Recent from talks
All channels
Be the first to start a discussion here.
Be the first to start a discussion here.
Be the first to start a discussion here.
Be the first to start a discussion here.
Welcome to the community hub built to collect knowledge and have discussions related to Alpha globulin.
Nothing was collected or created yet.
Alpha globulin
View on Wikipediafrom Wikipedia

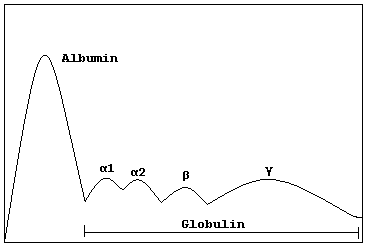
Alpha globulins are a group of globular proteins in plasma[1] that are highly mobile in alkaline or electrically charged solutions. They inhibit certain blood proteases and show significant inhibitor activity.
The alpha globulins typically have molecular weights of around 93 kDa.
Examples
[edit]Alpha globulins include certain hormones, proteins that transport hormones, and other compounds, including prothrombin and HDL.
Alpha 1 globulins
[edit]- α1-antitrypsin
- Alpha 1-antichymotrypsin
- Orosomucoid (acid glycoprotein)
- Serum amyloid A
- Alpha 1-lipoprotein
- Protein HC
Alpha 2 globulins
[edit]References
[edit]- ^ Alpha-Globulins at the U.S. National Library of Medicine Medical Subject Headings (MeSH)
Alpha globulin
View on Grokipediafrom Grokipedia