Recent from talks
Nothing was collected or created yet.
Aromatic sulfonation
View on WikipediaIn organic chemistry, aromatic sulfonation is a reaction in which a hydrogen atom on an arene is replaced by a sulfonic acid (−SO2OH) group. Together with nitration and chlorination, aromatic sulfonation is a widely used electrophilic aromatic substitutions.[1] Aryl sulfonic acids are used as detergents, dye, and drugs.
Stoichiometry and mechanism
[edit]
Typical conditions involve heating the aromatic compound with sulfuric acid:[2]
- C6H6 + H2SO4 → C6H5SO3H + H2O
Sulfur trioxide or its protonated derivative is the actual electrophile in this electrophilic aromatic substitution.
To drive the equilibrium, dehydrating agents such as thionyl chloride can be added:[2]
- C6H6 + H2SO4 + SOCl2 → C6H5SO3H + SO2 + 2 HCl
Historically, mercurous sulfate has been used to catalyze the reaction.[3]
Chlorosulfuric acid is also an effective agent:
- C6H6 + HSO3Cl → C6H5SO3H + HCl
In contrast to aromatic nitration and most other electrophilic aromatic substitutions this reaction is reversible. Sulfonation takes place in concentrated acidic conditions and desulfonation is the mode of action in a dilute hot aqueous acid. The reaction is very useful in protecting the aromatic system because of this reversibility. Due to their electron withdrawing effects, sulfonate protecting groups can be used to prevent electrophilic aromatic substitution. They can also be installed as directing groups to affect the position where a substitution may take place.[4]
Specialized sulfonation methods
[edit]Many method have been developed for introducing sulfonate groups aside from direction sulfonation.
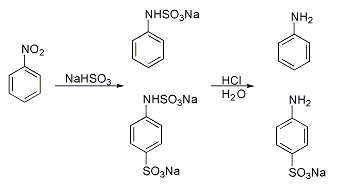
A classic named reaction is the Piria reaction (Raffaele Piria, 1851) in which nitrobenzene is treated with a metal bisulfite forming an aminosulfonic acid as a result of combined nitro group reduction and sulfonation.[2][5][6]
In the Tyrer sulfonation process (1917),[7] at some time of technological importance, benzene vapor is led through a vessel containing 90% sulfuric acid the temperature of which is increased from 100 to 180°C. Water and benzene are continuously removed and the benzene fed back to the vessel. In this way an 80% yield is obtained.

Applications
[edit]
Aromatic sulfonic acids are intermediates in the preparation of dyes and many pharmaceuticals. Sulfonation of anilines lead to a large group of sulfa drugs.
Sulfonation of polystyrene is used to make sodium polystyrene sulfonate, a common ion exchange resin for water softening.
See also
[edit]References
[edit]- ^ March, Jerry (1985). Advanced Organic Chemistry: Reactions, Mechanisms, and Structure (3rd ed.). New York: Wiley. ISBN 9780471854722. OCLC 642506595..
- ^ a b c Lindner, Otto; Rodefeld, Lars (2000). "Benzenesulfonic Acids and Their Derivatives". Ullmann's Encyclopedia of Industrial Chemistry. doi:10.1002/14356007.a03_507. ISBN 978-3-527-30385-4.
- ^ Sabatier, Paul (1922). Catalysis in Organic Chemistry. Translated by Reid, E. Emmet. New York, NY: Van Nostrand. p. 2.
- ^ T.W. Graham Solomons: Organic Chemistry, 11th Edition, Wiley, Hoboken, NJ, 2013, p. 676, ISBN 978-1-118-13357-6.
- ^ Piria, Raffaele (1851). "Über einige Produkte der Einwirkung des schwefligsäuren Ammoniaks auf Nitronaphtalin". Annalen der Chemie und Pharmacie. 78: 31–68. doi:10.1002/jlac.18510780103. ISSN 0075-4617.
- ^ The Piria Reaction. I. The Overall Reaction W. H. Hunter, Murray M. Sprung J. Am. Chem. Soc., 1931, 53 (4), pp 1432–1443 doi:10.1021/ja01355a037.
- ^ U.S. patent 1,210,725
- ^ Siegfried Hauptmann: Organische Chemie, 2nd Edition, VEB Deutscher Verlag für Grundstoffindustrie, Leipzig, 1985, p. 511, ISBN 3-342-00280-8.
Aromatic sulfonation
View on GrokipediaFundamentals
Overview and Importance
Aromatic sulfonation is a fundamental electrophilic aromatic substitution (EAS) reaction in which a hydrogen atom on an aromatic ring, or arene, is replaced by a sulfonic acid group (-SO₃H).[2] This process introduces a strongly electron-withdrawing substituent that significantly influences the electronic properties of the aromatic system, distinguishing it from other EAS reactions like nitration or halogenation.[5] The reaction was first reported in 1834 by German chemist Eilhard Mitscherlich, who obtained benzenesulfonic acid by treating benzene with fuming sulfuric acid.[6] During the 19th century, aromatic sulfonation played a pivotal role in the burgeoning field of synthetic dyes, enabling the production of water-soluble colorants essential for the textile industry and marking a key advancement in organic synthesis. Aromatic sulfonation holds broad industrial significance due to its versatility in manufacturing surfactants, detergents, dyes, pharmaceuticals, and polymers, where the sulfonic acid group imparts desirable solubility and reactivity.[4] Unlike many EAS reactions, such as nitration, sulfonation is reversible under acidic conditions, allowing the sulfonic acid group to serve as a temporary directing or blocking group in synthetic sequences.[7] This reversibility, combined with the group's strong meta-directing and deactivating effects, makes it invaluable for controlling regioselectivity in polysubstituted arenes. The reaction applies to a wide scope of aromatic substrates, including activated arenes like phenols and deactivated ones like nitrobenzene, with regioselectivity governed by existing substituents: electron-donating groups direct ortho/para, while electron-withdrawing groups favor meta substitution.[5]Stoichiometry and Reversibility
The stoichiometry of aromatic sulfonation typically involves the replacement of one hydrogen atom on the aromatic ring with a sulfonic acid group, following a 1:1 molar ratio between the arene and the sulfonating agent. For benzene, the reaction with sulfuric acid proceeds asyielding benzenesulfonic acid and water.[8] This balanced equation extends to general aromatic hydrocarbons (ArH), where
with the position of sulfonation influenced by the substituents on the ring.[8] To enhance the reaction rate and minimize side products, dehydrating agents such as sulfur trioxide (SO₃) or chlorosulfonic acid (HSO₃Cl) are employed, which avoid water formation or facilitate its removal. With SO₃, the sulfonation of benzene is
proceeding rapidly and exothermically in anhydrous conditions.[8] Similarly, chlorosulfonic acid reacts as
producing hydrogen chloride as a byproduct and allowing for controlled introduction of the sulfonic acid group.[8] Aromatic sulfonation is reversible, with desulfonation achieved by heating the sulfonic acid in dilute aqueous acid conditions, typically at 100–120°C, to shift the equilibrium toward the parent arene. The reverse reaction is represented as
where the sulfonic acid group is removed, regenerating the aromatic ring.[9] In concentrated sulfuric acid, the low water concentration drives the equilibrium toward sulfonation by Le Chatelier's principle, as water is a product that dilutes the medium.[8] Thermodynamically, the Gibbs free energy change (ΔG) for sulfonation is governed primarily by the water concentration in the reaction medium. Since sulfonation is exothermic and produces water, higher water levels favor desulfonation by Le Chatelier's principle, particularly under dilute conditions. Elevated temperatures also promote desulfonation as the reverse process is endothermic.[8][10] This reversibility makes the sulfonic acid group (-SO₃H) valuable as a temporary blocking group in multistep syntheses, where it directs subsequent electrophilic substitutions to desired positions before being selectively removed.[9]


