Recent from talks
Nothing was collected or created yet.
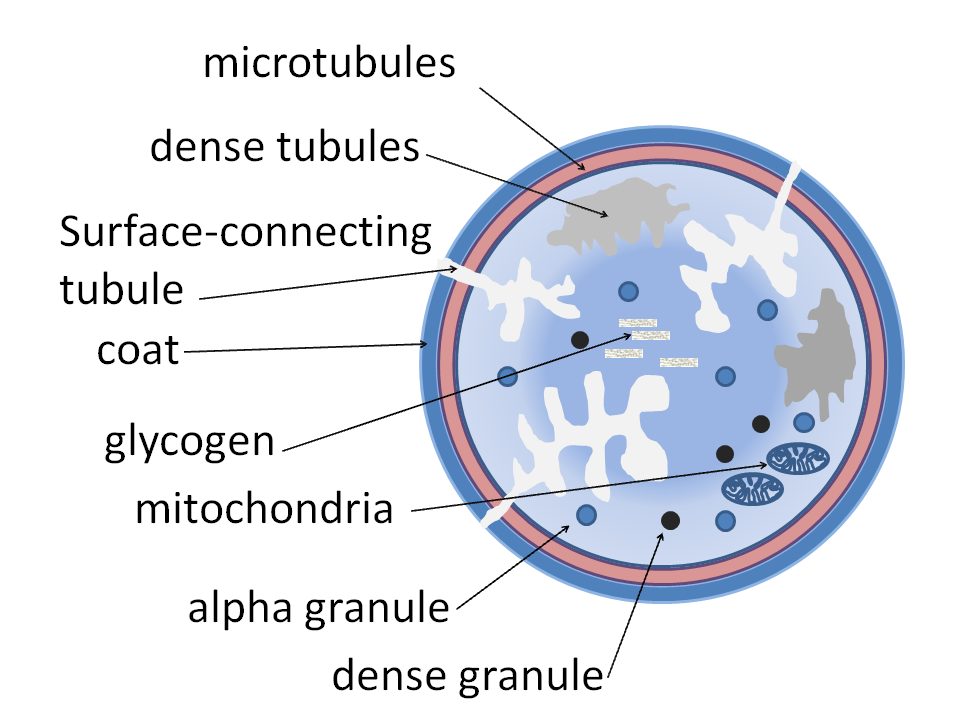
Alpha granule
View on Wikipedia| Alpha granule | |
|---|---|
 Alpha granules shown in a platelet | |
| Details | |
| Part of | Platelets |
| Identifiers | |
| Latin | granulum alpha |
| TH | H2.00.04.1.03005 |
| Anatomical terminology | |
Alpha granules, (α-granules) also known as platelet alpha-granules are a cellular component of platelets. Platelets contain different types of granules that perform different functions, and include alpha granules, dense granules, and lysosomes.[1] Of these, alpha granules are the most common,[1] making up 50% to 80% of the secretory granules.[2] Alpha granules contain several growth factors.[3]
Contents
[edit]Contents include insulin-like growth factor 1, platelet-derived growth factors, TGF beta, platelet factor 4 (which is a heparin-binding chemokine) and other clotting proteins (such as thrombospondin, fibronectin, factor V,[4] and von Willebrand factor).[5]
The alpha granules express the adhesion molecule P-selectin[6] and CD63.[7] These are transferred to the membrane after synthesis.
The other type of granules within platelets are called dense granules.
Clinical significance
[edit]A deficiency of alpha granules is known as gray platelet syndrome.
See also
[edit]References
[edit]- ^ a b Blair P, Flaumenhaft R (July 2009). "Platelet alpha-granules: basic biology and clinical correlates". Blood Reviews. 23 (4): 177–89. doi:10.1016/j.blre.2009.04.001. PMC 2720568. PMID 19450911.
- ^ Heijnen, H.; Sluijs, P. van der (2015). "Platelet secretory behaviour: as diverse as the granules … or not?". Journal of Thrombosis and Haemostasis. 13 (12): 2141–2151. doi:10.1111/jth.13147. PMID 26391322. S2CID 206159932. Retrieved 3 April 2021.
- ^ Harrison P, Cramer EM (March 1993). "Platelet alpha-granules". Blood Reviews. 7 (1): 52–62. doi:10.1016/0268-960X(93)90024-X. PMID 8467233.
- ^ Whiteheart SW (August 2011). "Platelet granules: surprise packages". Blood. 118 (5): 1190–1191. doi:10.1182/blood-2011-06-359836. PMID 21816838. S2CID 8273132.
- ^ Nurden AT (May 2011). "Platelets, inflammation and tissue regeneration". Thrombosis and Haemostasis. 105 (Suppl 1): S13–33. doi:10.1160/THS10-11-0720. PMID 21479340. S2CID 36934086.
- ^ Orkin SH, Nathan DG, Ginsburg D, Look AT (2009). Nathan and Oski's hematology of infancy and childhood. Elsevier Health Sciences. pp. 1386–. ISBN 978-1-4160-3430-8. Retrieved 2 November 2010.
- ^ Coleman WB, Tsongalis GJ (2009). Molecular pathology: the molecular basis of human disease. Academic Press. pp. 258–. ISBN 978-0-12-374419-7. Retrieved 2 November 2010.