Recent from talks
Nothing was collected or created yet.
Retrosynthetic analysis
View on WikipediaRetrosynthetic analysis is a technique for solving problems in the planning of organic syntheses. This is achieved by transforming a target molecule into simpler precursor structures regardless of any potential reactivity/interaction with reagents. Each precursor material is examined using the same method. This procedure is repeated until simple or commercially available structures are reached. These simpler/commercially available compounds can be used to form a synthesis of the target molecule. Retrosynthetic analysis was used as early as 1917 in Robinson's Tropinone total synthesis.[1] Important conceptual work on retrosynthetic analysis was published by George Vladutz in 1963.[2][3] E.J. Corey formalized and popularized the concept from 1967 onwards in his article General methods for the construction of complex molecules and his book The Logic of Chemical Synthesis.[4][5][6][7]
The power of retrosynthetic analysis becomes evident in the design of a synthesis. The goal of retrosynthetic analysis is a structural simplification. Often, a synthesis will have more than one possible synthetic route. Retrosynthesis is well suited for discovering different synthetic routes and comparing them in a logical and straightforward fashion.[8] A database may be consulted at each stage of the analysis, to determine whether a component already exists in the literature. In that case, no further exploration of that compound would be required. If that compound exists, it can be a jumping point for further steps developed to reach a synthesis.
There are both academic and commercial groups developing retrosynthesis tools. With the growing application of machine learning and artificial intelligence in chemistry, many research groups, such as the Coley Group from MIT, and companies, such as Chemical.AI, Reaxys, etc., have started to integrate deep learning into the conventional rule-based approaches.
Definitions
[edit]- Disconnection
- A retrosynthetic step involving the breaking of a bond to form two (or more) synthons.
- Retron
- A minimal molecular substructure that enables certain transformations.
- Retrosynthetic tree
- A directed acyclic graph of several (or all) possible retrosyntheses of a single target.
- Synthon
- A fragment of a compound that assists in the formation of a synthesis, derived from that target molecule. A synthon and the corresponding commercially available synthetic equivalent are shown below:
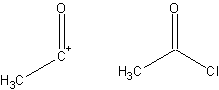
- Target
- The desired final compound.
- Transform
- The reverse of a synthetic reaction; the formation of starting materials from a single product.
Example
[edit]Shown below is a retrosynthetic analysis of phenylacetic acid:
In planning the synthesis, two synthons are identified. A nucleophilic "-COOH" group, and an electrophilic "PhCH2+" group. Both synthons do not exist as written; synthetic equivalents corresponding to the synthons are reacted to produce the desired product. In this case, the cyanide anion is the synthetic equivalent for the −COOH synthon, while benzyl bromide is the synthetic equivalent for the benzyl synthon.
The synthesis of phenylacetic acid determined by retrosynthetic analysis is thus:
- PhCH2Br + NaCN → PhCH2CN + NaBr
- PhCH2CN + 2 H2O → PhCH2COOH + NH3

In fact, phenylacetic acid has been synthesized from benzyl cyanide,[9] itself prepared by the analogous reaction of benzyl bromide with sodium cyanide.[10]
Strategies
[edit]Functional group strategies
[edit]Manipulation of functional groups can lead to significant reductions in molecular complexity.
Stereochemical strategies
[edit]Numerous chemical targets have distinct stereochemical demands. Stereochemical transformations (such as the Claisen rearrangement and Mitsunobu reaction) can remove or transfer the desired chirality thus simplifying the target.
Structure-goal strategies
[edit]Directing a synthesis toward a desirable intermediate can greatly narrow the focus of analysis. This allows bidirectional search techniques.
Transform-based strategies
[edit]The application of transformations to retrosynthetic analysis can lead to powerful reductions in molecular complexity. Unfortunately, powerful transform-based retrons are rarely present in complex molecules, and additional synthetic steps are often needed to establish their presence.
Topological strategies
[edit]The identification of one or more key bond disconnections may lead to the identification of key substructures or difficult to identify rearrangement transformations in order to identify the key structures.
- Disconnections that preserve ring structures are encouraged.
- Disconnections that create rings larger than 7 members are discouraged.
- Disconnection involves creativity.
See also
[edit]References
[edit]- ^ Robinson, R. (1917). "LXIII. A Synthesis of Tropinone". Journal of the Chemical Society, Transactions. 111: 762–768. doi:10.1039/CT9171100762.
- ^ Ugi, Ivar; Bauer, Johannes; Bley, Klemens; Dengler, Alf; Dietz, Andreas; Fontain, Eric; Gruber, Bernhard; Herges, Rainer; Knauer, Michael; Reitsam, Klaus; Stein, Natalie (1993). "Computer-Assisted Solution of Chemical Problems—The Historical Development and the Present State of the Art of a New Discipline of Chemistry". Angewandte Chemie International Edition in English. 32 (2): 201–227. doi:10.1002/anie.199302011.
- ^ Vléduts, G.É. (1963). "Concerning one system of classification and codification of organic reactions". Information Storage and Retrieval. 1 (2–3): 117–146. doi:10.1016/0020-0271(63)90013-5.
- ^ Corey, E. J. (1967). "General methods for the construction of complex molecules". Pure and Applied Chemistry. 14: 19–38. doi:10.1351/pac196714010019.
- ^ E. J. Corey, X-M. Cheng (1995). The Logic of Chemical Synthesis. New York: Wiley. ISBN 978-0-471-11594-6.
- ^ E. J. Corey (1988). "Retrosynthetic Thinking – Essentials and Examples". Chem. Soc. Rev. 17: 111–133. doi:10.1039/CS9881700111.
- ^ E. J. Corey (1991). "The Logic of Chemical Synthesis: Multistep Synthesis of Complex Carbogenic Molecules (Nobel Lecture)" (Reprint). Angewandte Chemie International Edition in English. 30 (5): 455–465. doi:10.1002/anie.199104553.
- ^ James Law et.al:"Route Designer: A Retrosynthetic Analysis Tool Utilizing Automated Retrosynthetic Rule Generation", Journal of Chemical Information and Modelling (ACS JCIM) Publication Date (Web): February 6, 2009; doi:10.1021/ci800228y, http://pubs.acs.org/doi/abs/10.1021/ci800228y
- ^ Wilhelm Wenner (1963). "Phenylacetamide". Organic Syntheses; Collected Volumes, vol. 4, p. 760.
- ^ Roger Adams; A. F. Thal (1941). "Benzyl Cyanide". Organic Syntheses
{{cite journal}}: CS1 maint: multiple names: authors list (link); Collected Volumes, vol. 1, p. 107.
External links
[edit]- ChemAIRS, AI-driven retrosynthesis tools by Chemical.AI
- Centre for Molecular and Biomolecular Informatics Archived 2005-02-12 at the Wayback Machine
- Presentation on ARChem Route Designer, ACS, Philadelphia, September 2008 for more info on ARChem see the SimBioSys pages.
- Manifold, Software freely available for academic users developed by PostEra Archived 2022-01-13 at the Wayback Machine
- Retrosynthesis planning tool: ICSynth by InfoChem Archived 2017-12-08 at the Wayback Machine
- Spaya, Software freely available proposed by Iktos Archived 2023-06-11 at the Wayback Machine
Retrosynthetic analysis
View on GrokipediaFundamentals
Definition and Principles
Retrosynthetic analysis is a systematic technique in organic chemistry used to plan the synthesis of complex molecules by mentally deconstructing a target molecule (TGT) into simpler precursor structures through the imagined reversal of synthetic reactions. This approach, also known as antithetic analysis, transforms the target into a sequence of progressively simpler intermediates that ultimately lead to readily available or commercially obtainable starting materials (SM). As defined by E. J. Corey, it constitutes "a problem-solving technique for transforming the structure of a synthetic target (TGT) molecule to a sequence of progressively simpler structures along a pathway which ultimately leads to simple or commercially available starting materials for a chemical synthesis."[1] The method emphasizes logical simplification rather than forward trial-and-error experimentation, providing a structured framework for devising efficient synthetic routes.[3] The fundamental principle of retrosynthetic analysis is to work backwards from the target molecule, applying disconnections—hypothetical bond cleavages that mirror the reverse of known synthetic transformations—to generate potential precursors. These disconnections are guided by chemical feasibility, focusing on substructural units that align with established reaction patterns, thereby reducing molecular complexity in a controlled manner. Corey described this as a process where "the target structure is subjected to a deconstruction process which corresponds to the reverse of a synthetic reaction, so as to convert that target structure to simpler precursor structures."[1] By iteratively applying such steps, chemists can explore a tree-like network of possible pathways, prioritizing those that maintain synthetic viability at each stage.[4] This backward-planning strategy is crucial for enabling the efficient design of multi-step syntheses, particularly for intricate natural products or pharmaceuticals, as it allows chemists to identify optimal routes that minimize steps, resources, and potential failures. Retrosynthetic analysis shifts the focus from empirical guessing to rational strategy, optimizing overall synthetic efficiency and convergence.[4] It forms the basis of a general logic for synthetic planning, as articulated by Corey, facilitating both manual and computational approaches to complex molecule construction.[1] In practice, the retrosynthetic process follows a basic flowchart: starting with the target molecule, performing a disconnection to yield immediate precursors, then recursively applying further disconnections to those precursors until simple, commercial starting materials are reached. This iterative deconstruction ensures a convergent pathway toward practical synthesis.[4]Historical Development
The origins of retrosynthetic analysis trace back to the early 20th century, when organic chemists began moving beyond trial-and-error approaches toward more systematic planning of syntheses, often drawing on insights from reaction mechanisms to anticipate synthetic routes.[1] Pioneering efforts, such as Robert Robinson's 1917 total synthesis of tropinone, implicitly employed backward-thinking strategies by identifying key bond disconnections based on known reactions, though without formal methodology.[5] Elias James Corey formalized retrosynthetic analysis as a structured technique in his 1967 paper, introducing the concept of systematically deconstructing target molecules into simpler precursors via retro-synthetic steps, represented by arrows pointing backward from products to reactants. This approach emphasized logical disconnection of bonds and functional group transformations, enabling efficient planning for complex molecules, as demonstrated in Corey's synthesis of longifolene published in 1961 and detailed further in 1964.[1] Corey's methodology rapidly gained traction, transforming organic synthesis from intuitive artistry to a disciplined science. In the 1970s, Corey extended retrosynthetic analysis through the development of the LHASA (Logic and Heuristics Applied to Synthetic Analysis) computer program, initiated in the late 1960s and first publicly demonstrated in 1969, which automated the generation of synthetic pathways using heuristic rules derived from retrosynthetic principles. LHASA allowed chemists to explore vast arrays of possible routes interactively, as described in key publications including a 1972 Journal of the American Chemical Society article, marking the integration of computational tools with human ingenuity in synthesis planning. Corey's contributions culminated in the 1990 Nobel Prize in Chemistry, awarded for his development of retrosynthetic analysis and its methodological impact on organic synthesis.[6] His seminal book, The Logic of Chemical Synthesis (1989), provided a comprehensive framework for applying retrosynthetic strategies, solidifying the approach as a cornerstone of the field. By the 1990s, retrosynthetic analysis had evolved through refinements in computational implementations, building on LHASA to incorporate more sophisticated databases of reactions and stereochemical considerations, facilitating broader application in academic and industrial synthesis without relying on emerging AI paradigms.Core Methodology
Disconnection Approach
The disconnection approach in retrosynthetic analysis involves the imaginary cleavage of a bond in the target molecule to generate simpler synthetic precursors through the application of a transform, which is the exact reverse of a known synthetic reaction.[4] This technique systematically reduces molecular complexity by identifying strategic bonds whose disconnection aligns with established synthetic pathways.[1] Disconnections are classified based on the position of the cleaved bond relative to functional groups in the target. A 1,1-disconnection breaks a bond adjacent to a single functional group, such as the reverse of a carbonyl addition where a tertiary alcohol or ketone is cleaved to a carbonyl compound and a carbanionic synthon.[4] In contrast, a 1,2-disconnection cleaves the bond between two adjacent functional groups or atoms within a functional group, as seen in the retrosynthesis of aldol products from β-hydroxy carbonyl compounds.[4] A 1,3-disconnection, meanwhile, involves breaking a bond two or three atoms removed from a functional group, corresponding to reactions like the Michael addition in 1,5-dicarbonyl systems.[4] For a disconnection to be valid, it must correspond to a known and reliable forward synthetic transform that simplifies the target structure by reducing its size, topological complexity, or number of stereocenters.[4] Additionally, valid disconnections require the presence of a retron—a structural subunit in the target that matches the transform—and prioritize simplicity by favoring convergent pathways over linear ones.[1] Heuristic rules further refine disconnection choices by emphasizing those that produce stable, commercially available, or easily synthesized synthons, which are the idealized reactive fragments resulting from the cleavage.[4] These rules also advise against disconnections that generate strained rings larger than seven members, uncorrectable stereocenters, or unstable intermediates, ensuring the retrosynthetic path remains practical.[4] As an illustrative example, consider a generic ketone target molecule of the form R–C(=O)–R'. Applying a 1,1-disconnection at the carbonyl carbon yields precursors such as an aldehyde (R–CHO) and an organometallic synthon (R'–M), which in the forward direction would react via nucleophilic addition to form the ketone. This disconnection highlights how the approach leverages common reactivity patterns for simplification.Target: R–C(=O)–R'
Disconnection: | (cleavage at C–R')
Precursors: R–C(=O)–H + ¯C–R' (synthons; M = metal)
Target: R–C(=O)–R'
Disconnection: | (cleavage at C–R')
Precursors: R–C(=O)–H + ¯C–R' (synthons; M = metal)
Synthons and Retrosynthetic Notation
In retrosynthetic analysis, synthons are defined as idealized, often charged molecular fragments that represent the reactive intermediates resulting from the disconnection of a target molecule, serving as synthetic equivalents to guide the identification of viable precursors. These fragments embody the polarity and reactivity patterns necessary for the corresponding forward synthetic reaction, allowing chemists to systematically explore bond-forming strategies without initially considering practical synthetic constraints. The concept of synthons was introduced by E. J. Corey to formalize the logical disconnection of complex structures into simpler components, emphasizing their role in antithetic (reverse) thinking. Synthons are classified based on their electronic nature, primarily as nucleophilic (electron-donor) or electrophilic (electron-acceptor) species, which mirrors the natural reactivity in organic transformations. Nucleophilic synthons act as electron-rich donors, while electrophilic ones function as electron-deficient acceptors, facilitating the pairing of complementary fragments during retrosynthetic planning. A key variant involves umpolung, or polarity reversal, where a synthon exhibits reactivity opposite to its typical behavior; for instance, an acyl anion equivalent serves as a nucleophilic synthon at the carbonyl carbon, enabling syntheses that would otherwise require incompatible polarities. This umpolung approach expands the scope of retrosynthetic disconnections by inverting functional group reactivities.[4][7] The retrosynthetic arrow provides a standardized symbolic notation to denote the backward transformation from a target structure to its precursors, typically represented as "⇒" or a similar double-headed arrow pointing leftward, distinguishing it from forward synthetic arrows. This notation underscores the iterative, hierarchical nature of retrosynthesis, where each step simplifies the molecular complexity toward commercially available starting materials. Complementing this is the retron, defined as the minimal substructural motif within the target molecule that matches the requirements for applying a specific synthetic transform, ensuring that disconnections are structurally feasible. Retrons often encompass functional groups, stereocenters, or ring systems that "key" the retrosynthetic operation.[4] Notation conventions in retrosynthetic analysis further enhance clarity and precision in representing these concepts. Disconnections are commonly illustrated with dashed or wavy lines across the cleaved bond in the target structure, visually indicating the site of potential bond formation in synthesis. Synthons are explicitly labeled with charges (e.g., positive for electrophilic, negative for nucleophilic) or polarity indicators to highlight their intended reactivity, while retrons may be bracketed or annotated to denote their enabling role. These conventions, rooted in systematic diagramming, facilitate the communication of retrosynthetic trees and the evaluation of synthetic routes.[4]Illustrative Examples
Simple Molecule Synthesis
Retrosynthetic analysis applied to simple molecules emphasizes fundamental disconnections that correspond to well-established synthetic transforms, allowing rapid identification of feasible routes from commercial precursors. A representative example is the synthesis of 1-phenylethanol, a secondary alcohol with the structure , which serves as an intermediate in various pharmaceutical and fragrance applications. The initial step involves a 1,1-disconnection at the bond between the carbinol carbon and the methyl group, transforming the target into benzaldehyde () as the electrophilic synthon and a methyl carbanion equivalent () as the nucleophilic synthon. This disconnection aligns with the general principle of carbonyl umpolung in retrosynthesis, where the alcohol functionality is traced back to an aldehyde precursor. In practice, the methyl nucleophile is realized as methylmagnesium bromide (), a readily prepared organometallic reagent.[8] To verify feasibility, the forward synthesis proceeds via nucleophilic addition of to benzaldehyde in anhydrous ether, followed by acidic workup to yield 1-phenylethanol in high efficiency (typically >90% yield under standard conditions). This reaction exemplifies a classic Grignard addition, tolerant of the aryl aldehyde and producing the desired C-C bond without over-addition issues common to ketones.[8] A two-level retrosynthetic tree for 1-phenylethanol is depicted below, illustrating the stepwise simplification to starting materials:Target: [1-Phenylethanol](/page/1-Phenylethanol) ($\ce{C6H5CH(OH)CH3}$)
|
+-- 1,1-Disconnection ([nucleophilic addition](/page/Nucleophilic_addition) transform)
|
+-- Precursor 1: [Benzaldehyde](/page/Benzaldehyde) ($\ce{C6H5CHO}$) [commercial availability]
|
+-- Precursor 2: $\ce{CH3^{-}}$ equivalent ($\ce{CH3MgBr}$)
|
+-- Further disconnection: $\ce{CH3Br}$ (or $\ce{CH3I}$) + Mg [both commercial]
Target: [1-Phenylethanol](/page/1-Phenylethanol) ($\ce{C6H5CH(OH)CH3}$)
|
+-- 1,1-Disconnection ([nucleophilic addition](/page/Nucleophilic_addition) transform)
|
+-- Precursor 1: [Benzaldehyde](/page/Benzaldehyde) ($\ce{C6H5CHO}$) [commercial availability]
|
+-- Precursor 2: $\ce{CH3^{-}}$ equivalent ($\ce{CH3MgBr}$)
|
+-- Further disconnection: $\ce{CH3Br}$ (or $\ce{CH3I}$) + Mg [both commercial]

