Recent from talks
Nothing was collected or created yet.
2-Bromopropane
View on Wikipedia | |||
 | |||
| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
2-Bromopropane[2] | |||
| Other names
Isopropyl bromide[1]
| |||
| Identifiers | |||
3D model (JSmol)
|
|||
| 741852 | |||
| ChEMBL | |||
| ChemSpider | |||
| ECHA InfoCard | 100.000.778 | ||
| EC Number |
| ||
| MeSH | 2-bromopropane | ||
PubChem CID
|
|||
| RTECS number |
| ||
| UNII | |||
| UN number | 2344 | ||
CompTox Dashboard (EPA)
|
|||
| |||
| |||
| Properties | |||
| C3H7Br | |||
| Molar mass | 122.993 g·mol−1 | ||
| Appearance | Colorless liquid | ||
| Density | 1.31 g mL−1 | ||
| Melting point | −89.0 °C; −128.1 °F; 184.2 K | ||
| Boiling point | 59 to 61 °C; 138 to 142 °F; 332 to 334 K | ||
| 3.2 g L−1 (at 20 °C) | |||
| log P | 2.136 | ||
| Vapor pressure | 32 kPa (at 20 °C) | ||
Henry's law
constant (kH) |
1.0 μmol Pa−1 mol−1 | ||
Refractive index (nD)
|
1.4251 | ||
| Viscosity | 0.4894 mPa s (at 20 °C) | ||
| Thermochemistry | |||
Heat capacity (C)
|
135.6 J K mol−1 | ||
Std enthalpy of
formation (ΔfH⦵298) |
−129 kJ mol−1 | ||
Std enthalpy of
combustion (ΔcH⦵298) |
−2.0537–−2.0501 MJ mol−1 | ||
| Hazards | |||
| GHS labelling: | |||
 
| |||
| Danger | |||
| H225, H360, H373 | |||
| P210, P308+P313 | |||
| NFPA 704 (fire diamond) | |||
| Flash point | 19 °C (66 °F; 292 K) | ||
| Related compounds | |||
Related alkanes
|
|||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||
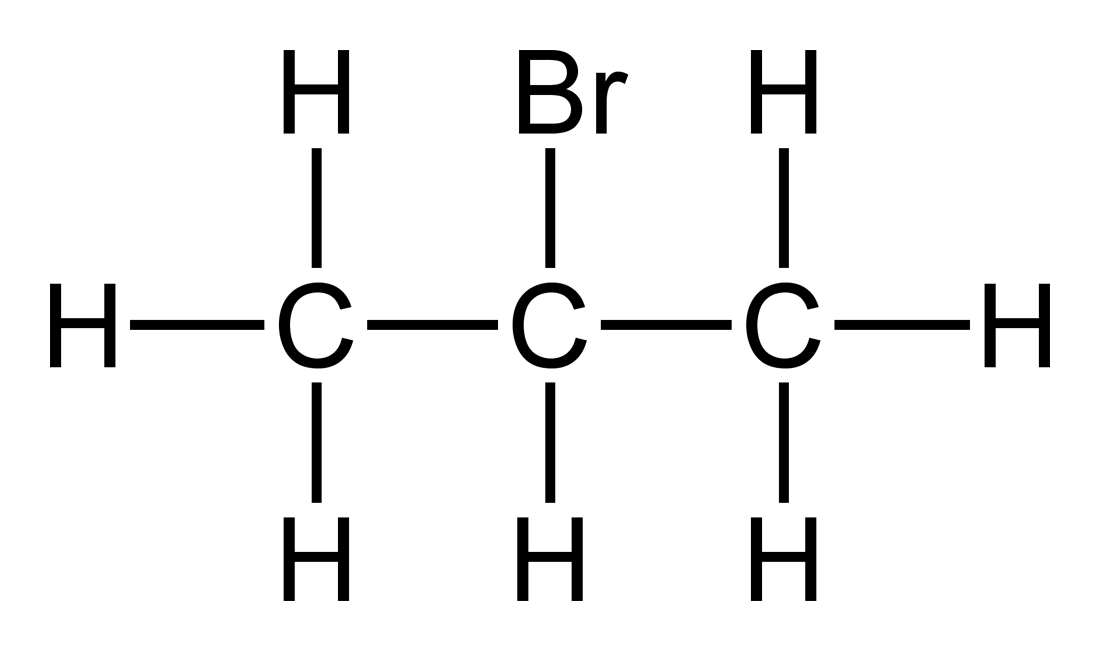
2-Bromopropane, also known as isopropyl bromide and 2-propyl bromide, is the halogenated hydrocarbon with the formula CH3CHBrCH3. It is a colorless liquid. It is used for introducing the isopropyl functional group in organic synthesis. 2-Bromopropane is prepared by heating isopropanol with hydrobromic acid.[3]
Preparation
[edit]2-Bromopropane is commercially available. It may be prepared in the ordinary manner of alkyl bromides, by reacting isopropanol with phosphorus and bromine,[4] or with phosphorus tribromide.[5]
Safety
[edit]Short-chain alkyl halides are often carcinogenic.
The bromine atom is at the secondary position, which allows the molecule to undergo dehydrohalogenation easily to give propene, which escapes as a gas and can rupture closed reaction vessels. When this reagent is used in base catalyzed reactions, potassium carbonate should be used in place of sodium or potassium hydroxide.
Further reading
[edit]- Max Gergel, “Excuse Me Sir, Would You Like to Buy a Kilo of Isopropyl Bromide?” Pierce Chemical Co. (1979). (story of start-up chemical company).
References
[edit]- ^ Armarego, Wilfred L.F.; Li Lin Chai, Christina (2013). Purification of laboratory chemicals (7th ed.). Butterworth-Heinemann. p. 176. ISBN 9780123821621.
- ^ "2-bromopropane - Compound Summary". PubChem Compound. USA: National Center for Biotechnology Information. 27 March 2005. Identification. Retrieved 15 June 2012.
- ^ "Monograph 6526". Merck Index of Chemicals and Drugs.
- ^ Oliver Kamm and C. S. Marvel (1941). "Alkyl and alkylene bromides". Organic Syntheses; Collected Volumes, vol. 1, p. 25.
- ^ C. R. Noller and R. Dinsmore (1943). "Isobutyl bromide". Organic Syntheses; Collected Volumes, vol. 2, p. 358.
2-Bromopropane
View on GrokipediaPhysical and Chemical Properties
2-Bromopropane has a molecular weight of 122.99 g/mol, a density of 1.31 g/mL at 25 °C, a boiling point of 59 °C, and a melting point of -89 °C.[1][2][3] Its flash point is 19 °C, making it highly flammable, and it has low solubility in water (0.3 g/100 mL) but is miscible with many organic solvents.[2][4] Chemically, it undergoes typical reactions of secondary alkyl bromides, including nucleophilic substitution (SN1 and SN2) and elimination to form propene.[1]Preparation
2-Bromopropane is commonly prepared by the reaction of isopropyl alcohol (isopropanol) with hydrobromic acid, often involving heating to facilitate the substitution.[2] This method yields the compound through protonation of the alcohol followed by bromide ion attack.[1]Uses
In organic synthesis, 2-bromopropane serves primarily as an alkylating agent to introduce the isopropyl group into molecules, such as in the preparation of ligands or other intermediates.[1][3] It is also employed as a solvent in industrial cleaning processes and as an intermediate in the production of amines and organometallic compounds.[2] Historically, it has been used in electronics manufacturing for cleaning, though such applications have raised health concerns.[5]Safety and Toxicity
2-Bromopropane is classified as highly flammable (Hazard Class 3, UN 2344) and poses risks of skin, eye, and respiratory irritation upon exposure.[2][6] It is a reproductive toxicant (Category 1B), capable of damaging fertility and the unborn child, and has been linked to hematopoietic disorders.[2][1] The International Agency for Research on Cancer (IARC) classifies it as probably carcinogenic to humans (Group 2A), based on sufficient evidence of carcinogenicity in experimental animals.[7] This classification stems from studies showing tumors in rodents and human epidemiological data.[7] A notable 1995 incident in South Korea involved occupational exposure to high levels of 2-bromopropane in a cleaning facility, resulting in an outbreak of reproductive and hematopoietic disorders among workers, which heightened global awareness of its hazards.[8] Exposure limits are recommended below 10 ppm (TWA) to minimize risks.[5]Structure and identification
Molecular structure
2-Bromopropane has the molecular formula C₃H₇Br and the condensed structural formula CH₃CHBrCH₃, in which a bromine atom is covalently bonded to the central carbon of a linear three-carbon chain flanked by two methyl groups.[1] This arrangement positions the bromine on a secondary carbon atom, classifying the compound as a secondary alkyl bromide, where the halogen-bearing carbon is directly attached to two alkyl groups. The molecule is also known by its common name, isopropyl bromide.[1] The central carbon atom in 2-bromopropane adopts a tetrahedral geometry due to sp³ hybridization, resulting in bond angles of approximately 109.5° around this atom. The C-Br bond length measures 1.957 Å, as determined by microwave spectroscopy.[9] In the Simplified Molecular Input Line Entry System (SMILES) notation, the structure is represented as CC(C)Br, reflecting the branched propane backbone with bromine substitution.[1] This compound exists as one of two isomers with the formula C₃H₇Br; the other is 1-bromopropane (CH₃CH₂CH₂Br), a primary alkyl bromide where bromine attaches to a terminal carbon, leading to distinct chemical behaviors despite sharing the same molecular formula.Nomenclature
2-Bromopropane is the systematic name according to the International Union of Pure and Applied Chemistry (IUPAC) nomenclature for halogenated hydrocarbons, where the parent chain is propane and the bromine substituent is located at the second carbon atom. This naming convention prioritizes the longest carbon chain and assigns the lowest possible number to the halogen substituent. Commonly, it is referred to as isopropyl bromide or 2-propyl bromide, names derived from the isopropyl group attached to the bromine atom, a traditional approach in organic chemistry for simple alkyl halides.[3] These alternative designations highlight its role as a secondary alkyl bromide and have been used historically in chemical literature to denote the branched structure corresponding to CH₃CHBrCH₃. Unique identifiers for 2-bromopropane include the CAS Registry Number 75-26-3, which uniquely catalogs the substance in chemical databases, and the European Community (EC) Number 200-855-1, assigned by the European Chemicals Agency for regulatory purposes.[10] Additionally, its International Chemical Identifier (InChI) is InChI=1S/C3H7Br/c1-3(2)4/h3H,1-2H3, providing a standardized textual representation of its molecular structure for computational and database applications.[1]Physical and thermodynamic properties
Appearance and phase behavior
2-Bromopropane is a colorless to slightly yellow liquid at room temperature.[1] Its molar mass is 122.993 g/mol.[1] The compound has a density of 1.31 g/mL at 20 °C.[4] It exhibits a low melting point of -89.0 °C, indicating it remains in the liquid phase under typical ambient conditions, and a boiling point ranging from 59 to 61 °C at standard pressure.[3] These phase transition temperatures highlight its volatility as a small alkyl halide. The flash point of 2-bromopropane is 19 °C, underscoring its flammability.[6] Its vapor pressure is 31.5 kPa at 25 °C, further contributing to its ease of evaporation and potential for vapor-phase hazards.[11]| Property | Value | Conditions |
|---|---|---|
| Molar mass | 122.993 g/mol | - |
| Density | 1.31 g/mL | 20 °C |
| Melting point | -89.0 °C | - |
| Boiling point | 59–61 °C | 101.3 kPa |
| Flash point | 19 °C | Closed cup |
| Vapor pressure | 31.5 kPa | 25 °C |