Recent from talks
Nothing was collected or created yet.
Vanilloid
View on WikipediaThe vanilloids are compounds which possess a vanillyl group. They include vanillyl alcohol, vanillin, vanillic acid, acetovanillon, vanillylmandelic acid, homovanillic acid, capsaicin, etc. Isomers are the isovanilloids.
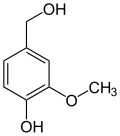
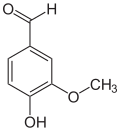

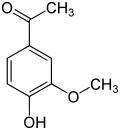
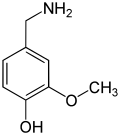
vanillyl alcohol vanillin vanillic acid acetovanillon Vanillylamine Capsaicin
A number of vanilloids, most notably capsaicin, bind to the transient receptor potential vanilloid type 1 (TRPV1) receptor, an ion channel which naturally responds to noxious stimuli such as high temperatures and acidic pH.[1] This action is responsible for the burning sensation experienced after eating spicy peppers. Endogenously generated chemicals that trigger the TRPV1 channel of the vanilloids class are referred to as endovanilloids[2] including anandamide, 20-hydroxyeicosatetraenoic acid (20-HETE),[3] N-arachidonoyl dopamine (NADA) and N-oleoyl-dopamine (CID 5282106 from PubChem).[4]
Fatty acid amide hydrolase (FAAH), is a crucial enzyme for endovanilloid, and the N-acylethanolamines (NAEs), catabolism at TRPV1, and other cannabinoid receptors.[5]
Outside the food industry vanilloids such as nonivamide are used commercially in pepper spray formulations.
Other vanilloids which act at TRPV1 include resiniferatoxin and olvanil.[6]
References
[edit]- ^ Pingle, SC; Matta, JA; Ahern, GP (2007). Capsaicin receptor: TRPV1 a promiscuous TRP channel. Handbook of Experimental Pharmacology. Vol. 179. pp. 155–171. doi:10.1007/978-3-540-34891-7_9. ISBN 978-3-540-34889-4. PMID 17217056.
- ^ Van Der Stelt M, Di Marzo V (2004). "Endovanilloids. Putative endogenous ligands of transient receptor potential vanilloid 1 channels". Eur J Biochem. 271 (10): 1827–34. doi:10.1111/j.1432-1033.2004.04081.x. PMID 15128293.
- ^ Hamers A, Primus CP, Whitear C, Kumar NA, Masucci M, Montalvo Moreira SA; et al. (2022). "20-hydroxyeicosatetraenoic acid (20-HETE) is a pivotal endogenous ligand for TRPV1-mediated neurogenic inflammation in the skin". Br J Pharmacol. 179 (7): 1450–1469. doi:10.1111/bph.15726. PMID 34755897. S2CID 243939400.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ De Petrocellis L, Chu CJ, Moriello AS, Kellner JC, Walker JM, Di Marzo V (2004). "Actions of two naturally occurring saturated N-acyldopamines on transient receptor potential vanilloid 1 (TRPV1) channels". Br J Pharmacol. 143 (2): 251–6. doi:10.1038/sj.bjp.0705924. PMC 1575334. PMID 15289293.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Silva, M.; Martins, D.; Charrua, A.; Piscitelli, F.; Tavares, I.; Morgado, C.; Di Marzo, V. (2016-08-01). "Endovanilloid control of pain modulation by the rostroventromedial medulla in an animal model of diabetic neuropathy". Neuropharmacology. 107: 49–57. doi:10.1016/j.neuropharm.2016.03.007. ISSN 0028-3908. PMID 26965218.
- ^ Carlson, Neil R.; Birkett, Melissa A. (2017). Physiology of Behavior (12 ed.). Pearson. p. 212. ISBN 9780134320823.
Literature
[edit]- Lee, Jeewoo; Uk Kang, Sang; Yeon Kim, Su; Eun Kim, Sung; Joon Jo, Yeong; Kim, Sunghoon (2001). "Vanilloid and Isovanilloid Analogues as Inhibitors of Methionyl-tRNA and Isoleucyl-tRNA Synthetases" (PDF). Bioorganic & Medicinal Chemistry Letters. 11 (8): 965–968. doi:10.1016/S0960-894X(01)00096-8. PMID 11327601.[permanent dead link]








