Recent from talks
Nothing was collected or created yet.
Adherens junction
View on Wikipedia| Adherens junction | |
|---|---|
| Details | |
| Identifiers | |
| Latin | junctio adhaesionis |
| MeSH | D022005 |
| TH | H1.00.01.1.02002 |
| FMA | 67400 |
| Anatomical terminology | |

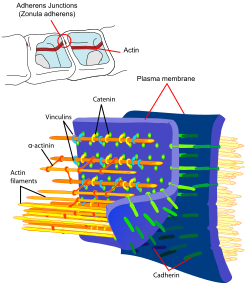
In cell biology, adherens junctions (or zonula adherens, intermediate junction, or "belt desmosome"[1]) are protein complexes that occur at cell–cell junctions and cell–matrix junctions in epithelial and endothelial tissues,[2] usually more basal than tight junctions. An adherens junction is defined as a cell junction whose cytoplasmic face is linked to the actin cytoskeleton. They can appear as bands encircling the cell (zonula adherens) or as spots of attachment to the extracellular matrix (focal adhesion).
Adherens junctions uniquely disassemble in uterine epithelial cells to allow the blastocyst to penetrate between epithelial cells.[3]
A similar cell junction in non-epithelial, non-endothelial cells is the fascia adherens. It is structurally the same, but appears in ribbonlike patterns that do not completely encircle the cells. One example is in cardiomyocytes.
Proteins
[edit]Adherens junctions are composed of the following proteins:[4]
- cadherins. The cadherins are a family of transmembrane proteins that form homodimers in a calcium-dependent manner with other cadherin molecules on adjacent cells.
- p120 (sometimes called delta catenin) binds the juxtamembrane region of the cadherin.
- γ-catenin or gamma-catenin (plakoglobin) binds the catenin-binding region of the cadherin.
- α-catenin or alpha-catenin binds the cadherin indirectly via β-catenin or plakoglobin and links the actin cytoskeleton with cadherin. Significant protein dynamics are thought to be involved.[5]
Models
[edit]Adherens junctions were, for many years, thought to share the characteristic of anchor cells through their cytoplasmic actin filaments.[citation needed]
Adherens junctions may serve as a regulatory module to maintain the actin contractile ring with which it is associated in microscopic studies.[citation needed]
See also
[edit]References
[edit]- ^ Pardo, JV, Craig, SW (1979). "alpha-Actinin localization in the junctional complex of intestinal epithelial cells". J Cell Biol. 80 (1): 203–210. doi:10.1083/jcb.80.1.203. PMC 2110298. PMID 370125. Retrieved 15 October 2014.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Guo, Renyong; Sakamoto, Hiroshi; Sugiura, Shigeki (October 2006). "Endothelial Cell Motility Is Compatible With Junctional Integrity". Journal of Cellular Physiology. 211 (2): 327–335. doi:10.1002/jcp.20937. PMID 17167782. S2CID 11590025.
- ^ Dowland S, Madawala R, Lindsay L, Murphy C (2016). "The adherens junction is lost during normal pregnancy but not during ovarian hyperstimulated pregnancy". Acta Histochemica. 118 (2): 137–143. doi:10.1016/j.acthis.2015.12.004. PMID 26738975.
- ^ Ferreri DM, Vincent PA (2008). "Signaling to and through the Endothelial Adherens Junction". In LaFlamme SE, Kowalczyck AP (eds.). Cell Junctions: Adhesion, Development, and Disease. Wiley VCH. ISBN 978-3-527-31882-7.
- ^ Farago B, Nicholl ID, Wang S, Cheng X, Callaway DJ, Bu Z (March 30, 2021). "Activated nanoscale actin-binding domain motion in the catenin-cadherin complex revealed by neutron spin echo spectroscopy". Proc Natl Acad Sci USA. 118 (13) e2025012118. Bibcode:2021PNAS..11825012F. doi:10.1073/pnas.2025012118. PMC 8020631. PMID 33753508.
External links
[edit]- MBInfo - Adherens Junction
- MBInfo - Adherens Junction Assembly
- Adherens+Junctions at the U.S. National Library of Medicine Medical Subject Headings (MeSH)
- Histology image: 20502loa – Histology Learning System at Boston University