Recent from talks
Nothing was collected or created yet.
Amaranth (dye)
View on Wikipedia | |
 | |
| Names | |
|---|---|
| IUPAC name
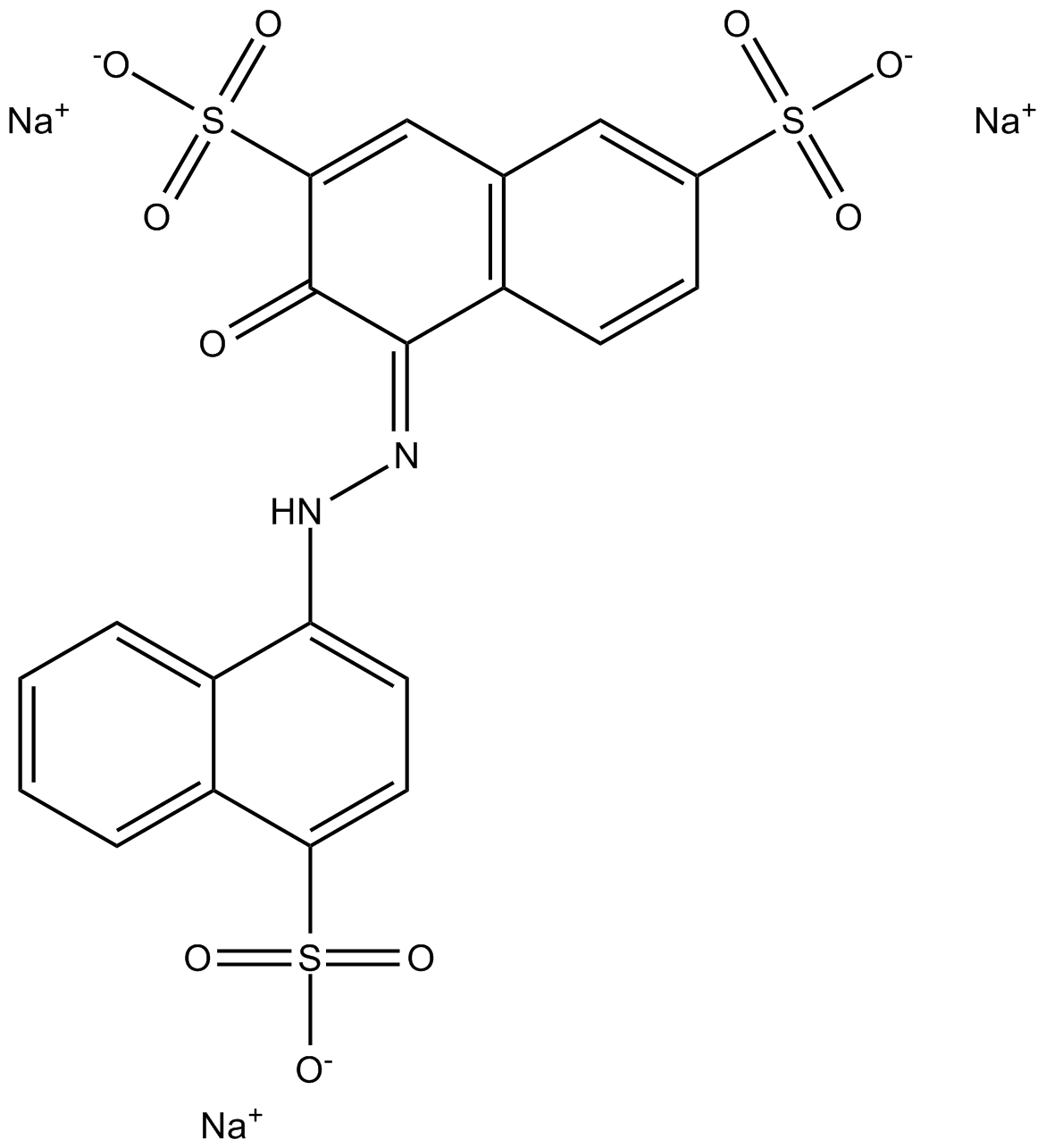
Trisodium (4E)-3-oxo-4-[(4-sulfonate-1-naphthyl)hydrazono]naphthalene-2,7-disulfonate
| |
Other names
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.011.839 |
| EC Number |
|
| E number | E123 (colours) |
| KEGG | |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C20H11N2Na3O10S3 | |
| Molar mass | 604.47305 |
| Appearance | Dark red solid |
| Melting point | 120 °C (248 °F; 393 K) (decomposes) |
| Hazards | |
| GHS labelling: | |

| |
| Warning | |
| H315, H319, H335 | |
| P261, P264, P271, P280, P302+P352, P304+P340, P305+P351+P338, P312, P321, P332+P313, P337+P313, P362, P403+P233, P405, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Amaranth, FD&C Red No. 2, E123, C.I. Food Red 9, Acid Red 27, Azorubin S, or C.I. 16185 is a modified red azo dye used as a food dye and to color cosmetics. The name was taken from amaranth grain, a plant distinguished by its red color and edible protein-rich seeds.
Amaranth is an anionic dye. It can be applied to natural and synthetic fibers, leather, paper, and phenol-formaldehyde resins. As a food additive it has E number E123. Amaranth usually comes as a trisodium salt. It has the appearance of reddish-brown, dark red to purple water-soluble powder that decomposes at 120 °C without melting. Its water solution has an absorption maximum of about 520 nm. Like all azo dyes, Amaranth was, during the middle of the 20th century, made from coal tar; modern synthetics are more likely to be made from petroleum byproducts.[1][2]
Since 1976, amaranth dye has been banned in the United States by the Food and Drug Administration (FDA)[3] as a suspected carcinogen.[4][5] Its use is still legal in some countries, notably in the United Kingdom where it is most commonly used to give glacé cherries their distinctive color.
History and health effects
[edit]After an incident in the 1950s involving Orange 1[6][7] in Clover brand "Spooky Lozenges" and other orange and red candies manufactured at that time, the FDA retested food colors. Later, in 1960, the FDA was given jurisdiction over color additives, limiting the amounts that could be added to foods and requiring producers of food color to ensure the safety and proper labeling of colors. Permission to use food additives was given on a provisional basis, which could be withdrawn should safety issues arise.[7] The FDA gave "generally recognized as safe" (GRAS) provisional status to substances already in use, and extended Red No. 2's provisional status 14 times.
In 1971, a Soviet study linked the dye to cancer.[8][7] By 1976, over 1,000,000 pounds (450,000 kg) of the dye worth $5 million was used as a colorant in $10 billion worth of foods, drugs and cosmetics.[9] Consumer activists in the United States, perturbed by what they perceived as collusion between the FDA and food conglomerates,[10] put pressure on the FDA to ban it.[9] FDA Commissioner Alexander M. Schmidt defended the agency's stance, as he had earlier defended the FDA against collusion accusations in his 1975 book, stating that the FDA found "no evidence of a public health hazard".[9] However, testing by the FDA found a statistically significant increase in the incidence of malignant tumors in female rats given a high dosage of the dye,[7] as well as an increase in cholecystitis, and concluded that since there could also no longer be a presumption of safety, that use of the dye should be discontinued.[7] The FDA banned FD&C Red No. 2 in 1976.[9][11] FD&C Red No. 40 (Allura Red AC) replaced the banned Red No. 2, as its toxicity was determined to be significantly lower due to the removal of one sodium sulfonate functional group, among other molecular adjustments to furthermore reduce the immediate toxicity of the specific azo dye upon consumption.
See also
[edit]References
[edit]- ^ "Amaranth E123". LOOKCUT, INC. 2004–2010. Retrieved August 15, 2014.
- ^ "Aniline Wood Dye, Coal Tar Dyes". Craftsman Style. International Styles Network. 2005–2012. Retrieved August 15, 2014.
- ^ Compliance Program Guidance Manual: Domestic Food Safety (FY07-08) (PDF), Food and Drug Administration, November 9, 2008, p. 6, archived from the original (PDF) on July 10, 2009,
Be aware that the following color additives are not on the list for use in food products in the United States. a. Amaranth (C.I. 16185, EEC No E123, formerly certifiable as FD&C Red No 2)
- ^ Color Additives Fact Sheet, U. S. Food and Drug Administration's Center for Food Safety and Applied Nutrition, July 30, 2001, archived from the original on November 17, 2001
- ^ Overview of Food Color Additives, Prepared for the USDA National Organic Program and the National Organic Standards Board, Agricultural Marketing Service (United States Department of Agriculture), October 14, 2005, pp. 1 and 7, archived from the original on March 9, 2010, retrieved August 15, 2014
- ^ Science News Letter, Volumes 65-66. Science Service. January 30, 1954. p. 95.
- ^ a b c d e Omaye, Stanley T. (2004). Food and Nutritional Toxicology. CRC Press LLC. p. 257. ISBN 0-203-48530-0.
- ^ "Why Red M&M's Disappeared for a Decade". Pricenomics. 12 May 2014. Retrieved 2016-08-16.
- ^ a b c d "Death of a Dye". Time. February 2, 1976. Archived from the original on January 11, 2005. Retrieved 2009-07-07.
- ^ Greenberg, Dan (4 December 1975). "Washington View: FDA in difficulties". New Scientist. 64 (978). London: New Science Publications: 600.
- ^ "Burger Backs Red Dye Ban Pending Rule". The Hartford Courant. February 14, 1976. Archived from the original on 2012-10-21. Retrieved 2009-07-07.
Amaranth (dye)
View on GrokipediaAmaranth, also designated as FD&C Red No. 2 or E123, is a synthetic red azo dye with the molecular formula C₂₀H₁₁N₂Na₃O₁₀S₃, employed historically as a colorant in foods, drugs, and cosmetics.[1][2]
Developed in 1878, it provided a stable reddish hue derived from coal tar, supplanting earlier natural pigments in industrial applications.[3]
Its certification by the U.S. Food and Drug Administration persisted until 1976, when delisting occurred following animal studies demonstrating tumor formation in female rats at high doses, prompting a precautionary ban on its use in ingested products despite debates over relevance to human exposure levels.[2][4][5]
Although prohibited in the United States for safety reasons, amaranth retains limited authorization in regions like the European Union for specific non-solid food categories, underscoring persistent regulatory divergences on synthetic dye tolerability.[5]
The episode exemplifies broader scrutiny of azo compounds' metabolic breakdown products, which can yield aromatic amines potentially linked to genotoxicity, fueling ongoing research into additive risks versus benefits in consumer goods.[6][5]