations are thought to influence tumor growth and metastasis, though their precise functional roles remain under investigation.[19]Environmental and lifestyle factors
Environmental and lifestyle factors play a role in the development of small intestine cancer, particularly through modifiable exposures that promote chronic inflammation or cellular damage. Dietary patterns, such as high consumption of animal fats and proteins, have been associated with increased risk of small bowel adenocarcinoma, with correlations observed between these dietary components and cancer incidence (r = 0.61 for fats, r = 0.75 for proteins).[20] Similarly, red and processed meat intake is linked to a 2- to 3-fold elevated risk for small intestine cancers, potentially due to carcinogenic compounds formed during processing or cooking.[20] In contrast, low-fiber diets may exacerbate risk by impairing gut health, while high fiber intake appears protective against adenocarcinoma.[21] Chronic inflammatory conditions triggered by diet, such as celiac disease, substantially raise the risk of small intestine lymphoma by 10- to 13-fold through persistent mucosal inflammation and immune dysregulation.[20] Lifestyle behaviors further contribute to susceptibility. Smoking is associated with a 1.5- to 2-fold higher risk of small bowel adenocarcinomas, with current smokers showing a hazard ratio of 1.77 compared to never smokers in large cohort studies.[22] Alcohol consumption demonstrates a weak positive correlation with adenocarcinoma risk, with a relative risk of approximately 1.5 for heavy drinkers.[21] Occupational exposures also pose risks, particularly for neuroendocrine tumors; workers in metal preparation and construction have shown elevated rates, with odds ratios up to 4.3 for structural metal workers developing carcinoid tumors.[23] Crohn's disease, often exacerbated by environmental triggers like diet and smoking, increases adenocarcinoma risk by 20- to 60-fold via chronic inflammation, with standardized incidence ratios reaching 34.9 in affected individuals.[21] Demographic factors intersect with these exposures to heighten vulnerability. Small intestine cancer incidence is higher in males, with a male-to-female ratio of 1.3:1, and predominantly affects individuals over 60 years of age, with a median diagnosis age of 66.[20] Obesity emerges as an additional modifiable factor, conferring a relative risk of about 1.3 for small intestine cancers, particularly through abdominal adiposity that promotes insulin resistance and inflammation.[20] These environmental and lifestyle elements can interact with genetic predispositions to amplify overall risk, though their effects are largely acquired rather than inherited.[20]Histopathologic classification
Adenocarcinoma
Adenocarcinoma is the most common histologic subtype of small intestine cancer, arising from glandular epithelial cells and accounting for approximately 30-40% of all small bowel malignancies.[24] It predominantly occurs in the duodenum, representing about 50% of cases, followed by the jejunum at around 30%, with the ileum being less frequently affected at 20%.[24] This subtype is histopathologically similar to colorectal adenocarcinoma but exhibits distinct molecular features that differentiate it from other gastrointestinal cancers.[21] Macroscopically, small bowel adenocarcinomas often present as annular constricting lesions that lead to intestinal obstruction, particularly in the jejunum and ileum, while duodenal tumors may appear more exophytic and polypoid.[24] These growth patterns contribute to early symptoms such as abdominal pain and bloating due to partial or complete blockage.[18] Microscopically, these tumors display tubular or mucinous architectural patterns, characterized by complex glandular structures embedded in a desmoplastic stromal reaction.[24] They can be further subclassified into intestinal-type (expressing CDX-2, MUC2, and CD10) or gastric-type (expressing MUC5AC and MUC6) phenotypes, with poorly differentiated forms being more common than in colorectal counterparts.[21] The molecular profile of small bowel adenocarcinoma includes frequent inactivating mutations in APC (seen in 7-27% of cases), activating mutations in KRAS (approximately 42-54%), and alterations in SMAD4 (10-17%), which drive tumorigenesis and progression similar to but less pronounced than in colorectal cancer.[18] HER2 (ERBB2) amplification occurs in 8-13% of tumors, particularly those in the duodenum, offering potential for targeted therapies.[18] Additional common alterations involve TP53 (around 58%) and PIK3CA (16%), with microsatellite instability in about 8% and high tumor mutational burden in 10%.[25] Clinically, small bowel adenocarcinoma demonstrates aggressive local invasion into surrounding tissues and is associated with metastasis at diagnosis in 30-40% of patients, most commonly to the liver and peritoneum.[25] Compared to neuroendocrine tumors, it carries a poorer prognosis, with 5-year overall survival rates of approximately 71%.[26] Patients with underlying conditions such as Crohn's disease face an elevated risk for this subtype.[21]Neuroendocrine tumors
Neuroendocrine tumors (NETs), formerly referred to as carcinoid tumors, account for approximately 40% of all small intestine malignancies and are the most common subtype. These neoplasms arise from enterochromaffin cells within the diffuse neuroendocrine system of the gut mucosa. They predominantly originate in the ileum, comprising about 60% of small bowel NETs, with the remainder occurring in the jejunum or duodenum. While most are well-differentiated (grades 1 or 2), poorly differentiated neuroendocrine carcinomas represent a more aggressive subset with higher proliferative activity. A notable feature of small bowel NETs is their multifocality, observed in up to 30% of cases, which underscores the need for thorough evaluation of the entire small intestine during diagnosis. In patients with advanced disease, particularly those with liver metastases, 10-20% develop carcinoid syndrome, manifesting as episodic flushing, diarrhea, and abdominal cramping due to excessive serotonin secretion by the tumor. This syndrome highlights the functional endocrine capacity of these tumors, distinguishing them from non-hormone-producing small bowel cancers. Histologically, small bowel NETs are characterized by nests or trabeculae of uniform polygonal cells with finely granular, salt-and-pepper chromatin and inconspicuous nucleoli. Immunohistochemical staining is typically positive for neuroendocrine markers such as chromogranin A and synaptophysin, aiding in confirmation of the diagnosis. These tumors generally exhibit indolent growth patterns, but 30-50% present with metastases at diagnosis, often to regional lymph nodes or the liver. Tumor grading relies on the Ki-67 proliferation index, where grade 1 tumors have a low index of less than 3%, indicating slower proliferation and better differentiation. Some small bowel NETs are associated with inherited syndromes like multiple endocrine neoplasia type 1 (MEN1).Lymphomas
Lymphomas account for approximately 15-20% of small intestine malignancies, with the vast majority being non-Hodgkin lymphomas, particularly diffuse large B-cell lymphoma and, less commonly, follicular lymphoma.[27] These tumors arise from the lymphoid tissue within the intestinal mucosa and are characterized by their potential association with underlying conditions such as immunosuppression, including in patients with HIV/AIDS.[27] A notable link exists with celiac disease, which predisposes to specific subtypes like enteropathy-associated T-cell lymphoma, though B-cell variants predominate overall.[28] These lymphomas most frequently originate in the ileum, comprising 60-65% of cases, often involving the Peyer's patches, which are aggregates of lymphoid follicles in the intestinal wall.[27] A distinctive variant, immunoproliferative small intestinal disease (IPSID), is prevalent in Mediterranean and Middle Eastern regions and typically affects the proximal small intestine, presenting as a low-grade B-cell lymphoma linked to chronic infection with Campylobacter jejuni.[28][29] Histologically, small intestine lymphomas feature dense lymphoid infiltrates that efface the normal mucosal architecture, with the majority showing CD20-positive B-cells indicative of B-cell lineage origin.[28] These infiltrates often extend transmurally and may include a plasma cell component in IPSID cases.[29] In terms of behavior, these tumors exhibit rapid growth, particularly in high-grade forms like diffuse large B-cell lymphoma, with B symptoms such as fever, night sweats, and weight loss occurring in 20-30% of patients.[28] Extranodal spread is frequent, commonly involving mesenteric lymph nodes or adjacent structures, contributing to their aggressive clinical course.[27]Sarcomas
Sarcomas represent rare mesenchymal tumors arising from the connective tissues of the small intestine, accounting for approximately 10-20% of all small bowel malignancies.[30][31] These tumors originate from smooth muscle, stromal cells, or other mesenchymal elements and are distinct from epithelial or lymphoid neoplasms due to their non-hematopoietic, connective tissue derivation. The primary subtypes include gastrointestinal stromal tumors (GISTs), which are the most common form of small intestine sarcoma, leiomyosarcoma, and less frequently liposarcoma.[7][32] GISTs arise from interstitial cells of Cajal and are characterized by activating mutations in the KIT gene, leading to c-kit protein expression.[33] Leiomyosarcomas typically develop from smooth muscle layers. Liposarcomas, though rarer in the small bowel, originate from adipose tissue precursors. These tumors predominantly occur in the jejunum and ileum, with the ileum being a favored site for leiomyosarcomas and both jejunum and ileum commonly affected by GISTs.[7][34] GISTs in the small intestine are typically c-kit positive on immunohistochemical staining, aiding in their identification.[35] Histologically, leiomyosarcomas consist of spindle-shaped cells with atypical nuclei, eosinophilic cytoplasm, and a high mitotic rate that correlates with aggressive behavior.[36] In contrast, GISTs often display epithelioid or spindle cell morphology, with epithelioid variants showing rounded cells and prominent nucleoli; a elevated mitotic rate further indicates malignancy potential.[34][37] Sarcomas of the small intestine are often diagnosed at a large size, with an average tumor diameter of about 10 cm, contributing to their insidious presentation.[38] They exhibit aggressive behavior through hematogenous spread, with metastasis to the liver occurring in approximately 40% of cases at diagnosis or recurrence.[39]Diagnosis
Imaging studies
Imaging studies play a crucial role in the initial detection, localization, and characterization of small intestine cancers, often prompted by symptoms such as abdominal pain or obstruction. These non-invasive modalities help identify tumor masses, assess bowel wall involvement, and evaluate for regional lymphadenopathy or distant spread, guiding subsequent diagnostic steps.[40] Computed tomography (CT) enterography is the preferred initial imaging test for suspected small intestine cancer due to its high resolution and ability to evaluate the entire bowel. It involves oral contrast to distend the small bowel loops, allowing detection of masses greater than 1 cm with a sensitivity of 80-90%, particularly for identifying circumferential wall thickening, luminal narrowing, and associated lymphadenopathy. This modality excels in delineating adenocarcinoma features like shouldering of the bowel wall and mesenteric involvement, as well as complications such as obstruction or perforation.[41][42][43] Magnetic resonance (MR) enteroclysis provides detailed soft tissue evaluation and is particularly useful in younger patients to minimize radiation exposure, offering an alternative to CT for comprehensive bowel assessment. By using oral and intravenous contrast with bowel distension, it achieves high accuracy (up to 96.6%) in detecting small bowel neoplasms, superior for characterizing tumor margins, vascular involvement, and multifocal lesions without ionizing radiation. It is especially beneficial for neuroendocrine tumors, where it highlights hypervascularity and desmoplastic reactions.[44][45][41] Positron emission tomography-computed tomography (PET-CT) is primarily employed for staging, particularly in neuroendocrine tumors using somatostatin receptor imaging (e.g., 68Ga-DOTATATE PET-CT) to detect primary lesions and metastases with high sensitivity due to receptor overexpression. For aggressive subtypes like adenocarcinoma or lymphoma, 18F-FDG PET-CT aids in identifying metabolically active disease and occult metastases, improving staging accuracy over CT alone, though it is not routine for initial detection.[46][47][48] Ultrasound has a limited role in small intestine cancer evaluation due to acoustic shadowing from bowel gas, but it can detect secondary signs such as ascites, bowel obstruction, or peritoneal spread with moderate sensitivity (around 50%). Endoscopic ultrasound (EUS) is more targeted for duodenal lesions, providing high-resolution images of the bowel wall layers to assess depth of invasion and guide biopsy, with utility in preoperative planning for ampullary or proximal tumors.[49][50][51]Endoscopic and biopsy procedures
Endoscopic procedures provide direct visualization and tissue sampling of the small intestine, often guided by prior imaging abnormalities such as masses or wall thickening identified on CT or MRI.[47] Upper endoscopy, also known as esophagogastroduodenoscopy (EGD), is the primary method for evaluating duodenal tumors in small intestine cancer. This procedure involves inserting a flexible endoscope through the mouth to examine the esophagus, stomach, and duodenum, allowing for real-time visualization and targeted biopsy of suspicious lesions. Biopsies obtained during EGD enable pathologic confirmation of malignancy, including adenocarcinomas.[4][24][52] Capsule endoscopy offers a non-invasive means to visualize the entire small bowel mucosa, particularly useful for detecting tumors in the jejunum or ileum that are beyond the reach of standard EGD. Patients swallow a small capsule containing a camera that transmits images as it traverses the digestive tract, providing comprehensive assessment of mucosal lesions with a sensitivity of approximately 80% for small bowel tumors. However, this modality lacks the ability to obtain biopsies, necessitating follow-up procedures for tissue confirmation if abnormalities are detected.[47][53] Double-balloon enteroscopy extends diagnostic and sampling capabilities deeper into the small intestine, targeting the jejunum and ileum for tumors not accessible by conventional endoscopy. This technique uses an endoscope with two balloons—one on the scope tip and one on a transparent overtube—that are alternately inflated to pleat the intestinal wall, advancing the scope while minimizing loops; it can be performed via oral (anterograde) or rectal (retrograde) routes. The procedure achieves total enteroscopy in about 86% of cases and facilitates biopsy in the majority of targeted lesions, with a diagnostic yield superior to capsule endoscopy for small or submucosal tumors.[4][54][55] Biopsy specimens from these endoscopic procedures undergo cytologic and histologic analysis to confirm malignancy and classify the tumor type. Pathologic examination reveals characteristic features such as glandular formations in adenocarcinomas, with immunohistochemistry playing a key role in subtyping; for instance, small bowel adenocarcinomas may show cytokeratin 7 (CK7) positivity, aiding differentiation from other gastrointestinal malignancies.[56][24]Staging
TNM classification
The TNM classification system, developed by the American Joint Committee on Cancer (AJCC), is used to stage small intestine cancers, primarily adenocarcinomas, based on the extent of the primary tumor (T), involvement of regional lymph nodes (N), and presence of distant metastasis (M).[57][58] This system, as outlined in the AJCC 8th edition (effective January 2018), provides a standardized framework for assessing disease progression and guiding clinical management.[59] The T category reflects the depth of tumor invasion into the small bowel wall. T1 tumors are confined to the lamina propria or submucosa (with T1a limited to the lamina propria and T1b to the submucosa). T2 tumors invade the muscularis propria. T3 tumors extend through the muscularis propria into the subserosa or nonperitonealized perimuscular tissue (such as the mesentery) without penetrating the serosa. T4 tumors perforate the visceral peritoneum or directly invade adjacent organs or structures, such as the pancreas or bile duct in duodenal cases.[57][60] The N category indicates regional lymph node involvement, with regional nodes including mesenteric and perigastric nodes for the small intestine. N0 denotes no regional lymph node metastasis. N1 indicates metastasis in 1 to 2 regional lymph nodes. N2 signifies metastasis in 3 or more regional lymph nodes.[57][58][60] The M category assesses distant spread. M0 indicates no distant metastasis. M1 denotes distant metastasis, commonly to sites such as the liver or peritoneum.[57][58] For neuroendocrine tumors of the small intestine (primarily jejunal and ileal), the AJCC 8th edition adapts the TNM system with modifications based on tumor size and node burden, while incorporating histologic grade (G1 to G3, determined by mitotic rate and Ki-67 index) as a prognostic factor that influences overall staging and outcomes, though grade is not directly integrated into the core T, N, or M descriptors.[61][62] In this context, T1 tumors invade the lamina propria or submucosa and are ≤1 cm; T2 tumors invade the muscularis propria or are >1 cm; T3 tumors extend through the muscularis propria into subserosal tissue without penetrating the serosa; and T4 tumors invade the serosa or adjacent structures. N1 involves fewer than 12 regional lymph nodes, while N2 involves 12 or more regional lymph nodes and/or extensive mesenteric masses (>2 cm).[61][62]Prognostic groupings
Prognostic groupings for small intestine cancer integrate the TNM classification elements into stage categories that predict patient outcomes, primarily for adenocarcinoma, the most common histology. These groupings, defined by the American Joint Committee on Cancer (AJCC), stratify disease based on tumor invasion depth (T), regional lymph node involvement (N), and distant metastasis (M), providing a framework for prognosis and treatment planning.[58][57] For adenocarcinoma, the 5-year relative survival rates based on SEER data (2015–2021) are approximately 86% for localized disease (Stages I and II), 80% for regional disease (Stage III), and 47% for distant disease (Stage IV).[26] The following table summarizes the AJCC prognostic stage groups for small intestine adenocarcinoma:| Stage | TNM Combination | Description |
|---|---|---|
| I | T1-2 N0 M0 | Tumor invades submucosa or muscularis propria; no regional lymph nodes or distant metastasis. |
| IIA | T3 N0 M0 | Tumor invades subserosa; no regional lymph nodes or distant metastasis. |
| IIB | T4 N0 M0 | Tumor perforates serosa or invades adjacent structures; no regional lymph nodes or distant metastasis. |
| IIIA | Any T N1 M0 | Any T stage with metastasis to 1-2 regional lymph nodes; no distant metastasis. |
| IIIB | Any T N2 M0 | Any T stage with metastasis to 3 or more regional lymph nodes; no distant metastasis. |
| IV | Any T Any N M1 | Distant metastasis present. |
Treatment
Surgical interventions
Surgical interventions represent the cornerstone of treatment for localized small intestine cancer, aiming to achieve complete tumor removal while preserving intestinal function. For resectable disease, the primary approach involves segmental resection of the affected bowel segment, including the tumor and a margin of surrounding healthy tissue, accompanied by lymphadenectomy to address regional lymph node involvement. This procedure typically includes removal of at least 8-10 lymph nodes for adequate staging and oncologic clearance. In cases of duodenal adenocarcinoma, a more extensive pancreaticoduodenectomy (Whipple procedure) is often required due to the tumor's proximity to the pancreas and bile duct, incorporating resection of the duodenum, head of the pancreas, and adjacent structures. Surgical margins of at least 5 cm proximally and distally are recommended to minimize local recurrence risk.[64][65][66] For neuroendocrine tumors of the small intestine, surgery focuses on curative intent for localized lesions through segmental resection or right hemicolectomy for ileal tumors, with mesenteric lymphadenectomy to resect involved nodes and desmoplastic reactions that may cause bowel obstruction. In advanced cases with liver metastases, which occur in 50-75% of patients at diagnosis, cytoreductive debulking surgery is performed to alleviate symptoms from hormone excess, such as carcinoid syndrome, particularly when hepatic tumor burden exceeds 50-90%. Liver resection, including hemihepatectomy or wedge resections, is feasible in approximately 20-50% of metastatic cases, depending on tumor distribution and patient fitness, often combined with ablation techniques for multifocal disease.[67][68][69] Palliative surgical options are employed for unresectable or advanced disease causing complications like intestinal obstruction or bleeding. Bypass procedures, such as gastrojejunostomy for duodenal lesions or enteroenterostomy for jejunal/ileal blockages, divert intestinal contents around the tumor to restore patency without attempting curative resection. For duodenal obstructions, endoscopic stenting with self-expandable metal stents provides an alternative to surgery, offering faster symptom relief and shorter hospital stays in select patients.[4][47] Oncologic outcomes of surgical resection vary by histology and stage, with R0 resection (negative margins) achieved in 50-70% of cases for primary small bowel tumors, correlating with improved local control. Postoperative complication rates range from 20-45%, including anastomotic leaks (5-10%), infections, and prolonged ileus, with higher risks in extensive procedures like pancreaticoduodenectomy. Morbidity is influenced by patient comorbidities and nutritional status, but overall 30-day mortality remains low at 2-5% in specialized centers.[70][71][72]Systemic and targeted therapies
Systemic therapies for small intestine cancer encompass chemotherapy, targeted agents, and immunotherapy, tailored to the histologic subtype such as adenocarcinoma, lymphoma, gastrointestinal stromal tumors (GIST), or neuroendocrine tumors (NETs). These approaches are primarily used in adjuvant settings following surgery or for metastatic disease, aiming to control tumor growth, alleviate symptoms, and improve survival. Selection of therapy depends on tumor biology, patient performance status, and molecular features like microsatellite instability (MSI) status.[73] For small bowel adenocarcinoma, the most common epithelial malignancy, systemic chemotherapy is a cornerstone. In adjuvant settings for locally advanced disease, regimens such as FOLFOX (fluorouracil, leucovorin, and oxaliplatin) or CAPEOX (capecitabine and oxaliplatin) are recommended for 6 months to reduce recurrence risk. In metastatic cases, first-line FOLFOX or CAPEOX yields objective response rates of approximately 45-50%, with median progression-free survival around 7 months. FOLFIRI (fluorouracil, leucovorin, and irinotecan) serves as a second-line option, achieving response rates of about 20%. Bevacizumab may be added to these fluoropyrimidine-based regimens to enhance efficacy in advanced disease.[73][74][75] Primary lymphomas of the small intestine, often diffuse large B-cell type, respond well to immunochemotherapy. The R-CHOP regimen (rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone), administered in 21-day cycles for 6-8 cycles, is the standard, achieving complete remission rates of 60-70% in aggressive non-Hodgkin lymphomas, including gastrointestinal subtypes. This combination targets B-cell proliferation and has demonstrated superior outcomes over CHOP alone in improving event-free survival.[76][77] Targeted therapies address specific molecular drivers in small intestine sarcomas and NETs. For GIST, which arises from interstitial cells of Cajal, imatinib mesylate—a tyrosine kinase inhibitor— is first-line at doses of 400-800 mg daily, inhibiting KIT or PDGFRA mutations present in over 80% of cases. Clinical trials report partial response rates of 50-70%, with many patients achieving stable disease and prolonged progression-free survival exceeding 2 years. For well-differentiated small intestine NETs, somatostatin analogs such as octreotide or lanreotide provide symptomatic relief from carcinoid syndrome by suppressing hormone secretion; they are administered as long-acting formulations monthly, stabilizing tumor growth in midgut NETs as shown in the PROMID trial. For progressive disease, everolimus (an mTOR inhibitor) is recommended, with peptide receptor radionuclide therapy (PRRT) using lutetium Lu 177 dotatate for somatostatin receptor-positive tumors, and cabozantinib (a multi-tyrosine kinase inhibitor) as a recent option for advanced gastroenteropancreatic NETs per NCCN guidelines version 1.2025.[78][79][80] Emerging immunotherapy options target immune checkpoints in select cases. Approximately 5-10% of small bowel adenocarcinomas exhibit MSI-high (MSI-H) status due to mismatch repair deficiency, rendering them responsive to PD-1 inhibitors like pembrolizumab or nivolumab. In MSI-H advanced gastrointestinal cancers, including small bowel subtypes, these agents yield objective response rates of 30-50%, with durable responses in refractory settings as evidenced by KEYNOTE-158 and CheckMate trials. Such therapies are typically reserved for later lines after chemotherapy failure.[24][81][82]Prognosis
Survival outcomes
The 5-year relative survival rate for small intestine cancer is 71.1% overall, based on diagnoses from 2015 to 2021.[3] Survival rates vary markedly by stage at diagnosis, with 86% for localized disease confined to the small intestine, 80% for regional involvement of nearby lymph nodes or structures, and 47% for distant metastasis, according to recent Surveillance, Epidemiology, and End Results (SEER) Program data as of 2025.[26] These outcomes differ substantially across histological subtypes. Neuroendocrine tumors generally exhibit the most favorable prognosis, with 5-year survival rates of 80-90%, reflecting their often indolent behavior and responsiveness to targeted therapies.[20] In contrast, adenocarcinomas, the most aggressive epithelial malignancies in the small intestine, have 5-year survival rates of 30-50%, influenced by late presentation and limited systemic options.[20] Small intestine lymphomas achieve 64% 5-year survival with chemotherapy regimens like CHOP, particularly when combined with surgery for localized disease (based on Canadian data).[83] Sarcomas, including gastrointestinal stromal tumors (GISTs), show 79% 5-year survival following resection and tyrosine kinase inhibitor therapy such as imatinib (based on Canadian data).[83] Survival has improved notably in recent decades, with increases in 5-year relative rates since 2000, attributable to enhanced imaging for earlier staging and multidisciplinary treatment approaches.[20]Influencing factors
Several tumor-related characteristics significantly influence the prognosis of small intestine cancer, particularly adenocarcinoma, the most common histological subtype. High-grade tumors, characterized by poor differentiation, are associated with worse outcomes due to increased aggressiveness and metastatic potential. Tumor location also plays a critical role, with duodenal adenocarcinomas exhibiting the poorest prognosis—median overall survival as low as 20 months—compared to ileal tumors, which show better outcomes owing to earlier detection and easier surgical access.[84] Additionally, resection margin status is a key determinant; R1 resections (microscopically positive margins) are linked to significantly diminished survival relative to R0 (negative margins) resections by promoting local recurrence.[85] Patient-specific factors further modulate prognosis beyond tumor biology. Advanced age, particularly over 70 years, correlates with reduced 5-year survival, attributed to diminished physiological reserve and higher comorbidity burden, as evidenced by SEER data showing progressively lower relative survival in older cohorts.[3] Poor performance status, defined as Eastern Cooperative Oncology Group (ECOG) score greater than 2, independently predicts inferior overall survival due to limited tolerance for aggressive therapies.[86] Comorbid conditions, such as Crohn's disease, exacerbate outcomes in small bowel adenocarcinoma, yielding 5-year survival rates around 30%—notably lower than in de novo cases—stemming from chronic inflammation and diagnostic delays.[87] Treatment-related variables can favorably alter prognosis when optimized. Achieving complete (R0) resection improves 5-year survival to 42% in localized adenocarcinoma, reflecting effective local control and reduced recurrence risk.[31] Evidence for adjuvant chemotherapy in node-positive cases is limited, with non-randomized studies showing no clear survival benefit.[88] Biomarkers offer prognostic and therapeutic insights, particularly microsatellite instability-high (MSI-H) status, present in 18-35% of small bowel adenocarcinomas. MSI-H tumors predict superior responses to immunotherapy, with objective response rates of 30-50% and prolonged progression-free survival in responders, contrasting with microsatellite stable tumors that show limited benefit.[89]Epidemiology
Incidence and prevalence
Small intestine cancer is a rare malignancy, accounting for less than 1% of all cancer diagnoses worldwide. Globally, an estimated 64,477 new cases were reported in 2020, corresponding to an age-standardized incidence rate (ASR) of 0.60 per 100,000 population. This represents approximately 3.5% of all gastrointestinal cancers. In the United States, projections for 2025 estimate about 13,920 new cases.00816-8/fulltext)[32][5] The disease exhibits geographic variation, with higher incidence rates in high-income Western countries compared to regions in Asia. In very high human development index (HDI) countries, which include many Western nations, the ASR reaches 1.0 per 100,000, while in low HDI regions such as parts of Asia, it is approximately 0.3 per 100,000. Prevalence remains low overall, with an estimated 89,327 individuals living with small intestine cancer in the United States as of 2022. The condition predominantly affects older adults, with a peak incidence in the 60-70 age group and a median age at diagnosis of around 66 years. There is a slight male predominance, with a male-to-female incidence ratio of approximately 1.2:1.00816-8/fulltext)[3] Histologically, small intestine cancers comprise a diverse group of subtypes, with neuroendocrine tumors (including carcinoids) and adenocarcinomas being the most common. The approximate distribution of major types based on recent population data is shown below:| Type | Percentage |
|---|---|
| Adenocarcinoma | 32% |
| Neuroendocrine tumors | 41% |
| Lymphoma | 14% |
| Sarcoma (including GIST) | 14% |