Recent from talks
Nothing was collected or created yet.
Crotonic acid
View on Wikipedia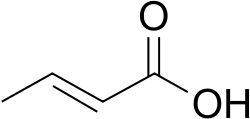 | |
 | |
| Names | |
|---|---|
| Preferred IUPAC name
(2E)-But-2-enoic acid | |
| Other names
(E)-But-2-enoic acid
(E)-2-Butenoic acid Crotonic acid trans-2-Butenoic acid β-Methylacrylic acid 3-Methylacrylic acid | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| DrugBank | |
| ECHA InfoCard | 100.003.213 |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C4H6O2 | |
| Molar mass | 86.090 g·mol−1 |
| Density | 1.02 g/cm3 |
| Melting point | 70 to 73 °C (158 to 163 °F; 343 to 346 K) |
| Boiling point | 185 to 189 °C (365 to 372 °F; 458 to 462 K) |
| Acidity (pKa) | 4.69 [1] |
| Hazards | |
| Safety data sheet (SDS) | SIRI.org |
| Related compounds | |
Other anions
|
crotonate |
Related carboxylic acids
|
propionic acid acrylic acid butyric acid succinic acid malic acid tartaric acid fumaric acid pentanoic acid tetrolic acid |
Related compounds
|
butanol butyraldehyde crotonaldehyde 2-butanone |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Crotonic acid ((2E)-but-2-enoic acid) is a short-chain unsaturated carboxylic acid described by the formula CH3CH=CHCO2H. The name crotonic acid was given because it was erroneously thought to be a saponification product of croton oil.[2] It crystallizes as colorless needles from hot water. With a cis-alkene, Isocrotonic acid is an isomer of crotonic acid. Crotonic acid is soluble in water and many organic solvents. Its odor is similar to that of butyric acid.
Production
[edit]Crotonic acid produced industrially by oxidation of crotonaldehyde:[3][4]: 230
- CH3CH=CHCHO + 1/2 O2 → CH3CH=CHCO2H
A number of other methods exist, including the Knoevenagel condensation of acetaldehyde with malonic acid in pyridine:[3]: 229
The alkaline hydrolysis of allyl cyanide followed by the intramolecular rearrangement of the double bond:[5][6]
Furthermore, it is formed during the distillation of 3-hydroxybutyric acid:[7]
Properties
[edit]Crotonic acid crystallizes in the monoclinic crystal system in the space group P21/a (space group 14, position 3) with the lattice parameters a = 971 pm, b = 690 pm, c = 775 pm and β = 104.0°. The unit cell contains four formula units.[8]
Reactions
[edit]Crotonic acid converts into butyric acid by hydrogenation or by reduction with zinc and sulfuric acid.[9]
Upon treatment with chlorine or bromine, crotonic acid converts to 2,3-dihalobutyric acids:[9]
Crotonic acid adds hydrogen bromide to form 3-bromobutyric acid.[9][10]
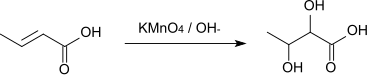
The reaction with alkaline potassium permanganate solution affords 2,3-dihydroxybutyric acid.[9]
Upon heating with acetic anhydride, crotonic acid converts to the acid anhydride:[11]
Esterification of crotonic acid using sulfuric acid as a catalyst provides the corresponding crotonate esters:
Crotonic acid reacts with hypochlorous acid to 2-chloro-3-hydroxybutyric acid. This can either be reduced with sodium amalgam to butyric acid, can form with sulfuric acid 2-chlorobutenoic acid, react with hydrogen chloride to 2,3-dichlorobutenoic acid or with potassium ethoxide to 3-methyloxirane-2-carboxylic acid.[12]
Crotonic acid reacts with ammonia at the alpha position in the presence of mercury(II) acetate. This reaction provides DL-threonine.[13]
Use
[edit]Crotonic acid is mainly used as a comonomer with vinyl acetate.[14] The resulting copolymers are used in paints and adhesives.[4]
Crotonyl chloride reacts with N-ethyl-2-methylaniline (N-ethyl-o-toluidine) to provide crotamiton, which is used as an agent against scabies.[15]
Safety
[edit]See also
[edit]References
[edit]- ^ Dawson, R. M. C.; et al. (1959). Data for Biochemical Research. Oxford: Clarendon Press.
- ^ Chisholm, Hugh, ed. (1911). . Encyclopædia Britannica. Vol. 7 (11th ed.). Cambridge University Press. p. 511.
- ^ a b Beyer, Hans; Walter, Wolfgang (1984). Organische Chemie (in German). Stuttgart: S. Hirzel Verlag. ISBN 3-7776-0406-2.
- ^ a b c Schulz, R. P.; Blumenstein, J.; Kohlpaintner, C. (2005). "Crotonaldehyde and Crotonic Acid". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a08_083. ISBN 978-3-527-30673-2.
- ^ Rinne, A.; Tollens, B. (1871). "Ueber das Allylcyanür oder Crotonitril" [On allyl cyanide or crotononitrile]. Justus Liebigs Annalen der Chemie. 159 (1): 105–109. doi:10.1002/jlac.18711590110.
- ^ Pomeranz, C. (1906). "Ueber Allylcyanid und Allylsenföl" [On allyl cyanide and allylic mustard oil]. Justus Liebigs Annalen der Chemie. 351 (1–3): 354–362. doi:10.1002/jlac.19073510127.
- ^ Beilstein, F. (1893). Handbuch der organischen Chemie (in German). Vol. 1 (3rd ed.). Verlag Leopold Voss. p. 506.
- ^ Shimizu, S.; Kekka, S.; Kashino, S.; Haisa, M. (1974). "Topochemical Studies. III. The Crystal and Molecular Structures of Crotonic Acid, CH3CH=CHCO2H, and Crotonamide, CH3CH=CHCONH2". Bulletin of the Chemical Society of Japan. 47 (7): 1627–1631. doi:10.1246/bcsj.47.1627.
- ^ a b c d Heilbron (1953). "Crotonic acid". Dictionary of Organic Compounds. 1: 615.
- ^ Lovén, J. M.; Johansson, H. (1915). "Einige schwefelhaltige β-Substitutionsderivate der Buttersäure" [Some sulfur-containing β-substitution derivatives of butyric acid]. Berichte der deutschen chemischen Gesellschaft. 48 (2): 1254–1262. doi:10.1002/cber.19150480205.
- ^ Clover, A. M.; Richmond, G. F. (1903). "The Hydrolysis of Organic Peroxides and Peracids". American Chemical Journal. 29 (3): 179–203.
- ^ Beilstein, F. (1893). Handbuch der organischen Chemie (in German). Vol. 1 (3rd ed.). Verlag Leopold Voss. p. 562.
- ^ Carter, H. E.; West, H. D. (1955). "dl-Threonine". Organic Syntheses; Collected Volumes, vol. 3, p. 813.
- ^ a b Entry on Butensäuren. at: Römpp Online. Georg Thieme Verlag, retrieved January 7, 2020.
- ^ Kleemann, A.; Engel, J. Pharmazeutische Wirkstoffe: Synthesen, Patente, Anwendungen. Vol. 5 (2nd rev. and updated ed.). Stuttgart & New York: Georg Thieme Verlag. p. 251. ISBN 3-13-558402-X.
Crotonic acid
View on GrokipediaChemical identity
Nomenclature
Crotonic acid is systematically named (2E)-but-2-enoic acid according to IUPAC nomenclature conventions for unsaturated carboxylic acids. The "but-2-enoic acid" portion indicates a four-carbon chain with a double bond between carbons 2 and 3, and the carboxyl group at carbon 1. The "(2E)" descriptor specifies the configuration at the double bond, where the E/Z system is based on Cahn-Ingold-Prelog priority rules: the higher-priority groups (the carboxyl group on C2 and the methyl group on C3) are on opposite sides of the double bond.[12] This contrasts with the cis isomer, named (2Z)-but-2-enoic acid, where the higher-priority groups are on the same side. Commonly, crotonic acid refers specifically to the trans (E) isomer, while the cis (Z) isomer is known as isocrotonic acid.[13] Historical synonyms include β-methacrylic acid or β-methylacrylic acid, reflecting early understandings of its structure as a derivative of acrylic acid with a methyl substituent.[12] The term "crotonic acid" originates from "croton," derived from its 19th-century association with croton oil extracted from Croton tiglium seeds, where it was initially thought to be a saponification product but is actually a natural constituent.[1] To avoid confusion, crotonic acid (the unbranched trans-but-2-enoic acid) is distinct from angelic acid, which is the (Z)-2-methylbut-2-enoic acid and occurs in plants like angelica root.[14]Structure and isomers
Crotonic acid has the molecular formula C₄H₆O₂ and the structural formula CH₃CH=CHCO₂H.[15] X-ray crystallographic analysis reveals typical bond lengths of approximately 1.34 Å for the C=C double bond, 1.50 Å for the adjacent C-C single bonds, and 1.20 Å for the carbonyl C=O bond. The molecule adopts a trans (E) configuration across the C=C double bond, with the methyl group and carboxylic acid group on opposite sides, and features a dihedral angle of approximately 180° between the C-C-C=O atoms, resulting in a nearly planar geometry. The cis (Z) isomer, known as isocrotonic acid, is less thermodynamically stable than the trans form due to steric interactions and has a melting point of 15 °C.[16] Crotonic acid crystallizes in the monoclinic crystal system with space group P2₁/a and lattice parameters a = 9.71 Å, b = 6.90 Å, c = 7.75 Å, β = 104.0°, and Z = 4. The skeletal formula of crotonic acid is commonly depicted as:
H3C-CH=CH-COOH
H3C-CH=CH-COOH
History and occurrence
Discovery and naming
Crotonic acid was first isolated in the early 19th century from croton oil obtained from the seeds of Croton tiglium through saponification. French chemists Pierre-Joseph Pelletier and Joseph-Bienaimé Caventou reported its discovery in 1818, initially believing it to represent the primary acidic component of the oil and attributing the oil's physiological effects to this substance—a historical misconception later revised as subsequent analyses revealed the acid to be only a minor constituent.[19][20] By the mid-19th century, amid rapid advancements in organic analysis, crotonic acid was accurately identified as a butenoic acid, an unsaturated carboxylic acid with the molecular formula C₄H₆O₂. This recognition aligned with broader efforts to elucidate the structures of unsaturated compounds, including the role of carbon-carbon double bonds, and contributed to foundational debates in structural organic chemistry.[6] The trans configuration of the double bond was established in the late 19th century through chemical studies of geometric isomerism, confirming the compound's stereochemistry as (2E)-but-2-enoic acid. The trivial name "crotonic acid," first documented around 1836, derived directly from its association with croton oil, perpetuating the early error regarding its prominence in the oil's composition. In the 20th century, nomenclature shifted to the systematic IUPAC designation.[21] Throughout the 19th century, crotonic acid served as a key exemplar in pioneering studies of unsaturated acids, facilitating investigations into isomerism, reactivity, and the behavior of conjugated systems in early organic synthesis.[22]Natural sources
Crotonic acid occurs naturally as a minor component in certain plant sources, primarily as the trans isomer. It is present in croton oil extracted from the seeds of Croton tiglium, where it contributes to the oil's composition alongside other fatty acids, though at low concentrations typically below 0.1% in unprocessed seed material (0.102 mg per 100 g seeds).[1][23] In tobacco leaves (Nicotiana tabacum), trans-crotonic acid has been isolated from the essential oil fraction, where it represents one of the unsaturated fatty acids contributing to the plant's volatile organic acid profile.[24] Additionally, crotonic acid is found in carrot seeds (Daucus carota), serving as a bioactive factor with plant growth inhibitory properties in water extracts, affecting germination and development of other species in a dose-dependent manner.[25] In biological contexts, crotonic acid plays a role in metabolism through its derivative, crotonyl-CoA, which acts as a key intermediate in the beta-oxidation pathway of fatty acids in both plants and animals. This step involves the dehydrogenation of acyl-CoA to form the trans-2-enoyl-CoA (crotonyl-CoA), facilitating the breakdown of even-chain fatty acids for energy production.[26] While crotonic acid itself is not a major fermentation byproduct in rumen bacteria, its structural analogs appear in microbial analyses of volatile fatty acids, highlighting its relevance in ruminant lipid metabolism studies. Natural concentrations remain low, with levels in plant extracts such as croton oil generally under 0.5%, and it does not serve as a significant dietary source for humans.[1]Physical properties
Appearance and phase behavior
Crotonic acid is a white to off-white crystalline solid that typically forms as colorless needles or prisms at room temperature.[1][4] It possesses a pungent odor.[4] The compound melts between 70 and 72 °C and boils at 185 to 189 °C under standard atmospheric pressure (760 mmHg).[1] Crotonic acid also exhibits sublimation behavior under moderate vacuum conditions.[27] The density of the solid is 1.02 g/cm³, and its vapor pressure is approximately 0.18 mmHg at 20 °C.[4][4]Solubility and thermodynamic data
Crotonic acid exhibits moderate solubility in water, with an experimental value of approximately 94 g/L at 25 °C, reflecting its polar carboxylic acid functionality that allows partial miscibility despite the hydrophobic alkyl chain. It is highly soluble in organic solvents such as ethanol, diethyl ether, and chloroform, which facilitate its dissolution in nonpolar environments due to favorable intermolecular interactions.[13] The octanol-water partition coefficient, expressed as log P = 0.72, indicates a slight preference for the aqueous phase, consistent with its amphiphilic nature.[1] The acidity of crotonic acid is characterized by a pKa of 4.69 for the carboxylic acid dissociation, which is lower than that of the saturated analog butyric acid (pKa = 4.82), attributable to the electron-withdrawing effect of the conjugated double bond stabilizing the conjugate base.[1] This enhanced acidity influences its behavior in aqueous solutions and biochemical contexts. Key thermodynamic properties include a standard enthalpy of formation (Δ_f H°) of -368.5 ± 1.4 kJ/mol in the gas phase, derived from combustion calorimetry and vapor pressure measurements, highlighting the energetic stability of its molecular structure.[28] The molar heat capacity (C_p) for the liquid phase is approximately 140 J/mol·K, reflecting contributions from vibrational and rotational modes. The molar mass is 86.09 g/mol, and the refractive index of the liquid (n_D) is 1.422 at 20 °C, useful for optical identification and purity assessment.[29]| Property | Value | Phase/Condition | Source |
|---|---|---|---|
| Water solubility | 94 g/L | 25 °C | Merck Millipore |
| log P (octanol-water) | 0.72 | - | PubChem |
| pK_a | 4.69 | Aqueous, 25 °C | PubChem |
| Δ_f H° | -368.5 kJ/mol | Gas | NIST |
| C_p | 140 J/mol·K | Liquid | Chemeo |
| Molar mass | 86.09 g/mol | - | Standard |
| Refractive index (n_D) | 1.422 | Liquid, 20 °C | ChemicalBook |
Synthesis
Industrial production
Crotonic acid is primarily produced on an industrial scale through the catalytic oxidation of crotonaldehyde using molecular oxygen or air. The reaction involves the selective oxidation of the aldehyde group in crotonaldehyde (CH₃CH=CHCHO) to the carboxylic acid, typically conducted in liquid phase with inert solvents such as heptane or toluene to facilitate heat transfer and product separation. Catalysts commonly employed include mixtures of cobalt acetate and copper acetate, with cobalt comprising up to 15% of the copper component, at concentrations of 0.02–2% by weight relative to the reaction mixture. This process operates at moderate temperatures of 25–40 °C and pressures of 50–100 psig, enabling high selectivity while minimizing over-oxidation to byproducts like acetic acid. Yields typically range from 60–90%, depending on catalyst composition and reaction conditions, with unreacted crotonaldehyde recycled to improve efficiency.[30][31] Crotonaldehyde feedstock is derived from petrochemical sources, primarily through aldol condensation of acetaldehyde (itself produced from propylene via Wacker oxidation) followed by dehydration, linking crotonic acid production to propylene-based routes. Alternative industrial routes focus on bio-based methods to reduce reliance on petrochemicals, notably the thermal pyrolysis or depolymerization of poly(3-hydroxybutyrate) (PHB), a biodegradable polyester accumulated by bacterial fermentation of renewable feedstocks like glucose or wastewater. PHB, produced as a byproduct in industrial fermentations (e.g., by engineered Cupriavidus necator strains), undergoes pyrolysis at 250–310 °C in inert atmospheres, yielding crotonic acid via dehydration and decarboxylation of 3-hydroxybutyric acid monomers, with overall yields up to 63%—about 30% higher than conventional petrochemical oxidation when accounting for purification losses. This route integrates with existing PHB production facilities, where distillation separates crotonic acid from oligomers and water, offering a sustainable alternative with lower carbon footprint but higher upfront costs due to biomass preprocessing.[32][33][34] Emerging developments emphasize engineered microbial pathways for direct crotonic acid biosynthesis, such as modified Escherichia coli strains optimized for PHB accumulation followed by in situ depolymerization, as outlined in recent patents exploring sustainable oxidation cascades. These bioengineered approaches aim to boost titers beyond 50 g/L in fermentation broths, potentially enabling integrated biorefineries by 2030.[35]Laboratory methods
Crotonic acid can be prepared in the laboratory via the Knoevenagel condensation of acetaldehyde with malonic acid under base catalysis, typically using pyridine or piperidine as the base, followed by decarboxylation to yield the α,β-unsaturated carboxylic acid. This method produces predominantly the thermodynamically favored trans-isomer and is suitable for small-scale syntheses due to its simplicity and use of readily available starting materials.[37] Hydrolysis routes provide alternative laboratory preparations. Alkaline hydrolysis of crotononitrile (CH₃CH=CHCN) with aqueous base such as sodium hydroxide converts the nitrile group to the carboxylic acid, yielding crotonic acid after acidification. Similarly, alkaline hydrolysis of allyl cyanide (CH₂=CHCH₂CN) initially forms 3-butenoic acid, which undergoes base-promoted double bond migration to the conjugated crotonic acid due to its greater stability.[38][39] Other preparations include the dehydration of 3-hydroxybutyric acid, achieved by heating the β-hydroxy acid in the presence of an acid catalyst or under thermal conditions, leading to elimination of water and formation of the α,β-unsaturated acid. For stereoselective access to the E-isomer, the Wittig reaction between acetaldehyde and a stabilized ylide such as (triphenylphosphoranylidene)acetate (derived from the phosphonium salt of ethyl glyoxylate or similar), followed by ester hydrolysis, favors the trans geometry due to the oxaphosphetane intermediate's conformational preferences.[40][41] Following synthesis, crotonic acid is commonly purified by recrystallization from hot water, exploiting its moderate solubility, or by vacuum distillation under reduced pressure (boiling point approximately 185 °C at atmospheric pressure) to isolate the pure trans form from isomers or impurities.[42]Chemical reactions
Addition reactions
Crotonic acid, as an α,β-unsaturated carboxylic acid, undergoes electrophilic addition reactions at the conjugated C=C double bond, with the electron-withdrawing carboxyl group influencing regioselectivity and rate. Halogenation proceeds via electrophilic addition of Br₂ or Cl₂ across the double bond. For bromine addition in ethylene chloride solvent, the reaction follows second-order kinetics and yields 2,3-dibromobutanoic acid through an anti addition mechanism involving a bromonium ion intermediate.[43] Similarly, chlorination in solvents like chloroform or water saturated with NaCl produces 2,3-dichlorobutanoic acid, with the reaction rate enhanced in nonpolar media due to reduced ionic solvation.[44] These additions are stereospecific, yielding racemic mixtures from the trans alkene. Hydrohalogenation with HBr occurs predominantly via an ionic mechanism, adding H to the β-carbon (C3) and Br to the α-carbon (C2) to form 2-bromobutanoic acid, consistent with Markovnikov orientation stabilized by the conjugating carbonyl.[45] The peroxide effect, which would promote anti-Markovnikov addition, is negligible here owing to the conjugation favoring the electrophilic pathway over radical initiation.[45] Hydrogenation of the double bond is achieved catalytically with H₂ and Pd/C, quantitatively converting crotonic acid to butanoic acid; the reaction exhibits autocatalysis by the product butyric acid, accelerating the rate.[46] In derivatives such as α,β-unsaturated esters, NaBH₄ enables selective 1,2-reduction of the carbonyl to an allylic alcohol while preserving the conjugated double bond, as the hydride does not typically affect isolated or conjugated alkenes under mild conditions.[47] Oxidative addition with cold, dilute alkaline KMnO₄ results in syn dihydroxylation, forming threo-2,3-dihydroxybutanoic acid (racemic (2R,3R)- and (2S,3S)-isomers) via a cyclic manganate ester intermediate. This reaction is stereospecific, reflecting the trans geometry of the alkene, and proceeds without cleavage under controlled low-temperature conditions. The conjugated system moderates reactivity in these additions, generally slowing rates compared to isolated alkenes but directing regiochemistry (detailed in Other transformations).Other transformations
Crotonic acid undergoes esterification with alcohols via the Fischer method, typically in the presence of an acid catalyst such as sulfuric acid, to yield crotonic esters. This reaction follows second-order kinetics, as demonstrated in studies with primary alcohols like octyl, decyl, and dodecyl alcohol, where the rate depends on the concentrations of both the acid and alcohol. The resulting esters, such as butyl crotonate, serve as monomers or precursors in the synthesis of polymers, including hydrogels for controlled release applications.[13] Heating crotonic acid with acetic anhydride below 100 °C leads to the formation of crotonic anhydride or mixed anhydrides, facilitating dehydration and activation of the carboxylic group for further synthetic transformations.[48] These anhydrides are valuable intermediates in organic synthesis, enhancing the reactivity of the acyl moiety toward nucleophiles. The α,β-unsaturation in crotonic acid imparts conjugation effects that enhance its acidity and reactivity compared to saturated carboxylic acids. The pKa of crotonic acid is 4.69 at 25 °C, lower than that of butanoic acid (pKa 4.82) or acetic acid (pKa 4.76), due to resonance stabilization of the conjugate base by the conjugated double bond, which delocalizes the negative charge.[49] This increased acidity influences its behavior in base-catalyzed reactions. Additionally, the electron-withdrawing carboxylic group activates the alkene as a dienophile in Diels-Alder cycloadditions; for instance, crotonic acid reacts with conjugated dienes derived from safflower fatty acids to form cyclic adducts, demonstrating its utility in stereoselective ring-forming processes.[50] A notable transformation involving the α-position is the multi-step synthesis of DL-threonine from crotonic acid, as described in a classic procedure. It begins with oxymercuration of crotonic acid using mercury(II) acetate in methanol to introduce a β-methoxy group, followed by bromination to form α-bromo-β-methoxybutyric acid. Subsequent treatment with ammonium hydroxide displaces the bromide with an amine group, yielding α-amino-β-methoxybutyric acid, which is then formylated and hydrolyzed under acidic conditions to replace the methoxy with hydroxy, producing DL-threonine.[42] This process yields the racemic amino acid without stereochemical control at the chiral centers and highlights crotonic acid's role in amino acid production, though modern methods often avoid mercury catalysts due to toxicity concerns.Applications
Industrial and material uses
Crotonic acid serves as a key comonomer in the synthesis of specialty polymers, particularly when copolymerized with vinyl acetate to form poly(vinyl acetate-co-crotonic acid). These copolymers typically incorporate 2–20 mol% crotonic acid, enhancing the material's hardness, adhesion, and film-forming properties, which make them suitable for acrylic resins used in paints, adhesives, and coatings.[51][52][53] Derivatives of crotonic acid further expand its utility in materials science. Crotonic anhydride acts as a crosslinking agent in the production of adhesives, coatings, and modified lignins, where it facilitates esterification to improve mechanical strength and thermal stability.[54][55] Esters derived from crotonic acid are employed in plasticizers for polyvinyl chloride (PVC), enabling superior plasticization through backbone functionalization that enhances compatibility and flexibility in blends like PVC/PBSA.[56][57] As of 2025, adhesive resins account for approximately 49% of global crotonic acid consumption, with coatings at 31% and plasticizers at 16%, reflecting significant use in polymer formulations including water-based emulsions for eco-friendly paints and adhesives.[58][59][60] Recent developments include the synthesis of biodegradable alternating copolymers from crotonic acid esters and 2-methylene-1,3-dioxepane, offering potential for sustainable packaging materials with controlled degradability under radical polymerization conditions.[61]Pharmaceutical applications
Crotonic acid serves as a key precursor in the synthesis of crotamiton, an antipruritic and scabicidal agent used to treat scabies and relieve itching associated with skin conditions. The process involves the conversion of crotonic acid to crotonyl chloride, which then reacts with N-ethyl-o-toluidine (N-ethyl-2-methylaniline) to form crotamiton. This compound is formulated as a 10% cream, such as Eucrema or Eurax, applied topically to affected areas for effective symptom relief.[62][63] In pharmaceutical synthesis, crotonic acid also plays a role in producing racemic threonine, an essential amino acid incorporated into nutritional supplements to support protein synthesis, immune function, and gut health. The synthesis begins with the addition of mercuric acetate to crotonic acid in methanol, followed by bromination to yield α-bromo-β-methoxy-n-butyric acid, which is then ammonolyzed and hydrolyzed to dl-threonine. This method provides a viable route for generating threonine for amino acid supplements, though industrial production typically relies on fermentation for the L-enantiomer.[42] Crotonic acid and its derivatives function as biochemical probes in studies of unsaturated fatty acid metabolism, particularly as enzyme inhibitors in β-oxidation pathways. For example, crotonyl-CoA, derived from crotonic acid, serves as a substrate analog to investigate the mechanism of enoyl-CoA hydratase, revealing the enzyme's catalytic dependence on sulfur in CoA thioesters through kinetic comparisons showing reduced activity with oxygen analogs. Similarly, 4-bromocrotonic acid acts as a potent inhibitor of 3-ketoacyl-CoA thiolase and acetoacetyl-CoA thiolase, blocking fatty acid oxidation and ketone body degradation in mitochondria, which has aided research into rate-limiting steps in these metabolic processes.[64][65] Historically, extracts from croton oil, which contains crotonic acid among other components, were employed in early 20th-century medicine as counterirritants and vesicants for treating inflammatory conditions, though their use declined due to toxicity concerns. These applications leveraged the oil's irritant properties for topical therapies, predating modern antiseptics but contributing to early understandings of skin permeation in drug delivery.[66]Safety and toxicology
Health hazards
Crotonic acid causes serious eye damage, potentially leading to corneal opacity upon contact. It is classified as causing skin irritation in many assessments, though some studies show no skin irritation. Acute dermal toxicity is low, with an LD50 greater than 2,000 mg/kg in rats.[67] Oral acute toxicity is low, with an LD50 of 2,610 mg/kg in rats.[67] Inhalation of crotonic acid dust or vapors acts as a respiratory irritant, potentially leading to symptoms such as coughing, choking, and shortness of breath; severe exposure may result in pulmonary edema. While specific LC50 values for inhalation are not widely reported, the compound is classified as causing serious respiratory tract irritation.[68][69] Chronic exposure to crotonic acid may lead to skin sensitization in some individuals, though it is primarily noted as a chronic irritant rather than a strong sensitizer. It is not classified as carcinogenic by the International Agency for Research on Cancer (IARC). Animal studies indicate limited reproductive toxicity.[70][71] Occupational exposure limits for crotonic acid are not specifically established by OSHA, but the ACGIH recommends a threshold limit value (TLV) of 5 mg/m³ as time-weighted average (TWA) with skin notation. Handling precautions include the use of protective gloves, eye protection, and adequate ventilation to prevent absorption and irritation.[72][73]Environmental impact
Crotonic acid exhibits low bioaccumulation potential, with a bioconcentration factor (BCF) estimated below 10 due to its low octanol-water partition coefficient (log Kow = 0.71 at 25 °C), which limits uptake in aquatic organisms. It is not classified as persistent, bioaccumulative, or toxic (PBT) under EU criteria. Ecotoxicity assessments show moderate effects on aquatic life. The 96-hour LC50 for fish (Pimephales promelas) is 31 mg/L, indicating acute toxicity at relatively high concentrations.[74] For invertebrates, the 48-hour EC50 for Daphnia magna is 150 mg/L, while for algae (Pseudokirchneriella subcapitata), the 72-hour EC50 is >57.5 mg/L, suggesting potential risks to primary producers in industrial effluents if concentrations exceed these thresholds.[74] Overall, these values classify crotonic acid as harmful to aquatic environments (H411 under EU CLP), particularly from point-source releases. The substance is expected to be readily biodegradable based on its structure. Under regulatory frameworks, crotonic acid (EC 203-533-9) is registered under the EU REACH regulation, requiring risk assessments for environmental releases.[75] In the United States, it is listed on the Toxic Substances Control Act (TSCA) inventory.[76] In wastewater treatment, the compound is effectively removed (>90%) via activated sludge processes, leveraging its biodegradability to minimize discharge to receiving waters.[77]References
- https://www.[researchgate](/page/ResearchGate).net/publication/266397945_Bio-based_production_of_crotonic_acid_by_pyrolysis_of_poly3-hydroxybutyrate_inclusions