Recent from talks
All channels
Be the first to start a discussion here.
Be the first to start a discussion here.
Be the first to start a discussion here.
Be the first to start a discussion here.
Welcome to the community hub built to collect knowledge and have discussions related to Muscle cell.
Nothing was collected or created yet.
Muscle cell
View on Wikipediafrom Wikipedia
Not found
Muscle cell
View on Grokipediafrom Grokipedia
A muscle cell, also known as a myocyte, is the specialized contractile cell that forms the basic structural and functional unit of all muscle tissues in the body, enabling movement, circulation, and other vital processes through the generation of force via contraction.[1] These cells are categorized into three distinct types—skeletal, cardiac, and smooth—each exhibiting unique structural features, control mechanisms, and physiological roles tailored to their locations and functions.[1] Skeletal muscle cells, which are striated and multinucleated, are responsible for voluntary movements and are attached to bones via tendons.[2] Cardiac muscle cells, also striated but involuntary, form the myocardium of the heart and are interconnected by intercalated discs to ensure synchronized contractions.[1] Smooth muscle cells, non-striated and involuntary, line the walls of hollow organs and blood vessels, facilitating functions such as peristalsis and vascular tone regulation.[1]
At the cellular level, all muscle cells share core components for contraction, including actin and myosin filaments that interact in a sliding mechanism triggered by calcium ions, though the organization and regulation differ by type.[1] Skeletal muscle cells are elongated, cylindrical fibers ranging from 10 to 100 micrometers in diameter and up to several centimeters long, containing multiple nuclei and organized into sarcomeres that give them their characteristic striations under microscopy.[2] These cells feature a highly developed sarcoplasmic reticulum for calcium storage and T-tubules that propagate action potentials deep into the fiber for rapid, uniform contraction.[1] In contrast, cardiac muscle cells are shorter and branched, with a single central nucleus, and rely on gap junctions in intercalated discs for electrical coupling, allowing the heart to function as a unified pump.[1] Smooth muscle cells are spindle-shaped with a single nucleus, lacking sarcomeres but possessing dense bodies and caveolae that anchor filaments and aid in calcium signaling, enabling slower, sustained contractions.[1]
Muscle cells develop from mesodermal precursors through myogenesis, a process involving transcription factors like MyoD and Myf-5 that drive cell fusion and differentiation.[1] Skeletal muscle arises from somites and limb bud mesoderm, forming multinucleated syncytia during embryogenesis.[1] Cardiac muscle differentiates from the cardiogenic mesoderm and becomes functional early in fetal development, around day 32 of gestation.[1] Smooth muscle originates from diverse embryonic layers depending on the organ, such as mesoderm for vascular smooth muscle.[1] Functionally, contraction in skeletal muscle follows the sliding filament theory, where neural excitation releases calcium to bind troponin, exposing myosin-binding sites on actin for cross-bridge cycling.[2] Cardiac contraction is modulated by the autonomic nervous system and hormones, featuring a prolonged action potential for tetanic prevention.[1] Smooth muscle contraction is often initiated by autonomic nerves or circulating factors, involving calmodulin-mediated myosin light chain phosphorylation.[1]
These adaptations underscore the versatility of muscle cells in maintaining homeostasis, with skeletal muscle comprising about 40% of body mass in adults and playing key roles in metabolism and posture.[3] Disruptions in muscle cell function contribute to conditions like muscular dystrophies, cardiomyopathies, and vascular disorders, highlighting their clinical significance.[1]
This models collective myosin head contributions during ATP-driven cycling.[58] The length-tension relationship, describing how force varies with sarcomere length, follows Hill's equation for force-velocity dynamics:
where is force, is velocity, is maximum isometric force, and , are muscle-specific constants shaping the hyperbolic curve.[59] This equation reveals molecular insights into cross-bridge attachment rates and energy efficiency.[59]
Structure and Classification
Skeletal muscle cells
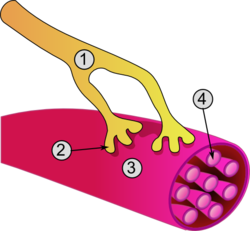
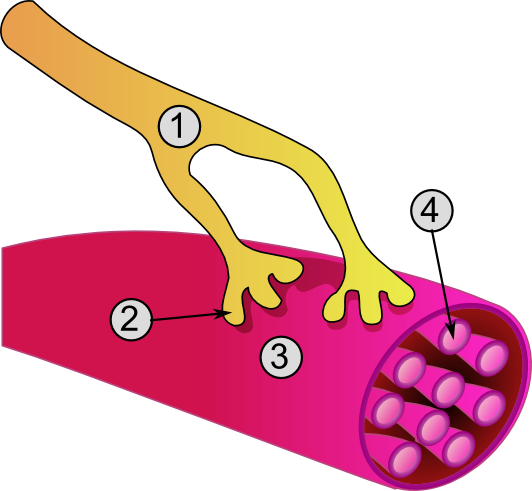
Skeletal muscle cells, also known as muscle fibers or myofibers, are elongated, cylindrical, multinucleated cells that form the functional units of skeletal muscles responsible for voluntary movement. These fibers arise from the fusion of multiple myoblasts during development, resulting in syncytial structures that can extend up to 30 cm in length in certain muscles like the sartorius, with diameters ranging from 10 to 100 micrometers. The multiple nuclei within each fiber are characteristically located at the periphery, just beneath the sarcolemma, the plasma membrane of the muscle cell. This multinucleated organization supports the large size and extensive contractile apparatus of the fibers, enabling powerful and coordinated contractions for locomotion and posture maintenance.[2][4][5] A defining feature of skeletal muscle cells is their striated appearance, caused by the highly organized arrangement of sarcomeres—the repeating units of the contractile apparatus—aligned within myofibrils that run parallel to the fiber's long axis. Skeletal muscle fibers are classified into two main types based on their contractile and metabolic properties: Type I (slow-twitch) fibers, which are oxidative and fatigue-resistant, suited for endurance activities like sustained posture or long-distance running; and Type II (fast-twitch) fibers, which are primarily glycolytic and generate rapid, powerful contractions for short bursts of activity, such as sprinting or weightlifting. Type II fibers can be further subdivided into Type IIa (fast oxidative-glycolytic, with moderate endurance) and Type IIx (fast glycolytic, with high power but low fatigue resistance), allowing muscles to adapt to diverse functional demands. The striations and fiber type distribution contribute to the precise control of force and speed in voluntary movements, with sarcomere organization detailed further in discussions of contractile proteins.[2][6][7] Human skeletal muscles contain varying numbers of these fibers; for example, the biceps brachii typically has approximately 253,000 fibers in young adults, organized into motor units where each fiber is innervated by a single motor neuron at a specialized synapse called the neuromuscular junction. This junction ensures precise, all-or-none activation of fibers within a motor unit, facilitating graded force production through recruitment of multiple units for voluntary actions like arm flexion. Surrounding the fibers are layers of connective tissue that provide structural support and transmit contractile forces: the endomysium envelops individual fibers, the perimysium bundles fibers into fascicles, and the epimysium encases the entire muscle. Additionally, skeletal muscle fibers are associated with satellite cells—quiescent stem cells located between the basal lamina and sarcolemma—that play a crucial role in muscle maintenance and repair by proliferating and fusing with damaged fibers to restore function.[8][9][10][11]Cardiac muscle cells
Cardiac muscle cells, also known as cardiomyocytes, are specialized striated muscle cells that form the contractile tissue of the heart. These cells are typically branched and cylindrical, with lengths ranging from 50 to 100 micrometers and diameters of about 25 micrometers, allowing for efficient packing within the myocardial wall. Unlike skeletal muscle fibers, each cardiac muscle cell contains a single, centrally located nucleus, which facilitates coordinated gene expression across the interconnected network. The striated appearance arises from the organized arrangement of contractile proteins within the cells, enabling powerful and rhythmic contractions essential for heart function.[12][13][14] A defining feature of cardiac muscle cells is the presence of intercalated discs, which are specialized junctions located at the ends of the cells where they connect to adjacent cardiomyocytes. These discs include desmosomes and fascia adherens for mechanical coupling, providing structural integrity to withstand the repetitive stretching and contracting of the heart. Gap junctions within the intercalated discs allow for electrical coupling by permitting the direct passage of ions and small molecules between cells, ensuring synchronized depolarization across the myocardium. This dual mechanical and electrical connectivity transforms individual cells into a functional syncytium, critical for efficient propagation of action potentials.[15][16][17] Within cardiac muscle cells, myofibrils are composed of repeating sarcomeres, similar to those in skeletal muscle, which consist of overlapping actin and myosin filaments responsible for contraction. However, the excitation-contraction coupling in cardiac cells features unique arrangements of T-tubules and the sarcoplasmic reticulum (SR) optimized for rapid calcium handling. T-tubules in cardiac muscle are larger and positioned at the Z-lines of sarcomeres, forming dyads with the SR rather than the triads seen in skeletal muscle, which enhances calcium influx from extracellular sources and release from intracellular stores. The SR surrounds the myofibrils closely, storing and releasing calcium ions efficiently to trigger contractions, with additional calcium entering via L-type channels during each beat to sustain the process.[18][19][13] Certain cardiac muscle cells exhibit autorhythmicity, the intrinsic ability to generate spontaneous action potentials without external stimulation, a property most prominent in pacemaker cells of the sinoatrial node. These specialized cells, derived from the same lineage as contractile cardiomyocytes, possess unique ion channel expressions, such as funny currents (If) and T-type calcium channels, that drive slow depolarization during diastole, leading to rhythmic firing at rates of 60-100 beats per minute. This autorhythmic capability initiates the heartbeat and propagates through the intercalated disc network to coordinate ventricular contraction.[13][20]Smooth muscle cells
Smooth muscle cells are fusiform, or spindle-shaped, uninucleated structures that typically measure 30 to 200 micrometers in length and 3 to 10 micrometers in width.[21] These cells lack the organized sarcomeres found in striated muscle, resulting in a smooth, non-striated appearance under light microscopy.[22] Instead, their contractile apparatus consists of thin actin filaments and thicker myosin filaments arranged in an oblique, crisscrossing pattern throughout the sarcoplasm, enabling a more diffuse and flexible force generation.[23] The actin filaments insert into dense bodies, which are discrete, electron-dense protein aggregates scattered throughout the cytoplasm and along the plasma membrane, serving to anchor the filaments and transmit contractile forces across the cell.[22] Intermediate filaments, primarily composed of desmin, link these dense bodies to one another and to the cell membrane, forming a cytoskeletal network that enhances mechanical stability and efficient force propagation to adjacent cells and extracellular matrix.[23] Smooth muscle is categorized into single-unit and multi-unit types based on cellular organization and coordination. Single-unit smooth muscle features cells electrically coupled by gap junctions containing connexins, promoting coordinated, wave-like activity resembling a functional syncytium, as observed in the tunica media of the small intestine.[22] Multi-unit smooth muscle, by contrast, comprises discrete cells with independent neural innervation and no widespread gap junctions, allowing precise, individual control, such as in the pupillary constrictor muscle of the iris.[23] These cells are predominantly situated in the tunica media of blood vessel walls, the muscularis layers of the digestive tract, and the bronchial walls of airways, where their elongated form and attachments facilitate sustained circumferential tension.[22] The plasma membrane of smooth muscle cells contains numerous caveolae, flask-shaped invaginations rich in cholesterol and sphingolipids that cluster L-type voltage-gated calcium channels, supporting localized calcium handling essential for structural integrity during prolonged activity.[23]Development and Regeneration
Embryonic development
Muscle cells originate from distinct regions of the mesoderm during early embryogenesis. Skeletal muscle cells derive from the paraxial mesoderm, specifically the somites, which form along the neural tube, while cardiac muscle cells and most smooth muscle cells arise primarily from the splanchnic layer of the lateral plate mesoderm, with some smooth muscle cells deriving from neural crest and other mesodermal sources.[24][25][26] Progenitor cells from these mesodermal origins undergo migration and commitment to the myogenic lineage, primarily regulated by the paired box transcription factors Pax3 and Pax7. In skeletal muscle development, Pax3-expressing cells in the dermomyotome of somites migrate to sites of muscle formation, such as the limb buds, where Pax7 further specifies satellite cell precursors and myogenic progenitors. Pax3 plays a predominant role in early embryonic myogenesis, driving delamination and migration of myoblasts, whereas Pax7 is essential for fetal muscle growth and the establishment of a progenitor pool.[27][28] Differentiation of these myogenic progenitors into mature muscle cells is orchestrated by the myogenic regulatory factors (MRFs), a family of basic helix-loop-helix transcription factors including Myf5, MyoD, myogenin, and MRF4. Myf5 and MyoD initiate commitment to the myogenic lineage by activating muscle-specific gene expression in proliferating myoblasts, while myogenin and MRF4 promote terminal differentiation and the withdrawal from the cell cycle. These factors function in a hierarchical and partially redundant manner, with Myf5 being the earliest expressed during somitogenesis to specify myoblasts.[29][30] In skeletal muscle, mononucleated myoblasts fuse to form multinucleated myotubes, a process critical for generating the syncytial structure of muscle fibers. This fusion occurs after MRF activation, involving cell adhesion molecules and cytoskeletal rearrangements to align and merge myoblasts. For cardiac muscle, cardioblasts from the splanchnic mesoderm coalesce bilaterally and fuse to form the primitive heart tube around the midline, establishing the linear structure that undergoes subsequent looping and chamber formation. Smooth muscle cells differentiate from mesenchymal progenitors primarily in the splanchnic mesoderm, with additional contributions from neural crest cells for certain vascular types; these progenitors respond to local inductive signals, such as epithelial-mesenchymal interactions and TGF-β signaling, to form contractile layers around developing organs and vessels without myoblast fusion.[31][32][33][26] In human embryos, somitogenesis begins during the third week post-fertilization, with the first somites appearing around day 20 and continuing until week 5, providing the initial pool of skeletal muscle progenitors. Myotube formation in skeletal muscle commences by weeks 7-8, marking the onset of primary myogenesis, while the primitive heart tube assembles by the end of week 3. These processes are modulated by signaling pathways such as Wnt and Notch, which refine progenitor specification; Wnt signaling promotes myogenic commitment in somitic cells, whereas Notch inhibits premature differentiation to maintain the progenitor state.[34][35][36][37]Postnatal growth and regeneration
Postnatal muscle growth primarily occurs through hypertrophy, where skeletal muscle fibers increase in size in response to mechanical stimuli such as resistance exercise. This process involves the addition of myofibrils and an expansion in their cross-sectional area, driven by signaling pathways activated by mechanical tension, metabolic stress, and muscle damage.[38] Hormones like insulin-like growth factor-1 (IGF-1) and nutrients further support protein synthesis, leading to net muscle mass gains without significant hyperplasia in adults.[39] In contrast, atrophy—characterized by reduced myofibril number and size—arises from disuse, such as immobilization, or aging-related sarcopenia, involving upregulated proteolysis via the ubiquitin-proteasome system and impaired mitochondrial function.[40] Sarcopenia, affecting up to 50% of individuals over 80, accelerates muscle loss through chronic inflammation and anabolic resistance, diminishing force production.[41] Muscle regeneration in postnatal life relies heavily on satellite cells, quiescent stem cells marked by Pax7 expression that reside between the basal lamina and sarcolemma of muscle fibers. Upon injury, such as strains or trauma, these cells activate, proliferate as Pax7-positive myoblasts, and differentiate into myocytes that fuse with damaged fibers or form new myofibers, restoring structure and function.[42] This process is robust in skeletal muscle, enabling repair after acute damage through coordinated expression of myogenic regulatory factors like MyoD and myogenin.[43] Cardiac muscle, however, exhibits minimal regenerative capacity postnatally; injury typically leads to cardiomyocyte apoptosis and replacement by fibrotic scar tissue via fibroblast activation, impairing contractility due to the limited proliferation of terminally differentiated cardiomyocytes.[44] Smooth muscle regeneration is intermediate, often involving dedifferentiation and proliferation of existing cells rather than dedicated stem cells.[45] Recent advances in muscle regeneration target satellite cell limitations and genetic defects. Stem cell therapies, particularly using mesenchymal stem cells (MSCs) derived from bone marrow or adipose tissue, enhance skeletal muscle repair by secreting paracrine factors that promote satellite cell activation and reduce inflammation in models of injury and dystrophy.[46] Ongoing clinical trials as of 2025 indicate that MSCs can improve functional outcomes in muscular dystrophies through mechanisms supporting myoblast fusion and vascularization.[47] For Duchenne muscular dystrophy (DMD), CRISPR-Cas9 editing of the dystrophin gene has progressed to phase I/II trials, where ex vivo editing of patient myoblasts restores dystrophin expression, with preclinical data indicating up to 60% functional protein recovery and reduced fibrosis in animal models.[48] A 2025 trial update reports safe delivery via AAV vectors, with initial human results showing modest dystrophin restoration in limb muscles.[49]Molecular Components
Contractile proteins and filaments
Muscle cells rely on specialized contractile proteins organized into filaments to generate force and enable contraction. The primary proteins include myosin and actin, which form thick and thin filaments, respectively, interacting via the cross-bridge cycle to produce mechanical work from ATP hydrolysis.[50] These filaments are arranged differently in striated (skeletal and cardiac) versus smooth muscle, influencing contractility across cell types.[51] Thick filaments consist of myosin II, a hexameric protein with two heavy chains and four light chains. Each heavy chain (~200-220 kDa) features a globular motor head with actin- and nucleotide-binding sites, a neck region serving as a lever arm, and a coiled-coil tail for filament assembly.[52] The light chains (essential and regulatory, ~15-20 kDa) stabilize the neck and modulate activity. Myosin II exhibits ATPase activity in its head domain, hydrolyzing ATP to ADP and inorganic phosphate, which powers conformational changes and force generation during the power stroke.[52] Isoforms vary by muscle type, with fast skeletal myosin showing higher ATPase rates (~30 s⁻¹) compared to slow cardiac (~5-6 s⁻¹), adapting to physiological demands like speed versus endurance.[52][50] Thin filaments are polymers of actin, associated with regulatory proteins tropomyosin and troponin in striated muscle. Actin exists as globular monomers (G-actin, ~42 kDa) that polymerize into double-helical filamentous structures (F-actin, ~7 nm diameter), forming the core of thin filaments anchored at Z-lines.[51] Tropomyosin, a coiled-coil dimer (~40 kDa), binds along F-actin, spanning seven actin subunits and sterically blocking myosin-binding sites in the relaxed state.[53] The troponin complex, comprising three subunits, regulates this interaction: troponin C (TnC, ~18 kDa) binds calcium ions to initiate contraction; troponin I (TnI, ~21 kDa) inhibits actin-myosin binding at low calcium by anchoring tropomyosin in a blocked position; and troponin T (TnT, ~31 kDa) links the complex to tropomyosin.[53] Calcium binding to TnC induces TnI release, pivoting tropomyosin to expose myosin sites.[53] In striated muscle, these filaments organize into sarcomeres, the basic contractile units (~2-3 μm long). Thin filaments anchor at Z-lines, defining sarcomere boundaries, and extend into the isotropic I-band before overlapping thick filaments in the anisotropic A-band. The A-band spans the full length of thick filaments, with the central H-zone (lacking thin filament overlap) flanked by regions of partial overlap.[54] Titin, a giant elastic protein (~3-4 MDa), spans from Z-line to M-line, aligning filaments and providing passive elasticity via its extensible I-band region (tandem immunoglobulin and PEVK domains), which generates restoring force (0-5 pN per molecule) to maintain sarcomere integrity during stretch.[54][55] Smooth muscle lacks sarcomeres, featuring a non-sarcomeric arrangement of actin-myosin filaments in dense bodies and oblique lattices for isotropic contraction. Regulatory proteins caldesmon and calponin modulate interactions: caldesmon (~87-93 kDa), an actin- and myosin-binding protein, cross-links filaments, inhibits ATPase activity, and maintains myosin spacing to balance force without calcium sensitization.[56][57] Calponin (~34 kDa), actin-associated, reduces shortening velocity and stabilizes filaments but does not directly regulate force or calcium sensitivity.[57] These adaptations support sustained, low-energy contractions in organs like blood vessels.[56] The cross-bridge cycle kinetics underpin force generation, where total force arises from the number of attached bridges , force per bridge , and displacement :This models collective myosin head contributions during ATP-driven cycling.[58] The length-tension relationship, describing how force varies with sarcomere length, follows Hill's equation for force-velocity dynamics:
where is force, is velocity, is maximum isometric force, and , are muscle-specific constants shaping the hyperbolic curve.[59] This equation reveals molecular insights into cross-bridge attachment rates and energy efficiency.[59]