Recent from talks
Nothing was collected or created yet.
Bromoethane
View on Wikipedia
| |||
| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
Bromoethane[2] | |||
| Other names | |||
| Identifiers | |||
3D model (JSmol)
|
|||
| 1209224 | |||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
| ECHA InfoCard | 100.000.751 | ||
| EC Number |
| ||
| KEGG | |||
| MeSH | bromoethane | ||
PubChem CID
|
|||
| RTECS number |
| ||
| UNII | |||
| UN number | 1891 | ||
CompTox Dashboard (EPA)
|
|||
| |||
| |||
| Properties | |||
| C2H5Br | |||
| Molar mass | 108.966 g·mol−1 | ||
| Appearance | Colorless liquid | ||
| Odor | ether-like | ||
| Density | 1.46 g mL−1 | ||
| Melting point | −120 to −116 °C; −184 to −177 °F; 153 to 157 K | ||
| Boiling point | 38.0 to 38.8 °C; 100.3 to 101.8 °F; 311.1 to 311.9 K | ||
| 1.067 g/100 mL (0 °C) 0.914 g/100 mL (20 °C) 0.896 g/100 mL (30 °C) | |||
| Solubility | miscible with ethanol, ether, chloroform, organic solvents | ||
| log P | 1.809 | ||
| Vapor pressure | 51.97 kPa (at 20 °C) | ||
Henry's law
constant (kH) |
1.3 μmol Pa−1 kg−1 | ||
| −54.70·10−6 cm3/mol | |||
Refractive index (nD)
|
1.4225 | ||
| Viscosity | 402 Pa.s (at 20 °C) | ||
| Thermochemistry | |||
Heat capacity (C)
|
105.8 J K−1 mol−1 | ||
Std enthalpy of
formation (ΔfH⦵298) |
−97.6–93.4 kJ mol−1 | ||
| Hazards | |||
| GHS labelling: | |||
  
| |||
| Danger | |||
| H225, H302, H332, H351 | |||
| P210, P281 | |||
| NFPA 704 (fire diamond) | |||
| Flash point | −23 °C (−9 °F; 250 K) | ||
| 511 °C (952 °F; 784 K) | |||
| Explosive limits | 6.75–11.25% | ||
| Lethal dose or concentration (LD, LC): | |||
LD50 (median dose)
|
1.35 g kg−1 (oral, rat) | ||
LC50 (median concentration)
|
26,980 ppm (rat, 1 hr) 16,230 ppm (mouse, 1 hr) 4681 ppm (rat) 2723 ppm (mouse)[3] | ||
LCLo (lowest published)
|
3500 ppm (mouse) 24,000 ppm (guinea pig, 30 min) 7000 ppm (guinea pig, >4.5 hr)[3] | ||
| NIOSH (US health exposure limits): | |||
PEL (Permissible)
|
TWA 200 ppm (890 mg/m3)[1] | ||
REL (Recommended)
|
None established[1] | ||
IDLH (Immediate danger)
|
2000 ppm[1] | ||
| Related compounds | |||
Related alkanes
|
|||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||
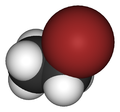
Bromoethane, also known as ethyl bromide, is a chemical compound of the haloalkanes group. It is abbreviated by chemists as EtBr (which is also used as an abbreviation for ethidium bromide). This volatile compound has an ether-like odor.
Preparation
[edit]The preparation of EtBr stands as a model for the synthesis of bromoalkanes in general. It is usually prepared by the addition of hydrogen bromide to ethene:
- H2C=CH2 + HBr → H3C-CH2Br
Bromoethane is inexpensive and would rarely be prepared in the laboratory. A laboratory synthesis includes reacting ethanol with a mixture of hydrobromic and sulfuric acids. An alternate route involves refluxing ethanol with phosphorus and bromine; phosphorus tribromide is generated in situ.[4]
Uses
[edit]In organic synthesis, EtBr is the synthetic equivalent of the ethyl carbocation (Et+) synthon.[5] In reality, such a cation is not actually formed. For example, carboxylates salts are converted to ethyl esters,[6] carbanions to ethylated derivatives, thiourea into ethylisothiouronium salts,[7] and amines into ethylamines.[8]
Safety
[edit]Short chain monohalocarbons in general are potentially dangerous alkylating agents. Bromides are better alkylating agents than chlorides, thus exposure to them should be minimized.
References
[edit]- ^ a b c d e NIOSH Pocket Guide to Chemical Hazards. "#0265". National Institute for Occupational Safety and Health (NIOSH).
- ^ "bromoethane - Compound Summary". PubChem Compound. USA: National Center for Biotechnology Information. 26 March 2005. Identification. Retrieved 15 June 2012.
- ^ a b "Ethyl bromide". Immediately Dangerous to Life or Health Concentrations. National Institute for Occupational Safety and Health.
- ^ Oliver Kamm & C. S. Marvel (1941). "Alkyl and alkylene bromides". Organic Syntheses; Collected Volumes, vol. 1, p. 25.
- ^ Makosza, M.; Jonczyk, A. "Phase-Transfer Alkylation of Nitriles: 2-Phenylbutyronitrile". Organic Syntheses. 55: 91; Collected Volumes, vol. 6, p. 897.
- ^ Petit, Y.; Larchevêque, M. "Ethyl Glycidate from (S)-Serine: Ethyl (R)-(+)-2,3-Epoxypropanoate". Organic Syntheses. 75: 37; Collected Volumes, vol. 10, p. 401.
- ^ E. Brand; Brand, F. C. "Guanidodacetic Acid". Organic Syntheses. 22: 440; Collected Volumes, vol. 3.
- ^ Brasen, W. R; Hauser, C. R. "o-Methylethylbenzyl Alcohol". Organic Syntheses. 34: 58; Collected Volumes, vol. 4, p. 582.
External links
[edit]Bromoethane
View on GrokipediaChemical Identity and Properties
Nomenclature and Molecular Formula
Bromoethane, also known as ethyl bromide, is the preferred IUPAC name for this haloalkane, with the abbreviation EtBr commonly used in chemical notation. The compound represents a simple alkyl bromide where bromine substitutes one hydrogen in ethane. Its molecular formula is C₂H₅Br, and the structural formula is CH₃CH₂Br, indicating a two-carbon chain with the bromine atom attached to the terminal carbon. The molar mass of bromoethane is 108.966 g/mol.[5] Bromoethane was first synthesized in 1827 by the French chemist Georges-Simon Serullas through the reaction of ethanol with phosphorus and bromine.[3] This preparation occurred shortly after the discovery of elemental bromine in 1826 by Antoine Jérôme Balard. In terms of molecular structure, the carbon atom bonded to the bromine exhibits sp³ hybridization, resulting in a tetrahedral geometry with bond angles near 109.5°. The C-Br bond length is approximately 1.94 Å.[6]Physical Properties
Bromoethane appears as a colorless, volatile liquid at room temperature, exhibiting an ether-like odor. Its boiling point ranges from 38.0 to 38.8 °C at 1 atm, reflecting its low boiling volatility suitable for laboratory handling under ambient conditions. The melting point is -119 °C, indicating it remains liquid over a wide temperature range above this threshold. The density of bromoethane is 1.46 g/mL at 20 °C, which is higher than that of water, contributing to its behavior in mixed solvent systems. It shows limited solubility in water, at 0.914 g/100 mL at 20 °C, but is fully miscible with common organic solvents such as ethanol, ether, and chloroform, facilitating its use in extraction processes. Vapor pressure is approximately 442 mmHg at 20 °C, underscoring its tendency to evaporate readily and produce vapors heavier than air.[7] The refractive index is 1.423 at 20 °C, a value typical for halogenated hydrocarbons of similar structure.| Property | Value | Conditions | Source |
|---|---|---|---|
| Appearance | Colorless volatile liquid, ether-like odor | Room temperature | PubChem |
| Boiling point | 38.0–38.8 °C | 1 atm | PubChem |
| Melting point | -119 °C | - | PubChem |
| Density | 1.46 g/mL | 20 °C | PubChem |
| Solubility in water | 0.914 g/100 mL | 20 °C | PubChem |
| Miscibility | With ethanol, ether, chloroform | - | PubChem |
| Vapor pressure | ~442 mmHg | 20 °C | Fisher Scientific SDS |
| Refractive index | 1.423 | 20 °C | PubChem |
Thermodynamic Properties
Bromoethane exhibits thermodynamic properties that reflect its molecular structure and intermolecular forces, particularly the polar C-Br bond, which contributes to its relatively high boiling point compared to nonpolar hydrocarbons of similar size. These properties are crucial for predicting phase behavior, stability, and energy changes in processes involving the compound. Key energetic data include the standard enthalpy of formation for the liquid phase at 298 K, which is -95.5 ± 2.1 kJ/mol, derived from combustion calorimetry measurements.[8] This value indicates moderate exothermicity in forming the liquid from elements in their standard states (carbon as graphite, hydrogen gas, and bromine liquid). For the gas phase, the standard enthalpy of formation is -61.9 ± 1.0 kJ/mol, less negative due to the endothermic vaporization process.[9] The standard Gibbs free energy of formation for gaseous bromoethane at 298 K is -23.9 kJ/mol, signifying that the formation reaction is spontaneous under standard conditions despite the entropy decrease associated with bond formation.[10] The heat capacity at constant pressure for the liquid phase at 25 °C is 105.8 J/mol·K, allowing for efficient heat absorption in thermal processes near room temperature.[11] This value, obtained from calorimetric studies, is higher than that of nonpolar analogs, attributable to dipole-dipole interactions enhancing vibrational and rotational contributions to heat capacity. Phase transition energetics are characterized by the enthalpy of vaporization, which is 27.0 ± 0.1 kJ/mol at the normal boiling point of approximately 311 K, reflecting the energy required to overcome intermolecular forces during evaporation.[12] Beyond the critical point, defined by a critical temperature of 231 °C and critical pressure of 6.15 MPa, distinct liquid and vapor phases cease to exist, marking the end of the vapor-liquid equilibrium curve.[13] The molecule's dipole moment of 2.04 D further underscores its polarity, arising from the electronegativity difference between carbon and bromine (C-Br bond polarity ≈ 0.4–0.5 on the Pauling scale), which influences dielectric properties and solubility in polar solvents.[13]| Property | Value | Phase/Conditions | Source |
|---|---|---|---|
| Standard enthalpy of formation (Δ_f H°) | -95.5 ± 2.1 kJ/mol | Liquid, 298 K | NIST [Ashcroft et al., 1965][8] |
| Standard Gibbs free energy of formation (Δ_f G°) | -23.9 kJ/mol | Gas, 298 K | Engineering Toolbox[10] |
| Heat capacity (C_p) | 105.8 J/mol·K | Liquid, 25 °C | NIST [Shehatta, 1993][11] |
| Enthalpy of vaporization (Δ_vap H) | 27.0 ± 0.1 kJ/mol | At boiling point (311 K) | NIST [Svoboda et al., 1977][12] |
| Critical temperature (T_c) | 231 °C | - | Stenutz Chemical Database[13] |
| Critical pressure (P_c) | 6.15 MPa | - | Stenutz Chemical Database[13] |
| Dipole moment (μ) | 2.04 D | Gas/liquid | Stenutz Chemical Database[13] |
Synthesis
Laboratory Synthesis
Bromoethane is commonly prepared in laboratory settings through the nucleophilic substitution of ethanol with a bromide source, yielding the alkyl halide via an SN2 mechanism under mild conditions suitable for small-scale synthesis.[14][15] One standard method involves the reaction of ethanol with hydrobromic acid, which can be used directly or generated in situ. The balanced equation is: This reaction is often catalyzed by concentrated sulfuric acid to facilitate protonation of the alcohol, enhancing the leaving group departure, or alternatively employs phosphorus tribromide (PBr3) as the brominating agent for cleaner conversion without excess acid.[14][15] In practice, hydrobromic acid is prepared by adding concentrated sulfuric acid to solid potassium bromide in the presence of ethanol; the sulfuric acid protonates the bromide to release HBr gas, which then reacts with the alcohol upon gentle heating and distillation. The equation for the in situ generation and reaction is: The mixture is typically warmed under reflux before distillation to ensure complete reaction, with the low-boiling bromoethane (b.p. 38°C) collected in a cooled receiver.[14][15] An alternative approach utilizes red phosphorus and bromine to form phosphorus tribromide in situ, which then brominates the alcohol. The overall reaction is: Here, red phosphorus reacts with bromine to generate PBr3, which substitutes the hydroxyl group; the mixture is heated under reflux to drive the reaction, followed by distillation of the product. This method avoids strong acids and is preferred for sensitive substrates, though it requires careful handling of elemental bromine.[14][15] Regardless of the synthesis route, the crude bromoethane contains impurities such as unreacted ethanol, water, sulfuric acid residues, and minor elimination products. Purification typically involves sequential washing steps: first with water to remove water-soluble byproducts, then with aqueous sodium carbonate to neutralize acids, followed by drying over anhydrous calcium chloride. The purified liquid is then isolated by fractional distillation under reduced pressure to minimize volatilization losses and decomposition, collecting the fraction boiling at approximately 38°C at atmospheric pressure (or lower under vacuum).[14][15]Industrial Production
Bromoethane is primarily produced on an industrial scale through two main processes: the hydrobromination of ethylene and the reaction of ethanol with hydrogen bromide. The hydrobromination method involves the direct addition of hydrogen bromide (HBr) to ethylene gas, following the reaction . This process is highly efficient due to the availability of ethylene as a petrochemical feedstock and operates under controlled pressure and temperature conditions in continuous-flow reactors, making it suitable for large-scale production. The reaction proceeds via an electrophilic addition mechanism, yielding high-purity product with minimal byproducts when optimized.[16][17] The alternative industrial route utilizes ethanol and concentrated aqueous HBr, derived from bromide salts and sulfuric acid, in the substitution reaction . This method is conducted in distillation setups where the bromoethane is continuously removed as it forms, typically at temperatures of 45–50°C, followed by neutralization and phase separation. It leverages inexpensive bio-derived or fermented ethanol, enhancing economic viability in regions with abundant alcohol supplies, though it requires careful control to avoid side reactions forming diethyl ether. Raw material consumption is approximately 557 kg of 95% ethanol and 1610 kg of 48% HBr per metric ton of product.[16][17] Both processes achieve yields of 90–96% and produce bromoethane with 98–99% purity after distillation and washing steps, such as treatment with sulfuric acid and sodium carbonate to remove impurities like unreacted alcohol or acids. Economic considerations favor these methods for their scalability and low-cost catalysts, with global production centered in Europe, the US, and Asia by a handful of specialized chemical firms. Byproducts are minimized through precise temperature and ratio control, ensuring high efficiency and compliance with environmental standards.[16] Historically, bromoethane production expanded in the early 20th century to meet demand as a refrigerant and anesthetic, but contemporary manufacturing focuses on its role as a versatile intermediate in organic synthesis and pharmaceuticals, reflecting shifts in regulatory and market priorities.[2][16]Chemical Reactions
Nucleophilic Substitution
Bromoethane, as a primary alkyl halide, undergoes nucleophilic substitution reactions predominantly via the bimolecular SN2 mechanism, in which a nucleophile attacks the carbon atom bonded to the bromine from the backside, leading to a concerted displacement of the bromide ion.[18][19] This process results in inversion of configuration at the carbon center, though bromoethane is achiral and thus does not exhibit observable stereoisomerism in the product.[20] The reaction rate follows second-order kinetics, expressed as rate = [CHCHBr][Nu], depending on the concentrations of both the substrate and the nucleophile.[21][22] The preference for the SN2 pathway in bromoethane arises from steric factors associated with its primary carbon, which minimizes hindrance to the nucleophile's approach compared to secondary or tertiary halides, thereby disfavoring the unimolecular SN1 mechanism that involves a carbocation intermediate.[18][19] Bromide serves as an effective leaving group due to its weak basicity and polarizability, facilitating clean departure during substitution.[21] Additionally, solvent effects play a key role; polar aprotic solvents, such as dimethyl sulfoxide (DMSO) or acetone, accelerate SN2 reactions by solvating cations but leaving the nucleophile anion relatively unsolvated and thus more reactive, in contrast to polar protic solvents that stabilize the nucleophile through hydrogen bonding.[23][24] Representative examples of SN2 reactions with bromoethane include its hydrolysis with hydroxide ion to form ethanol: CHCHBr + OH CHCHOH + Br.[25] Reaction with ammonia yields ethylamine: CHCHBr + NH CHCHNH + HBr.[26][27] Similarly, treatment with cyanide ion produces propanenitrile: CHCHBr + CN CHCHCN + Br, a common step in nitrile synthesis for extending carbon chains.[28][29] These reactions highlight bromoethane's utility in SN2 processes under mild conditions.[21]Elimination Reactions
Bromoethane undergoes elimination reactions primarily through the E2 mechanism, a concerted bimolecular process in which a strong base abstracts a β-hydrogen while the bromide ion departs simultaneously, forming a carbon-carbon double bond.[30] This reaction requires anti-periplanar geometry between the β-hydrogen and the leaving group for optimal orbital overlap in the transition state.[31] The rate law follows second-order kinetics: rate = k [CH₃CH₂Br][base], reflecting the involvement of both the substrate and the base in the rate-determining step.[30] A representative example is the dehydrohalogenation of bromoethane with hydroxide ion in alcoholic potassium hydroxide solution, yielding ethylene as the major product: This reaction proceeds efficiently under heating, with the alcoholic medium providing the strong, non-nucleophilic base conditions necessary for elimination.[31] In accordance with Zaitsev's rule, the elimination favors the more stable alkene product; however, for bromoethane, only one type of β-hydrogen is available on the methyl group, leading exclusively to the terminal alkene, ethylene.[30] These reactions are typically conducted at elevated temperatures in non-aqueous solvents, such as ethanol, to promote elimination over the competing nucleophilic substitution pathway.[31]Reactions with Metals and Oxidants
Bromoethane undergoes reductive coupling when treated with alkali metals such as sodium, forming butane via the Wurtz reaction. The balanced equation for this process is: This reaction is highly exothermic and requires careful control to manage the heat generated, often conducted in an inert solvent like ether to facilitate the coupling.[32] In the presence of magnesium metal, bromoethane forms the Grignard reagent ethylmagnesium bromide, a key organometallic compound used in synthetic organic chemistry. The reaction occurs in anhydrous ether as the solvent: The ether stabilizes the highly reactive Grignard species, and the reaction initiates with the oxidative addition of magnesium to the carbon-bromine bond, typically requiring dry conditions to prevent side reactions with moisture.[33] Bromoethane can form explosive mixtures with powdered aluminum or magnesium, particularly under conditions favoring rapid organometallic formation or ignition. These interactions may lead to violent decompositions, emphasizing the need for inert atmospheres during handling with such metals.[34] Beyond typical elimination pathways, bromoethane can engage in violent reactions with strong bases, potentially leading to rapid decomposition or explosive gas evolution under forcing conditions.[34]Applications
In Organic Synthesis
Bromoethane functions as a key ethylating agent in organic synthesis, enabling the formation of ethyl esters from carboxylate salts through nucleophilic substitution reactions. In this process, the carboxylate anion acts as a nucleophile, displacing the bromide leaving group to yield the corresponding ester. A representative example is the reaction of sodium benzoate with bromoethane, producing ethyl benzoate and sodium bromide:This approach is valued in laboratory esterifications for its straightforward conditions and compatibility with a range of carboxylic acid derivatives. In the alkylation of amines, bromoethane facilitates the conversion of primary amines to secondary amines, and further to tertiary amines or quaternary ammonium salts, by sequential addition of ethyl groups. The initial step involves the amine nitrogen attacking the electrophilic carbon of bromoethane, resulting in the formation of an ethylated amine and hydrogen bromide. For instance, a primary amine such as methylamine reacts as follows:
This method is particularly useful for preparing amine-based intermediates, with reaction outcomes influenced by stoichiometry and conditions to control the degree of alkylation.[35] Bromoethane plays a significant role in pharmaceutical synthesis by introducing ethyl groups into molecular frameworks, aiding the development of drug precursors with enhanced solubility or bioactivity. It is also employed in the production of agrochemicals, such as certain insecticides and herbicides, and in dye manufacturing, where ethyl substitution modifies chromophore properties for desired color and stability. These applications underscore its utility in constructing complex carbon chains for high-value compounds.[36] As a primary alkyl bromide, bromoethane exhibits high selectivity for SN2 pathways, benefiting from minimal steric hindrance at the reaction center and bromide's favorable leaving group ability, which promotes clean substitutions over competing elimination reactions. Yields are often optimized in polar aprotic solvents like dimethylformamide, achieving efficiencies above 80% in model alkylations.[37]