Recent from talks
Nothing was collected or created yet.
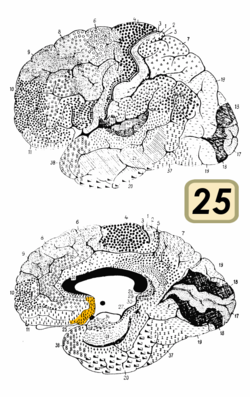
Brodmann area 25
View on Wikipedia| Brodmann area 25 | |
|---|---|
 Brodmann area 25 is shown in orange. | |
 Medial surface of the brain with Brodmann's areas numbered. | |
| Details | |
| Identifiers | |
| Latin | area subgenualis |
| NeuroNames | 1029 |
| FMA | 68622 |
| Anatomical terms of neuroanatomy | |
Brodmann area 25 (BA25) is the subgenual area, area subgenualis or subgenual cingulate area in the cerebral cortex of the brain and delineated based on its cytoarchitectonic characteristics.
It is the 25th "Brodmann area" defined by Korbinian Brodmann. BA25 is located in the cingulate region as a narrow band in the caudal portion of the subcallosal area adjacent to the paraterminal gyrus. The posterior parolfactory sulcus separates the paraterminal gyrus from BA25. Rostrally it is bound by the prefrontal area 11 of Brodmann.[1]
History
[edit]Brodmann described this area as it is labeled now in 1909. Originally in 1905, Brodmann labeled the area as part of area 24. In 1909, he divided the area into area 24 and 25.[2]
Function
[edit]This region is extremely rich in serotonin transporters and is considered as a governor for a vast network involving areas like hypothalamus and brain stem, which influences changes in appetite and sleep; the amygdala and insula, which affect the mood and anxiety; the hippocampus, which plays an important role in memory formation; and some parts of the frontal cortex responsible for self-esteem.[3] This region is particularly implicated in the normal processing of sadness.[4][5]
Involvement in depression
[edit]The subcallosal cingulate gyrus CG25 which consists of BA25 as well as parts of BA24 and BA32 has been implicated as playing an important role in major depression and has been the target of deep brain stimulation to treat the disorder.[6]
One study found that BA25 is metabolically overactive in treatment-resistant depression.[7] A different study found that metabolic hyperactivity in this area is associated with poor therapeutic response of persons with major depressive disorder to cognitive-behavioral therapy and venlafaxine.[8]
In 2005 Helen S. Mayberg and collaborators described how they successfully treated a number of depressed people—individuals virtually catatonic with depression despite years of talk therapy, drugs, and electroconvulsive therapy—with pacemaker-like electrodes (deep brain stimulation) in area 25.[9]
In 2013, Walker and Lawson conducted neurofeedback research with 183 subjects experiencing drug resistant depression. The neurofeedback specifically targeted Brodmann area 25. The treatment consisted of 6 neurofeedback sessions that were 20min long each over the course of 3 weeks. 1 year after the treatment 84% of subjects experienced over a 50% reduction in depression symptoms. 9% reported a 20-50% reduction in depression symptoms. 7% reported a 0-20% improvement. 1% initially reported positive effects, but had a depression relapse before the year follow up completed. There were no side effects or complications reported from the treatment.[10]
A study found that transcranial magnetic stimulation is more clinically effective treating depression when targeted specifically to Brodmann area 46, because this area has intrinsic functional connectivity (negative correlation) with area 25.[11]
Another study has found that the responses of area 25 to viewing sad stimuli are affected by cortisol.[12] This suggests that depression related changes in the activity in area 25 could be due to hypothalamic–pituitary–adrenal axis dysregulation.
Image
[edit]See also
[edit]Notes and references
[edit]- ^ subgen1ual area 25. Archived January 21, 2007, at the Wayback Machine braininfo.rprc.washington.edu, retrieved November 18, 2006.
- ^ area 25 of Brodmann-1909. Archived January 21, 2007, at the Wayback Machine braininfo.rprc.washington.edu, retrieved November 19, 2006.
- ^ "Faulty Circuits", Scientific American, April 2010
- ^ Mayberg, HS; Liotti M; Brannan SK; McGinnis S; Mahurin RK; Jerabek PA; et al. (May 1999). "Reciprocal limbic-cortical function and negative mood: converging PET findings in depression and normal sadness". Am J Psychiatry. 156 (5): 675–82. doi:10.1176/ajp.156.5.675. PMID 10327898. S2CID 16258492.
- ^ Phan, KL; Wager T; Taylor SF; Liberzon I (June 2002). "Functional neuroanatomy of emotion: a meta-analysis of emotion activation studies in PET and fMRI". NeuroImage. 16 (2): 331–48. doi:10.1006/nimg.2002.1087. PMID 12030820. S2CID 7150871.
- ^ Hamani C, Mayberg H, Stone S, Laxton A, Haber S, Lozano AM (15 February 2011). "The subcallosal cingulate gyrus in the context of major depression". Biological Psychiatry. 69 (4): 301–309. doi:10.1016/j.biopsych.2010.09.034. PMID 21145043. S2CID 35458273.
- ^ Deep Brain Stimulation for Treatment-Resistant Depression neuron.org, March 3, 2005. Retrieved November 18, 2018.
- ^ Konarski JZ, Kennedy SH, Segal ZV, Lau MA, Bieling PJ, McIntyre RS, Mayberg HS (2009). "Predictors of nonresponse to cognitive behavioural therapy or venlafaxine using glucose metabolism in major depressive disorder". J Psychiatry Neurosci. 34 (3): 175–80. PMC 2674969. PMID 19448846.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Lozano, Andres M; Peter Giacobbe; Clement Hamani; Sakina J Rizvi; Sidney H Kennedy; Theodore T Kolivakis; Guy Debonnel; Abbas F Sadikot; Raymond W Lam; Andrew K Howard; Magda Ilcewicz-Klimek; Christopher R Honey; Helen S Mayberg (2011-11-18). "A multicenter pilot study of subcallosal cingulate area deep brain stimulation for treatment-resistant depression". Journal of Neurosurgery. 116 (2): 315–22. doi:10.3171/2011.10.JNS102122. PMID 22098195. S2CID 9778445.
- ^ Walker, Jonathan (2013). "FP02 Beta Training for Drug-Resistant Depression—A New Protocol That Usually Reduces Depression and Keeps It Reduced". Journal of Neurotherapy. 17:3 (3): 198–200. doi:10.1080/10874208.2013.785784.
- ^ Fox, MD; Buckner, RL; White, MP; Greicius, MD; Pascual-Leone, A (2012). "Efficacy of transcranial magnetic stimulation targets for depression is related to intrinsic functional connectivity with the subgenual cingulate". Biological Psychiatry. 72 (7): 595–603. doi:10.1016/j.biopsych.2012.04.028. PMC 4120275. PMID 22658708.
- ^ Sudheimer, KD; Abelson JL; Taylor SF; Martis B; Welsh RC; Warner C; et al. (April 2013). "Exogenous glucocorticoids decrease subgenual cingulate activity evoked by sadness". Neuropsychopharmacology. 38 (5): 826–45. doi:10.1038/npp.2012.249. PMC 3599059. PMID 23303057.