Recent from talks
Contribute something
Nothing was collected or created yet.
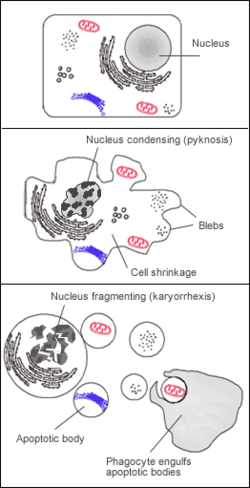
Karyorrhexis
View on Wikipedia

Karyorrhexis (from Greek κάρυον karyon, "kernel, seed, nucleus," and ῥῆξις rhexis, "bursting") is the destructive fragmentation of the cell nucleus that occurs in a dying cell.[1] It is characterized by the breakdown of the nuclear envelope and the dispersal of condensed chromatin into the cytoplasm.[2] The process is usually preceded by pyknosis (irreversible chromatin condensation) and followed by karyolysis (enzymatic dissolution of chromatin). It may occur during programmed cell death (apoptosis), cellular senescence, or necrosis. [citation needed]
In apoptosis, karyorrhexis is mediated by Ca2+- and Mg2+-dependent endonucleases, ensuring that nuclear fragments are packaged into apoptotic bodies and removed by phagocytosis. In necrosis, by contrast, nuclear fragmentation occurs in a less orderly fashion, leaving behind cellular debris that can contribute to tissue damage and inflammation.[3]
-
Morphological features of pyknosis and other forms of nuclear destruction
-
Microscopy of an apoptotic neutrophil with nuclear fragmentation (H&E stain)
Nuclear envelope dissolution
[edit]In the intrinsic pathway of apoptosis, cellular stressors such as oxidative stress activate pro-apoptotic members of the Bcl-2 protein family, leading to permeabilization of the mitochondrial outer membrane.[4] This releases cytochrome c into the cytoplasm, triggering a signaling cascade that culminates in the activation of multiple caspase enzymes.[4] Among these, caspase-6 cleaves nuclear lamina proteins such as lamin A/C, structural components that maintain the integrity of the nuclear envelope. Their cleavage facilitates the controlled dissolution of the nuclear envelope during apoptosis.[5]
Chromatin fragmentation
[edit]During karyorrhexis in apoptosis, nuclear DNA is cleaved in an orderly fashion by endonucleases such as caspase-activated DNase, producing discrete nucleosomal fragments.[6] This organization is possible because DNA has already undergone condensation during pyknosis, being tightly wrapped around histone proteins in repeating units of ≈180 bp. Activated endonucleases cleave the linker DNA between histones, generating short, regularly sized fragments that correspond to nucleosomal units.[7] These DNA fragments can be visualized by gel electrophoresis, where they produce a characteristic “ladder” pattern, a hallmark used to distinguish apoptosis from other forms of cell death.[8]
In other forms of cell death
[edit]In apoptosis, karyorrhexis is a controlled process in which caspases degrade lamin proteins, leading to the orderly breakdown of the nuclear envelope. In less regulated forms of cell death, such as necrosis, nuclear degradation occurs through different mechanisms. Necrotic cells are characterized by rupture of the plasma membrane, lack of caspase activation, and the induction of an inflammatory response.[3] Because necrosis is caspase-independent, the nucleus may remain intact during early stages before rupturing as a result of osmotic stress and membrane damage.
A specialized form of necrosis, necroptosis, involves a more regulated pathway but still results in plasma membrane rupture. Here, nuclear destabilization is mediated by the protease calpain, which cleaves lamins and promotes nuclear envelope breakdown.[3]
Unlike karyorrhexis in apoptosis, which generates apoptotic bodies subsequently removed by phagocytosis, karyorrhexis in necroptosis leads to the uncontrolled release of intracellular contents into the extracellular space, where they are cleared primarily through pinocytosis.[9]
Mechanism
[edit]Apoptotic pathways
[edit]Apoptosis, and the associated nuclear degradation through karyorrhexis, can be triggered by a variety of physiological and pathological stimuli. DNA damage, oxidative stress, hypoxia, and infections activate signaling cascades that converge on the intrinsic apoptotic pathway. This pathway may also be induced by external factors such as ethanol, which promotes activation of apoptosis-related proteins including BAX and caspases.[10]
In addition to intrinsic signals, activation of cell-surface death receptors such as CD95 can initiate the extrinsic apoptotic pathway, also resulting in caspase activation and nuclear envelope degradation.[5] In both pathways, executioner caspases, particularly caspase-3, cleave nuclear lamins and promote chromatin fragmentation, driving karyorrhexis.[3]
Necrotic pathways
[edit]In contrast to apoptosis, nuclear degradation during necrosis is a largely unregulated process. Necrotic cells are characterized by rupture of the plasma membrane, uncontrolled calcium influx, and activation of proteases such as calpain, which accelerate nuclear disintegration.[11] These features highlight the mechanistic differences between necrotic and apoptotic karyorrhexis.
Senescence and DNA damage response
[edit]The extent of DNA damage can also determine whether a cell undergoes apoptosis or enters cellular senescence. Senescence involves a permanent cessation of cell division and is typically observed after approximately 50 doublings in primary cells.[12]
One major cause of senescence is telomere shortening, which triggers a persistent DNA damage response (DDR). This response activates the kinases ATR and ATM, which in turn activate Chk1 and Chk2. These signaling events stabilize the transcription factor p53. When DNA damage is mild, p53 induces CIP proteins that inhibit CDKs, enforcing cell-cycle arrest. In cases of severe DNA damage, however, p53 activates apoptotic pathways, leading to caspase activity and nuclear envelope dissolution via karyorrhexis.[13]
Clinical significance
[edit]Karyorrhexis is a hallmark of cell death observed in a range of pathological conditions, including ischemia and neurodegenerative disorders. It has been documented in myocardial infarction and stroke, where nuclear fragmentation contributes to tissue damage during acute stress responses.[14] In obstetric pathology, placental vascular malperfusion has been linked to karyorrhexis and implicated in cases of fetal demise, reflecting its role in disrupted tissue homeostasis.[15]
In oncology, apoptotic karyorrhexis has a dual significance. On one hand, it contributes to controlled cell death and tumor suppression; on the other, resistance to apoptosis allows cancer cells to evade this process, promoting malignancy. Therapeutic strategies that target apoptotic pathways aim to restore nuclear degradation and trigger tumor regression.[16]
See also
[edit]References
[edit]- ^ Zamzami N, Kroemer G (September 1999). "Condensed matter in cell death". Nature. 401 (6749): 127–128. Bibcode:1999Natur.401..127Z. doi:10.1038/43591. PMID 10490018. S2CID 36673000.
- ^ Advances in Mutagenesis Research. Springer Science & Business Media. 2012. p. 11. ISBN 978-3-642-78193-3. Retrieved 11 November 2017.
- ^ a b c d Nikoletopoulou V, Markaki M, Palikaras K, Tavernarakis N (December 2013). "Crosstalk between apoptosis, necrosis and autophagy". Biochimica et Biophysica Acta (BBA) - Molecular Cell Research. 1833 (12): 3448–3459. doi:10.1016/j.bbamcr.2013.06.001. PMID 23770045.
- ^ a b Kaloni D, Diepstraten ST, Strasser A, Kelly GL (February 2023). "BCL-2 protein family: attractive targets for cancer therapy". Apoptosis. 28 (1–2): 20–38. doi:10.1007/s10495-022-01780-7. PMC 9950219. PMID 36342579.
- ^ a b Lindenboim L, Zohar H, Worman HJ, Stein R (2020-04-27). "The nuclear envelope: target and mediator of the apoptotic process". Cell Death Discovery. 6 (1) 29. doi:10.1038/s41420-020-0256-5. PMC 7184752. PMID 32351716.
- ^ Nagata S (April 2000). "Apoptotic DNA fragmentation". Experimental Cell Research. 256 (1): 12–18. doi:10.1006/excr.2000.4834. PMID 10739646.
- ^ Arends MJ, Morris RG, Wyllie AH (March 1990). "Apoptosis. The role of the endonuclease". The American Journal of Pathology. 136 (3): 593–608. PMC 1877493. PMID 2156431.
- ^ Gong J, Traganos F, Darzynkiewicz Z (May 1994). "A selective procedure for DNA extraction from apoptotic cells applicable for gel electrophoresis and flow cytometry". Analytical Biochemistry. 218 (2): 314–319. doi:10.1006/abio.1994.1184. PMID 8074286.
- ^ Wu Y, Dong G, Sheng C (September 2020). "Targeting necroptosis in anticancer therapy: mechanisms and modulators". Acta Pharmaceutica Sinica. B. 10 (9): 1601–1618. doi:10.1016/j.apsb.2020.01.007. PMC 7563021. PMID 33088682.
- ^ Fernández-Solà J (February 2020). "The Effects of Ethanol on the Heart: Alcoholic Cardiomyopathy". Nutrients. 12 (2): 572. doi:10.3390/nu12020572. PMC 7071520. PMID 32098364.
- ^ Priante G, Gianesello L, Ceol M, Del Prete D, Anglani F (July 2019). "Cell Death in the Kidney". International Journal of Molecular Sciences. 20 (14): 3598. doi:10.3390/ijms20143598. PMC 6679187. PMID 31340541.
- ^ Hayflick L, Moorhead PS (December 1961). "The serial cultivation of human diploid cell strains". Experimental Cell Research. 25 (3): 585–621. doi:10.1016/0014-4827(61)90192-6. PMID 13905658.
- ^ Surova O, Zhivotovsky B (August 2013). "Various modes of cell death induced by DNA damage". Oncogene. 32 (33): 3789–3797. doi:10.1038/onc.2012.556. PMID 23208502.
- ^ Zhang D, Jiang C, Feng Y, Ni Y, Zhang J (July 2020). "Molecular imaging of myocardial necrosis: an updated mini-review". Journal of Drug Targeting. 28 (6): 565–573. doi:10.1080/1061186X.2020.1725769. PMID 32037899.
- ^ Stanek J, Drach A (March 2022). "Placental CD34 immunohistochemistry in fetal vascular malperfusion in stillbirth". The Journal of Obstetrics and Gynaecology Research. 48 (3): 719–728. doi:10.1111/jog.15169. PMID 35092332.
- ^ Wong RS (September 2011). "Apoptosis in cancer: from pathogenesis to treatment". Journal of Experimental & Clinical Cancer Research. 30 (1) 87. doi:10.1186/1756-9966-30-87. PMC 3197541. PMID 21943236.
Karyorrhexis
View on GrokipediaFundamentals
Definition and Etymology
Karyorrhexis is the destructive fragmentation of the cell nucleus that occurs during cell death, characterized by the breakdown of the nuclear envelope and the dispersal of condensed chromatin into the cytoplasm as irregularly distributed basophilic nuclear fragments.[7][8] The term derives from the Greek words karyon, meaning "nucleus" or "kernel," and rhexis, meaning "bursting" or "rupture," reflecting the process of nuclear disintegration.[9] As a hallmark of irreversible cell death, karyorrhexis is typically observed under light microscopy as shrunken, fragmented nuclei that stain intensely with hematoxylin, distinguishing it from viable cells.[10] It follows pyknosis and precedes karyolysis in the sequence of nuclear changes during necrosis.[2]Role in Cell Death Pathways
Karyorrhexis occupies a central position as an intermediate stage in the nuclear alterations characteristic of classical necrosis, occurring after pyknosis—in which chromatin undergoes irreversible clumping and condensation—and before karyolysis, the final complete dissolution and fading of nuclear chromatin. This sequence reflects the progression from early reversible injury to irreversible cell demise in unregulated necrotic pathways, where cellular swelling and membrane rupture lead to inflammatory responses.[11][12] In apoptotic cell death, karyorrhexis manifests as a highly regulated nuclear fragmentation, integral to the controlled disassembly of the cell without eliciting inflammation, in stark contrast to the passive, damage-induced process in necrosis. Here, it follows chromatin condensation and contributes to the formation of apoptotic bodies containing nuclear fragments, ensuring orderly clearance by phagocytes. Similarly, in necroptosis—a programmed necrotic pathway—karyorrhexis appears as a morphological hallmark akin to classical necrosis, signaling commitment to lytic cell death, while in senescence-associated processes, it serves as an indicator of terminal progression toward death in irreparably damaged cells.[12][13][14] The process of karyorrhexis exhibits evolutionary conservation across eukaryotic organisms, underscoring its fundamental role in cell death cascades as a mechanism to sequester and eliminate damaged genetic material, thereby preventing its propagation and maintaining genomic integrity at the organismal level.[5][15]Morphological Features
Nuclear Envelope Dissolution
Karyorrhexis begins with the progressive loss of nuclear membrane integrity, which compromises the barrier between the nucleus and cytoplasm, resulting in the blurring of nuclear boundaries observable under microscopy. This initial breakdown disrupts the structural continuity of the nuclear envelope, allowing for the subsequent disintegration of nuclear architecture. The process marks a critical early event in nuclear fragmentation during cell death, distinguishing it from reversible cellular stresses. Visualization of nuclear envelope dissolution in karyorrhexis relies on established microscopy techniques that reveal these morphological alterations. Electron microscopy highlights fragmented remnants of the nuclear envelope, including dilated pore spaces and invaginations, providing high-resolution evidence of structural disassembly. In contrast, light microscopy of histological sections shows indistinct nuclear margins and loss of clear boundary definition, often appearing as hazy or irregular outlines around the condensing chromatin. These imaging approaches confirm the envelope's breakdown as a hallmark of karyorrhexis, preceding the dispersal of nuclear material.[17][18]Chromatin Condensation and Fragmentation
In karyorrhexis, chromatin undergoes a progressive transformation beginning with hypercondensation following pyknosis, where the chromatin aggregates into a dense, shrunken mass within the nucleus.[19] This stage is marked by intense basophilia due to the compaction of nuclear material, reflecting irreversible cellular damage in necrotic processes.[19] Subsequently, the hypercondensed chromatin fragments into discrete, basophilic chunks that disperse irregularly throughout the cytoplasm, appearing as small pyknotic masses under light microscopy.[7] These fragments stain darkly with basic dyes such as hematoxylin, highlighting their dense composition and aiding histological identification of karyorrhectic cells.[11] The dispersal is facilitated by concurrent nuclear envelope dissolution, which permits the chromatin pieces to scatter without containment.[7] Biochemically, this fragmentation results from non-specific DNA cleavage into irregular pieces, producing a heterogeneous smear pattern on gel electrophoresis rather than the uniform nucleosomal units seen in apoptotic DNA degradation.[20] Such irregular breaks disrupt the overall nuclear architecture, contributing to the complete loss of nuclear integrity and the cell's terminal disintegration in necrosis.[19]Molecular Mechanisms
Apoptotic Pathways
Karyorrhexis in apoptosis is initiated through two primary regulated pathways: the intrinsic mitochondrial pathway, triggered by cellular stresses such as DNA damage or oxidative stress, and the extrinsic death receptor pathway, activated by extracellular signals from ligands binding to death receptors like Fas or TNF receptors.[5][21] Both pathways converge on the activation of initiator caspases (such as caspase-8 in the extrinsic route or caspase-9 in the intrinsic route), which subsequently trigger a caspase cascade involving effector caspases like caspase-3 and caspase-6.[22][15] This enzymatic amplification ensures the orderly dismantling of nuclear components, culminating in karyorrhexis as a hallmark of programmed cell death.[23] A critical step in nuclear envelope breakdown during karyorrhexis is the cleavage of lamin A/C by caspase-6, which disrupts the nuclear lamina and facilitates fragmentation of the nucleus into discrete pieces.[24] Studies in caspase-6-deficient models demonstrate that this cleavage is essential for proper nuclear disassembly, as uncleaved lamin A/C inhibits the progression to karyorrhexis.[25] Concurrently, caspase-3 activates DNase caspase-activated DNase (CAD) by cleaving its inhibitor ICAD, allowing CAD to enter the nucleus and fragment chromatin into nucleosomal units of approximately 180 base pairs.[26] This internucleosomal cleavage produces the characteristic DNA "ladder" pattern observable on agarose gel electrophoresis, distinguishing apoptotic karyorrhexis from other cell death modes.[27][28] Additional chromatin cleavage is mediated by calcium- and magnesium-dependent endonucleases, which further degrade DNA into smaller fragments following CAD activity.[5] These enzymes contribute to the complete nuclear fragmentation while the process remains controlled, preventing release of intracellular contents.[29] The resulting nuclear fragments are packaged into membrane-bound apoptotic bodies, which are rapidly phagocytosed by neighboring cells or macrophages, ensuring a non-inflammatory resolution of cell death.[23]Necrotic and Necroptotic Pathways
In classical necrosis, karyorrhexis arises from uncontrolled cellular injury leading to plasma membrane rupture and massive influx of extracellular calcium ions into the cytoplasm. This calcium overload activates calcium-dependent proteases known as calpains, which proteolytically cleave nuclear lamina proteins such as lamins, compromising the integrity of the nuclear envelope and facilitating nuclear fragmentation. Concurrently, the elevated calcium levels promote the activity of endonucleases like DNase I, resulting in irregular, non-specific cleavage of genomic DNA and contributing to the disorganized nuclear breakdown characteristic of karyorrhexis.[30][31][32] In necroptosis, a form of regulated necrosis, karyorrhexis is triggered through the receptor-interacting protein kinase (RIPK) pathway involving RIPK1, RIPK3, and mixed lineage kinase domain-like (MLKL) proteins. Upon activation, RIPK3 phosphorylates MLKL, inducing its oligomerization and translocation to the plasma membrane, where it disrupts membrane integrity and causes permeabilization, allowing ion influx including calcium. This secondary calcium elevation activates calpains, which mediate nuclear envelope degradation and chromatin fragmentation, while the process also promotes the release of inflammatory cytokines such as IL-1β through NLRP3 inflammasome activation. Unlike the uniform DNA processing in apoptotic pathways, this leads to chaotic nuclear dispersal.[33][34]00058-7.pdf) The DNA fragments generated during both necrotic and necroptotic karyorrhexis are heterogeneous in size, ranging from high molecular weight smears to random breaks without a defined pattern, dispersing into the cytoplasm and exacerbating secondary inflammation via damage-associated molecular pattern (DAMP) release. This irregular fragmentation contrasts with more structured processes in other cell death modes and underscores the pro-inflammatory nature of these pathways.[35][36]Senescence-Associated Processes
Cellular senescence is often triggered by progressive telomere shortening, which limits cell proliferation to approximately 50 divisions in human fibroblasts, known as the Hayflick limit.[37] This telomere attrition generates DNA damage signals that activate the p53/p21 pathway, leading to cell cycle arrest and the formation of persistent DNA damage foci marked by γ-H2AX.[38] These foci represent sites of uncapped telomeres recognized as double-strand breaks, sustaining a chronic DNA damage response without progressing to full cell death. In senescent cells, nuclear architecture undergoes reorganization, including the formation of senescence-associated heterochromatin foci (SAHF), which are compact, macroH2A-enriched domains that repress proliferation-associated genes.[39] SAHF assembly contributes to partial nuclear envelope instability, as increased nuclear fragility promotes blebbing and localized disruptions without complete dissolution.[40] This results in chromatin fragmentation, manifesting as DNA breaks and micronuclei, yet cells remain viable and do not undergo full apoptosis.[40] Such changes overlap briefly with apoptotic DNA damage triggers but persist in a non-lethal state.[41] This incomplete karyorrhexis-like process, characterized by ongoing nuclear blebbing and unresolved DNA breaks, sustains the senescence-associated secretory phenotype (SASP), where proinflammatory factors are secreted to influence the tissue microenvironment.[41] Persistent nuclear alterations in senescent cells thus drive tissue dysfunction and aging by promoting chronic inflammation and impairing regenerative capacity.[41]Comparisons to Related Processes
Distinctions from Pyknosis and Karyolysis
Karyorrhexis represents a distinct mid-stage nuclear alteration in the progression of necrosis, characterized by the active fragmentation of the previously condensed chromatin mass into discrete, irregularly shaped nuclear fragments that are visible under light microscopy as shrunken, basophilic particles scattered throughout the cytoplasm. This process follows pyknosis, the initial irreversible condensation and clumping of chromatin into a dense, homogeneous, basophilic mass, which shrinks the nucleus and increases its density, often appearing as a small, dark-staining structure. Unlike pyknosis, which primarily involves compaction without breakdown, karyorrhexis involves enzymatic and mechanical disruption leading to irreversible nuclear disintegration.[19][11] In contrast, karyolysis occurs as the terminal stage, involving the complete enzymatic dissolution of the nuclear chromatin through digestion by endonucleases such as DNase γ, resulting in a pale, faded nucleus that loses its affinity for nuclear stains like hematoxylin, eventually appearing as an empty or ghost-like structure. This differs markedly from karyorrhexis, where distinct, fragmented nuclear pieces remain identifiable for a period, allowing for clear microscopic differentiation before full dissolution. Karyolysis thus signifies advanced autolysis or heterolysis, with the chromatin being progressively degraded into soluble nucleotides, unlike the particulate remnants in karyorrhexis.[42][43] Within the necrotic sequence, these changes unfold in a temporal progression observable via microscopy: pyknosis emerges early, typically within 12-24 hours post-injury as seen in models like myocardial infarction, marking the onset of irreversible damage; karyorrhexis follows as the intermediate phase, becoming prominent between 1-3 days with visible fragmentation; and karyolysis dominates in the late stage, extending to several days where nuclear outlines blur and dissolve entirely. This timeline varies by tissue type, injury severity, and environmental factors but generally spans hours to days, aiding pathologists in estimating injury duration.[44]Nuclear Changes in Other Cell Death Modes
In autophagy, a process often associated with cellular self-digestion rather than overt cell death, the nucleus is typically spared from significant morphological alterations, with the formation of perinuclear vacuoles and autophagosomes occurring without chromatin fragmentation or nuclear breakdown.[45] Karyorrhexis, characterized by the nuclear fragmentation prominent in necrotic cell death, is absent in pure autophagic responses unless the process progresses to secondary necrosis, where nuclear disintegration may ensue as a terminal event.[46] Ferroptosis, an iron-dependent form of regulated cell death driven by lipid peroxidation, exhibits minimal nuclear involvement, preserving nuclear integrity without condensation, margination, or fragmentation that defines karyorrhexis.[47] This contrasts sharply with the prominent nuclear pyknosis and subsequent karyorrhexis observed in necrosis, as ferroptotic cells prioritize cytoplasmic and mitochondrial damage over nuclear changes.[48] In pyroptosis, an inflammasome-mediated lytic cell death, gasdermin family proteins form plasma membrane pores that lead to cellular swelling and rupture, but the nucleus generally remains intact without the fragmentation typical of karyorrhexis.[49] Instead, late-stage pyroptotic events may include nuclear rounding or random DNA fragmentation, though these do not progress to the disseminated nuclear pieces seen in necrosis; hybrid features, such as partial karyorrhexis, can emerge in mixed death modes involving pyroptosis and necroptosis.[50][51]Clinical and Pathological Relevance
Associations with Diseases
Karyorrhexis, as a hallmark of nuclear fragmentation in necrotic and apoptotic cell death, is prominently observed in ischemic conditions such as myocardial infarction, where it appears in the histology of affected cardiac tissue around 3 to 4 days post-infarction, coinciding with neutrophil infiltration and early phagocytosis by macrophages.[52] In stroke pathology, particularly in ischemic infarcts, karyorrhexis contributes to the nuclear breakdown in neuronal necrosis, often following pyknosis in the evolving infarct core, serving as an indicator of irreversible tissue damage in both adult and perinatal cases.[53] Hypoxia-induced necrosis, a common sequela of ischemia, frequently exhibits karyorrhexis in vulnerable brain regions like the hippocampus and basal ganglia, with histological studies showing its prevalence in perinatal hypoxic-ischemic injuries where fragmented nuclei correlate with the extent of neuronal loss.[2][54] In neurodegenerative diseases, karyorrhexis is linked to nuclear fragmentation driven by apoptotic pathways, as seen in Alzheimer's disease where cleaved caspase-3 activation leads to karyorrhexis alongside tau-related pathology, contributing to neuronal loss in affected brain regions.[55] Similarly, in Parkinson's disease, evidence of apoptosis in the subventricular zone from animal models includes karyorrhexis, reflecting dopaminergic neuron degeneration and supporting its role as a biomarker of progressive cellular demise.[56] Beyond ischemia and neurodegeneration, karyorrhexis features in other pathological contexts, such as placental malperfusion leading to fetal demise, where clustered villous stromal-vascular karyorrhexis indicates fetal vascular obstruction and reduced perfusion, often correlating with adverse outcomes like intrauterine growth restriction or stillbirth.[57] In infections causing acute necrosis, such as Kikuchi-Fujimoto disease, karyorrhexis accompanies patchy necrotic areas in lymph nodes, highlighting its utility in identifying histiocytic necrotizing processes.[58] Toxin-induced cell death, including apoptosis from mitochondrial toxin exposures like 3-nitropropionic acid, displays karyorrhexis during the fragmentation phase in affected cells.[59] In cancer, evasion of karyorrhexis-associated apoptosis enables tumor cell survival and progression, with dysregulated anti-apoptotic pathways allowing neoplastic cells to resist nuclear fragmentation and promote uncontrolled proliferation.[60]Implications for Diagnosis and Therapy
Karyorrhexis, as a hallmark of nuclear fragmentation in regulated cell death pathways, plays a key role in clinical diagnostics through targeted assays that detect associated molecular changes. Immunohistochemical staining for cleaved lamins, particularly lamin A/C fragmented by caspase-6, serves as a reliable marker for apoptotic karyorrhexis in tissue biopsies, enabling quantification of caspase-dependent cell death in tumors and enabling correlation with prognostic factors.[61][62] Similarly, the TUNEL assay detects DNA strand breaks characteristic of karyorrhexis in apoptotic and necrotic processes, facilitating its use in biopsies for assessing cell death in conditions like kidney injury or skin disorders, though it requires combination with morphological evaluation to distinguish pathways.[63][64] In oncology, the mitosis-karyorrhexis index (MKI), which counts mitotic figures and karyorrhectic nuclei per 5,000 tumor cells, provides independent prognostic value in neuroblastoma, stratifying risk and guiding therapy intensity, with high MKI (>200/5,000 cells) associated with poorer outcomes independent of age and tumor grade.[65] Recent advances include artificial intelligence-based models for automated morphological classification and prediction of MKI status in neuroblastoma, enhancing diagnostic efficiency and prognostic assessment.[66] Therapeutically, promoting karyorrhexis via apoptosis induction offers a strategy for targeting cancer cells, exemplified by BH3 mimetics like venetoclax, which inhibit BCL-2 to unleash pro-apoptotic proteins, leading to mitochondrial outer membrane permeabilization and subsequent nuclear fragmentation in tumors such as chronic lymphocytic leukemia and acute myeloid leukemia.[15] Ongoing research on BCL-2 family modulation continues to explore enhancements in apoptotic nuclear disassembly for improved efficacy against resistant cancers, including solid tumors. Conversely, inhibiting necroptotic karyorrhexis mitigates inflammatory damage in ischemic conditions; RIPK1 kinase blockers, such as necrostatin-1, prevent RIPK1-mediated necroptosis, reducing neuronal and myocardial injury post-ischemia-reperfusion by blocking downstream MLKL activation and nuclear breakdown.[67][68] In senescence-associated aging diseases, senolytics such as dasatinib and quercetin selectively induce apoptosis in senescent cells, potentially triggering karyorrhexis to clear dysfunctional cells and alleviate pathologies like neurodegeneration, though clinical translation remains investigational.[69]References
- https://www.sciencedirect.com/topics/[neuroscience](/page/Neuroscience)/karyorrhexis