Recent from talks
Nothing was collected or created yet.
Pulse pressure
View on Wikipedia| Pulse pressure | |
|---|---|
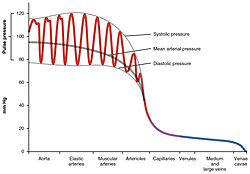 Pulse pressure variation (PPV) in different arteries and veins |
Pulse pressure is the difference between systolic and diastolic blood pressure.[1] It is measured in millimeters of mercury (mmHg). It represents the force that the heart generates each time it contracts. Healthy pulse pressure is around 40 mmHg.[1][2] A pulse pressure that is consistently 60 mmHg or greater is likely to be associated with disease, and a pulse pressure of 50 mmHg or more increases the risk of cardiovascular disease.[1][3] Pulse pressure is considered low if it is less than 25% of the systolic. (For example, if the systolic pressure is 120 mmHg, then the pulse pressure would be considered low if it were less than 30 mmHg, since 30 is 25% of 120.)[2] A very low pulse pressure can be a symptom of disorders such as congestive heart failure.[3]
Calculation
[edit]Pulse pressure is calculated as the difference between the systolic blood pressure and the diastolic blood pressure.[3][4]
The systemic pulse pressure is approximately proportional to stroke volume, or the amount of blood ejected from the left ventricle during systole (pump action) and inversely proportional to the compliance (similar to elasticity) of the aorta.[5]
- Systemic pulse pressure (most commonly measured at the brachial artery in the upper arm using a Sphygmomanometer) = Psystolic − Pdiastolic
- e.g. normal 120 mmHg – 80 mmHg = 40 mmHg[3]
- low: 100 mmHg − 80 mmHg = 20 mmHg
- high: 160 mmHg − 80 mmHg = 80 mmHg
- Pulmonary pulse pressure is normally much lower than systemic blood pressure due to the higher compliance of the pulmonary system compared to the arterial circulation.[6] It is measured by right heart catheterization or may be estimated by transthoracic echocardiography. Normal pulmonary artery pressure is 8 mmHg–20 mmHg at rest.[7]
- e.g. normal 15mmHg – 8mmHg = 7mmHg
- high 25mmHg – 10mmHg = 15mmHg
Values and variation
[edit]Low (narrow) pulse pressure
[edit]A pulse pressure is considered abnormally low if it is less than 25% of the systolic value.[2] If the pulse pressure is extremely low, i.e. 25 mmHg or less, it may indicate low stroke volume, as in congestive heart failure.[3]
The most common cause of a low (narrow) pulse pressure is a drop in left ventricular stroke volume. In trauma, a low or narrow pulse pressure suggests significant blood loss.[8]
A narrow pulse pressure is also caused by aortic stenosis.[3] This is due to the decreased stroke volume in aortic stenosis.[9] Other conditions that can cause a narrow pulse pressure include blood loss (due to decreased blood volume), and cardiac tamponade (due to decreased filling time). In the majority of these conditions, systolic pressure decreases, while diastolic pressure remains normal, leading to a narrow pulse pressure.[9]
In the Postural Orthostatic Tachycardia Syndrome it is postulated that declining venous return reduces stroke volume and frequently results in low pulse pressure. In extreme cases, patients experience a drop in pulse pressure to 0 mm Hg upon standing, rendering them practically pulseless while upright. This condition leads to significant morbidity, as many affected individuals struggle to remain standing.[10]
High (wide) pulse pressure
[edit]Consistently high
[edit]A pulse pressure of 50 mmHg or more can increase the risk of heart disease, heart rhythm disorders, stroke and other cardiovascular diseases and events. Higher pulse pressures are also thought to play a role in eye and kidney damage from diseases such as diabetes.[3] There are currently no drugs approved to lower pulse pressure, but some antihypertensive drugs have been shown to modestly lower pulse pressure, while other drugs used for hypertension can actually have the counterproductive side effect of increasing resting pulse pressure.[11]
The aorta has the highest compliance in the arterial system due in part to a relatively greater proportion of elastin fibers versus smooth muscle and collagen. This serves to dampen the pulsatile ejection fraction of the left ventricle, thereby reducing the initial systolic pulse pressure, but slightly raising the subsequent diastolic phase. If the aorta becomes rigid, stiff and inextensible because of disorders, such as arteriosclerosis, atherosclerosis or elastin defects (in connective tissue diseases), the pulse pressure would be higher due to less compliance of the aorta.[12]
In hypertensive patients, a high pulse pressure can often be an indicator of conduit artery stiffness (stiffness of the major arteries).[13] When the arterial walls are stiffer (less compliant), the heart has to beat harder to overcome the resistance from the stiff arteries, resulting in an increased pulse pressure.[14]
Other conditions that can lead to a high pulse pressure include aortic regurgitation,[15] aortic sclerosis, severe iron-deficiency anemia (due to decreased blood viscosity), arteriosclerosis (due to loss of arterial compliance), and hyperthyroidism[15] (due to increased systolic pressure), or arteriovenous malformation, among others.[9] In aortic regurgitation, the aortic valve insufficiency results in the backward flow of blood (regurgitation) that is ejected during systole, and its return to the left ventricle during diastole. This increases the systolic blood pressure, and decreases the diastolic blood pressure, leading to a widened pulse pressure.[9][3]
A high pulse pressure combined with bradycardia and an irregular breathing pattern is associated with increased intracranial pressure, a condition called Cushing's triad seen in people after head trauma with increased intracranial pressure.[16]
Common causes of widening pulse pressure include:[3]
- Anemia
- Aortic dissection
- Atherosclerosis[15]
- Arteriovenous fistula[15]
- Chronic aortic regurgitation
- Aortic root aneurysm[17]
- Aortic root dilation[17]
- Beri beri[15]
- Distributive shock[15]
- Endocarditis
- Fever
- Heart block
- Increased intracranial pressure[16][15]
- Patent ductus arteriosus
- Pregnancy[15]
- Thyrotoxicosis[15]
From exercise
[edit]For most individuals, during aerobic exercise, the systolic pressure progressively increases while the diastolic pressure remains about the same, thereby widening the pulse pressure. These pressure changes facilitate an increase in stroke volume and cardiac output at a lower mean arterial pressure, enabling greater aerobic capacity and physical performance. The diastolic drop reflects a reduced systemic vascular resistance of the muscle arterioles in response to the exercise.[18]
Clinical significance
[edit]Pulse pressure has implications for both cardiovascular disease as well as many non-cardiovascular diseases. Even in people without other risk factors for cardiovascular disease, a consistently wide pulse pressure remains a significant independent predictor of all-cause, cardiovascular, and, in particular, coronary mortality.[19] There is a positive correlation between high pulse pressure and markers of inflammation, such as c-reactive protein.[20]
Cardiovascular disease and pulse pressure
[edit]Awareness of the effects of pulse pressure on morbidity and mortality is lacking relative to the awareness of the effects of elevated systolic and diastolic blood pressure. However, pulse pressure has consistently been found to be a stronger independent predictor of cardiovascular events, especially in older populations, than has systolic, diastolic, or mean arterial pressure.[3][13] This increased risk has been observed in both men and women and even when no other cardiovascular risk factors are present. The increased risk also exists even in cases in which high pulse pressure is caused by diastolic pressure decreasing over time while systolic remains steady or even slightly decreases.[21][19]
A meta-analysis in 2000 showed that a 10 mmHg increase in pulse pressure was associated with a 20% increased risk of cardiovascular mortality, and a 13% increase in risk for all coronary end points. The study authors also noted that, while risks of cardiovascular end points do increase with higher systolic pressures, at any given systolic blood pressure the risk of major cardiovascular end points increases, rather than decreases, with lower diastolic levels.[22] This suggests that interventions that lower diastolic pressure without also lowering systolic pressure (and thus lowering pulse pressure) could actually be counterproductive.[9]
People who simultaneously have a resting diastolic pressure of less than 60 mmHg and a pulse pressure of greater than 60 mmHg have double the risk of subclinical myocardial ischaemia and a risk of stroke that is 5.85 times greater than normal.[23] For such patients, it may be dangerous to target a peripheral systolic pressure below 120 mmHg due to the fact that this could cause the diastolic blood pressure in the cerebral cortex in the brain to become so low that perfusion (blood flow) is insufficient, leading to white matter lesions. Nearly all coronary perfusion and more than half of cerebral perfusion occurs during diastole, thus a diastolic pressure that is too low can cause harm to both the heart and the brain.[24]
Increased pulse pressure is also a risk factor for the development of atrial fibrillation.[25]
Effects of medications on pulse pressure
[edit]There are no drugs currently approved to lower pulse pressure. Although some anti-hypertensive drugs currently on the market may have the effect of modestly lowering pulse pressure, others may actually have the counterproductive effect of increasing pulse pressure. Among classes of drugs currently on the market, a 2020 review stated that thiazide diuretics and long‐acting nitrates are the two most effective at lowering pulse pressure.[15]
It has been hypothesized that vasopeptidase inhibitors and nitric oxide donors may be useful at lowering pulse pressure in patients with elevated pulse pressure by increasing the distensibility of the large arteries.[22][13] There is evidence that glyceryl trinitrate, a nitric oxide donor, may be effective at lowering both pulse pressure and overall blood pressure in patients with acute and sub-acute stroke.[26]
A 2001 randomized, placebo-controlled trial of 1,292 males, compared the effects of hydrochlorothiazide (a thiazide diuretic), atenolol (a beta-blocker), captopril (an ACE inhibitor), clonidine (a central α2-agonist), diltiazem (a calcium channel blocker), and prazosin (an α1-blocker) on pulse pressure and found that, after one year of treatment, hydrochlorothiazide was the most effective at lowering pulse pressure, with an average decrease of 8.6 mmHg. Captopril and atenolol were equal as least effective, with an average decrease of 4.1 mmHg. Clonidine (decrease of 6.3 mmHg), diltiazem (decrease of 5.5 mmHg), and prazosin (decrease of 5.0 mmHg) were intermediate.[11]
Pulse pressure and sepsis
[edit]Diastolic blood pressure falls during the early stages of sepsis, causing a widening of pulse pressure. If sepsis becomes severe and hemodynamic compromise advances, the systolic pressure also decreases, causing a narrowing of pulse pressure.[27] A pulse pressure of over 70 mmHg in patients with sepsis is correlated with an increased chance of survival. A widened pulse pressure is also correlated with an increased chance that someone with sepsis will benefit from and respond to IV fluids.[28]
See also
[edit]References
[edit]- ^ a b c Homan TD, Bordes SJ, Cichowski E (12 July 2022). "Physiology, Pulse Pressure". StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing. PMID 29494015. Retrieved 2019-07-21 – via NCBI Bookshelf.
- ^ a b c Liaw SY, Scherpbier A, Klainin-Yobas P, Rethans JJ (September 2011). "A review of educational strategies to improve nurses' roles in recognizing and responding to deteriorating patients". International Nursing Review. 58 (3): 296–303. doi:10.1111/j.1466-7657.2011.00915.x. PMID 21848774.
- ^ a b c d e f g h i j "Pulse pressure". Cleveland Clinic. 28 July 2021. Retrieved 10 February 2023.
If you check your blood pressure regularly and notice you have an unusually wide (60 mmHg or more) or narrow pulse pressure (where your pulse pressure is less than one-quarter of the top blood pressure number), you should schedule an appointment with your healthcare provider to talk about it. [...] Pulse pressures of 50 mmHg or more can increase your risk of heart disease, heart rhythm disorders, stroke and more. Higher pulse pressures are also thought to play a role in eye and kidney damage from diseases like diabetes.
- ^ Weber CO (24 February 2022). Shah A (ed.). "Pulse Pressure". about.com. Archived from the original on 17 February 2009.
- ^ Klabunde RE (29 March 2007). "Arterial pulse pressure". Cardiovascular Physiology Concepts. Archived from the original on 16 May 2008.
- ^ Blacher J, Evans A, Arveiler D, et al. (January 2010). "Residual cardiovascular risk in treated hypertension and hyperlipidaemia: the PRIME Study" (PDF). Journal of Human Hypertension. 24 (1): 19–26. doi:10.1038/jhh.2009.34. PMID 19474798. S2CID 24409022.
- ^ Parasuraman S, Walker S, Loudon BL, Gollop ND, Wilson AM, Lowery C, Frenneaux MP (September 2016). "Assessment of pulmonary artery pressure by echocardiography-A comprehensive review". International Journal of Cardiology. Heart & Vasculature. 12: 45–51. doi:10.1016/j.ijcha.2016.05.011. PMC 5454185. PMID 28616542.
- ^ Advanced Trauma Life Support (ATLS) Program for Doctors. Chicago, IL: American College of Surgeons. 2008. p. 58. ISBN 978-1-880696-31-6.
- ^ a b c d e Homan, Travis D.; Bordes, Stephen J.; Cichowski, Erica (2023), "Physiology, Pulse Pressure", StatPearls, Treasure Island (FL): StatPearls Publishing, PMID 29494015, retrieved 2023-10-25
- ^ Homan TD, Bordes SJ, Cichowski E (2024), "Physiology, Pulse Pressure", StatPearls, Treasure Island (FL): StatPearls Publishing, PMID 29494015, archived from the original on 2024-04-21, retrieved 2024-04-22
- ^ a b Cushman, William C.; Materson, Barry J.; Williams, David W.; Reda, Domenic J. (1 Oct 2001). "Pulse Pressure Changes With Six Classes of Antihypertensive Agents in a Randomized, Controlled Trial". Hypertension. 38 (4): 953–957. doi:10.1161/hy1001.096212. PMID 11641316. S2CID 19241872.
- ^ See also: Central aortic blood pressure
- ^ a b c Mitchell, Gary F.; Izzo, Joseph L.; Lacourcière, Yves; Ouellet, Jean-Pascal; Neutel, Joel; Qian, Chunlin; Kerwin, Linda J.; Block, Alan J.; Pfeffer, Marc A. (25 Jun 2002). "Omapatrilat Reduces Pulse Pressure and Proximal Aortic Stiffness in Patients With Systolic Hypertension". Circulation. 105 (25). Ovid Technologies (Wolters Kluwer Health): 2955–2961. doi:10.1161/01.cir.0000020500.77568.3c. ISSN 0009-7322. PMID 12081987. S2CID 7092379.
- ^ "What is Arterial Stiffness?". News-Medical.net. 23 Nov 2009. Retrieved 18 Nov 2023.
- ^ a b c d e f g h i j Tang, KS; Medeiros, ED; Shah, AD (November 2020). "Wide pulse pressure: A clinical review". Journal of Clinical Hypertension (Greenwich, Conn.). 22 (11): 1960–1967. doi:10.1111/jch.14051. PMC 8029839. PMID 32986936.
- ^ a b Dinallo S, Waseem M (2022). "Cushing reflex". StatPearls. Treasure Island (FL): StatPearls Publishing. PMID 31747208.
- ^ a b Nataf P, Lansac E (September 2006). "Dilation of the thoracic aorta: medical and surgical management". Heart. 92 (9): 1345–1352. doi:10.1136/hrt.2005.074781. PMC 1861150. PMID 16908722.
- ^ Bertovic DA, Waddell TK, Gatzka CD, Cameron JD, Dart AM, Kingwell BA (June 1999). "Muscular strength training is associated with low arterial compliance and high pulse pressure". Hypertension. 33 (6). Dallas, Texas: 1385–91. doi:10.1161/01.hyp.33.6.1385. PMID 10373221.
- ^ a b Benetos, Athanase; Safar, Michel; Rudnichi, Annie; Smulyan, Harold; Richard, Jacques-Lucien; Ducimetière, Pierre; Guize, Louis (1997). "Pulse Pressure". Hypertension. 30 (6). Ovid Technologies (Wolters Kluwer Health): 1410–1415. doi:10.1161/01.hyp.30.6.1410. ISSN 0194-911X. PMID 9403561.
- ^ Abramson, Jerome L.; Vaccarino, Viola (2006). "Pulse Pressure and Inflammatory Process in Atherosclerosis". Atherosclerosis, Large Arteries and Cardiovascular Risk. Advances in Cardiology. Vol. 44. Basel: KARGER. pp. 223–233. doi:10.1159/000096733. ISBN 3-8055-8176-9. PMID 17075211.
- ^ Franklin, Stanley S.; Khan, Shehzad A.; Wong, Nathan D.; Larson, Martin G.; Levy, Daniel (27 Jul 1999). "Is Pulse Pressure Useful in Predicting Risk for Coronary Heart Disease?". Circulation. 100 (4). Ovid Technologies (Wolters Kluwer Health): 354–360. doi:10.1161/01.cir.100.4.354. ISSN 0009-7322. PMID 10421594.
- ^ a b Blacher J, Staessen JA, Girerd X, Gasowski J, Thijs L, Liu L, et al. (April 2000). "Pulse pressure not mean pressure determines cardiovascular risk in older hypertensive patients". Archives of Internal Medicine. 160 (8): 1085–1089. doi:10.1001/archinte.160.8.1085. PMID 10789600.
- ^ Spence, J David (10 Jun 2020). "Risk from low blood pressure in frail older adults: diastolic pressure and pulse pressure are important". Age and Ageing. 50 (6). Oxford University Press (OUP): e5 – e6. doi:10.1093/ageing/afaa084. ISSN 0002-0729. PMID 32520996.
- ^ Spence, J David; Müller, Lucas O; Blanco, Pablo J (11 Sep 2021). "How to identify which patients should not have a systolic blood pressure target of <120 mmHg". European Heart Journal. 43 (6). Oxford University Press (OUP): 538–539. doi:10.1093/eurheartj/ehab552. ISSN 0195-668X. PMID 34508627.
- ^ Staerk L, Sherer JA, Ko D, Benjamin EJ, Helm RH (April 2017). "Atrial Fibrillation: Epidemiology, Pathophysiology, and Clinical Outcomes". Circulation Research. 120 (9): 1501–1517. doi:10.1161/CIRCRESAHA.117.309732. PMC 5500874. PMID 28450367.
- ^ Gray, LJ; Sprigg, N; Rashid, PA; Willmot, MR; Bath, PM (2006). "Effect of nitric oxide donors on blood pressure and pulse pressure in acute and subacute stroke". Journal of Stroke and Cerebrovascular Diseases. 15 (6). Centre for Reviews and Dissemination (UK): 245–249. doi:10.1016/j.jstrokecerebrovasdis.2006.06.002. PMID 17904083. Retrieved 18 Nov 2023.
- ^ Khilnani P, Singhi S, Lodha R, Santhanam I, Sachdev A, Chugh K, Jaishree M, Ranjit S, Ramachandran B, Ali U, Udani S, Uttam R, Deopujari S (January 2010). "Pediatric Sepsis Guidelines: Summary for resource-limited countries". Indian J Crit Care Med. 14 (1): 41–52. doi:10.4103/0972-5229.63029. PMC 2888329. PMID 20606908.
- ^ Al-Khalisy H, Nikiforov I, Jhajj M, Kodali N, Cheriyath P (11 December 2015). "A widened pulse pressure: a potential valuable prognostic indicator of mortality in patients with sepsis". J Community Hosp Intern Med Perspect. 5 (6) 29426. doi:10.3402/jchimp.v5.29426. PMC 4677588. PMID 26653692.

