Recent from talks
Nothing was collected or created yet.
Natrix
View on WikipediaThis article needs additional citations for verification. (March 2017) |
| Natrix | |
|---|---|

| |
| Natrix natrix | |
| Scientific classification | |
| Kingdom: | Animalia |
| Phylum: | Chordata |
| Class: | Reptilia |
| Order: | Squamata |
| Suborder: | Serpentes |
| Family: | Colubridae |
| Subfamily: | Natricinae |
| Genus: | Natrix Laurenti, 1768 |
| Type species | |
| Natrix natrix | |
Natrix is a genus of Old World snakes found mainly across Eurasia (although the range of Natrix tessellata extends into Egypt and those of N. astreptophora and N. maura into north-west Africa) in the subfamily Natricinae of the family Colubridae. They are commonly called grass snakes and water snakes, but some other snake species also known commonly as "grass snakes" and "water snakes" are not in the genus.
Species
[edit]The genus Natrix contains five extant species[1] and at least five extinct (fossil-only) species.
| Image | Scientific name | Common name | Distribution |
|---|---|---|---|
 |
Natrix astreptophora (Seoane, 1885) | Iberian grass snake[2] | Iberian peninsula (Spain and Portugal), southern France, coastal north-west Africa (Morocco, Algeria, Libya, Tunisia)
|
 |
Natrix helvetica (Lacépède, 1789) | barred grass snake[3] | Western Europe, including southern Great Britain
|
 |
Natrix maura (Linnaeus, 1758) | viperine water snake[4] | Portugal, Spain, France, north-west Italy and into Switzerland; north-west Africa (Morocco, Algeria, Libya, Tunisia)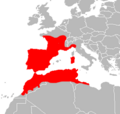
|
 |
Natrix natrix (Linnaeus, 1758) | grass snake[4] | Mainland Europe from mid Scandinavia to southern Italy, to northern Middle East and Central Asia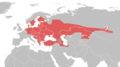
|
 |
Natrix tessellata (Laurenti, 1768) | dice snake[4] | Much of Eurasia, and Egypt
|
| †Natrix longivertebrata (Szyndlar, 1984) | extinct species (Pliocene, Miocene) | Poland, Austria, France[5] | |
| †Natrix merkurensis Ivanov, 2002 | extinct species (Miocene)[6] | Czech Republic, France[7] | |
| †Natrix mlynarskii Rage, 1988 | extinct species (Eocene–Miocene) | France[8] | |
| †Natrix parva Szyndlar, 1984 | extinct species (Miocene) | Poland[9] | |
| †Natrix sansaniensis (Lartet, 1851) | extinct species (Miocene)[6] | Czech Republic, France |
Nota bene: A binomial authority in parentheses indicates that the species was originally described in a genus other than Natrix.
Etymology
[edit]Natrix is classical Latin for a water snake. The word comes from a Proto-Indo-European root meaning "snake", with cognates in the Celtic and Germanic languages, the latter including the English adder. It was probably influenced through folk etymology by the Latin nare and natare meaning "swim";[10][11] it appears to be a grammatically feminine word for "swimmer".
Geography
[edit]The refuge of a widely distributed Western European lineage regarding the barred grass snake commonly known as Natrix helvetica was most likely located in southern France and outside the classical refuges in the southern European peninsulas. One genetic lineage of the common grass snake (N. natrix) is also distributed in Scandinavia, Central Europe, and the Balkan Peninsula.[12]
References
[edit]- ^ Genus Natrix at The Reptile Database. www.reptile-database.org.
- ^ de Lazaro, Enrico (February 23, 2016). "Iberian Grass Snake: Cryptic New Species of Snake Identified". Sci-News.com.
- ^ "New snake species identified in the UK". BBC News. 7 August 2017.
- ^ a b c "Natrix Laurenti, 1768". Encyclopedia of Life. http://eol.org/pages/35261/overview
- ^ Rage, JC; Szyndlar, Z (1986). "Natrix longivertebrata from the European Neogene, a snake with one of the longest known stratigraphic ranges". N. Jb. Geol. Palaonth. Mh. 1986 (1). Stuttgart: 56–64. Retrieved 15 September 2022.
- ^ a b Ivanov, Martin (2002). "The oldest known Miocene snake fauna from Central Europe: Merkur-North locality, Czech Republic". Acta Palaeontologica Polonica. 47 (3): 513–534. Retrieved 16 September 2022.
- ^ "†Natrix merkurensis Ivanov 2002 (water snake)". Fossilworks. n.d. Retrieved 16 September 2022.
- ^ "†Natrix mlynarskii Rage 1988 (water snake)". Fossilworks. n.d. Retrieved 16 September 2022.
- ^ "†Natrix parva Szyndlar 1984 (water snake)". Fossilworks. n.d. Retrieved 16 September 2022.
- ^ "adder, n.1". OED Online. Oxford University Press. March 2019. Retrieved 6 May 2019.
- ^ "adder". Online Etymology Dictionary. Retrieved 6 May 2019.
- ^ Kindler, Carolin; Graciá, Eva; Fritz, Uwe (29 January 2018). "Extra-Mediterranean glacial refuges in barred and common grass snakes (Natrix helvetica , N. natrix)". Scientific Reports. 8 (1): 1821. Bibcode:2018NatSR...8.1821K. doi:10.1038/s41598-018-20218-2. PMC 5788984. PMID 29379101.
Further reading
[edit]- Laurenti JN (1768). Specimen medicum, exhibens synopsin reptilium emendatam cum experimentis circa venena et antidota reptilium austriacorum. Vienna: "Joan. Thom. Nob. de Trattnern". 214 pp. + Plates I-V. (Natrix, new genus, p. 73). (in Latin).
Natrix
View on GrokipediaTaxonomy
Classification
The genus Natrix is classified within the kingdom Animalia, phylum Chordata, class Reptilia, order Squamata, suborder Serpentes, family Colubridae, and subfamily Natricinae.[5] The genus was established by Josephus Nicolaus Laurenti in 1768, with Natrix natrix designated as the type species (originally described as Coluber natrix by Linnaeus in 1758).[6] Phylogenetically, Natrix forms a monophyletic group within the Natricinae subfamily, closely related to other Eurasian natricine genera such as Rhabdophis and Amphiesma (formerly grouped under Tropidonotus in older classifications).[7] Molecular analyses, including sequences from mitochondrial genes like 16S rRNA and cytochrome b, have robustly supported this monophyly, revealing Natrix as a distinct clade that diverged during the Miocene.[7] These studies highlight the genus's evolutionary ties to semiaquatic colubrids in the Palearctic region. Taxonomic revisions in recent decades have refined the genus's composition, notably elevating former subspecies to species level based on integrated genetic and morphological data. For instance, in 2017, the barred grass snake (Natrix helvetica) was recognized as a full species distinct from N. natrix, supported by mitochondrial DNA phylogenies showing deep divergence and limited hybridization across contact zones like the Rhine River.[8] Currently, Natrix includes five extant species: N. astreptophora, N. helvetica, N. maura, N. natrix, and N. tessellata.[9] The fossil record documents at least five extinct species, such as N. longivertebrata from the Neogene, indicating a longer evolutionary history in Europe.[10]Etymology
The genus name Natrix derives from the classical Latin nātrīx, meaning "water snake" or "swimming snake," reflecting the semi-aquatic habits of many species in the genus.[11] This term traces back to the Proto-Indo-European root *(s)nh₁-tr-ih₂-, denoting "snake," with cognates in other Indo-European languages such as Old High German natar (viper) and English "adder."[12] The suffix in nātrīx likely emphasizes an aquatic association, distinguishing it from more general Latin terms for snakes like serpens.[13] The genus Natrix was formally established by Austrian naturalist Josephus Nicolaus Laurenti in his 1768 publication Specimen Medicum, Exhibens Synopsin Reptilium Emendatam cum Experimentis Circa Venena et Antidota Reptilium Austriacorum, where he classified several snake species under this name.[14] Laurenti's nomenclature drew influence from ancient Roman descriptions of natricine snakes in works by authors like Pliny the Elder, who referenced similar aquatic colubrids in natural history texts.[15] Species within Natrix bear common names that highlight their habitats and appearances, such as "grass snake" for the more terrestrial N. natrix and "viperine water snake" for the semi-aquatic N. maura.[11][16] Regional variations include "ringed snake" for N. natrix in parts of Europe, alluding to its distinctive yellow collar pattern.[17] To avoid confusion, Natrix species should be distinguished from North American "water snakes" in the genus Nerodia, which were formerly classified under Natrix but separated based on phylogenetic differences in the 1970s; Nerodia represents a distinct New World lineage within the Natricinae subfamily.[18]Description
Physical Characteristics
Natrix species are medium-sized colubrid snakes characterized by a slender, cylindrical body form adapted for semi-aquatic and terrestrial locomotion. Their dorsal scales are keeled and arranged in 17-23 rows at mid-body, providing some texture for grip on varied substrates, while the anal scale is divided.[6] Females are typically larger than males, with adults generally reaching 60–150 cm in total length, though exceptional individuals can exceed 2 m, as recorded in N. natrix; neonates hatch at 11–22 cm.[6][19][2][20] The head is distinctly separated from the neck, featuring large eyes with round pupils indicative of diurnal vision, and lacking heat-sensing pits typical of pit vipers.[21][22] These snakes are non-venomous rear-fanged colubrids, with no enlarged rear fangs for venom delivery. They have 155–189 ventral scales along the body. Males possess hemipenes for copulation, and the genus exhibits an oviparous reproductive system involving egg-laying.[23][2] Sensory adaptations in Natrix emphasize chemical and visual cues suited to their active lifestyle. The Jacobson's organ, a vomeronasal structure, facilitates chemoreception by processing scents gathered via the forked tongue.[24] Their eyesight supports diurnal foraging, with round pupils allowing better light regulation compared to nocturnal species.[22]Coloration and Patterns
Species in the genus Natrix exhibit a range of dorsal colorations, typically olive-green, brown, or gray, which provide effective camouflage in their wetland and riparian habitats. The ventral surface is generally yellowish or white, often marked with a black checkered pattern that aids in blending with substrate during foraging. These base colors vary geographically and by species, with northern populations tending toward darker gray tones and southern ones showing more reddish or olive hues.[2][25] Common patterns include transverse bands, spots, or blotches arranged in four longitudinal rows along the dorsum, creating a mottled appearance that disrupts the snake's outline. Many species feature a distinct yellow, white, or orange collar bordered in black behind the head, serving as a key identifying trait. For instance, N. tessellata displays a more tessellated pattern of alternating dark and light scales. Melanistic forms, where the body is predominantly black, and rare albino variants with reduced pigmentation are documented across the genus, though they occur sporadically and increase in frequency at higher latitudes.[2][25][26] Dorsal scales in Natrix are weakly to strongly keeled, arranged in 17–23 rows at midbody, contributing to a relatively smooth gliding motion over surfaces despite the keeling. This scale structure, combined with the cryptic coloration, supports ambush predation by minimizing visibility to prey and predators in vegetated or aquatic environments. Sexual dichromatism is minimal, with no pronounced color differences between sexes, though subtle variations in brightness may occur in males during the breeding season in some populations.[2][27][28] Ontogenetic changes are evident, particularly in juveniles, which often display bolder patterns and more contrasting collars for enhanced camouflage against avian and mammalian predators. In adults, these markings typically fade, resulting in a more uniform coloration that aligns with their larger size and altered microhabitat use. Such shifts are observed across species like N. helvetica and N. astreptophora, where juvenile spots diminish with age.[2][29]Distribution and Habitat
Geographic Range
The genus Natrix is primarily distributed across the Palearctic region, encompassing temperate Eurasia from western Europe—including the Iberian Peninsula and the United Kingdom—eastward to western China and Pakistan, with northern limits reaching southern Scandinavia and southern extensions into Egypt and northwest Africa.[30] This range spans diverse continental areas without evidence of transoceanic dispersal, relying instead on land bridges for expansion.[31] Biogeographically, the genus dominates temperate Eurasian zones, with disjunct populations in North Africa, such as N. maura in Morocco.[30] For instance, N. tessellata extends to Egypt, marking the southernmost extent in Africa.[27] The historical spread involved post-glacial colonization of northern Europe approximately 10,000 years ago, originating from Mediterranean and Caucasian refugia, with expansions following river systems like the Danube into central Europe.[31][32] Introduced populations are rare and typically not established; for example, N. maura was likely introduced to Mallorca by human activity, while escapes in non-native areas like UK islands have not led to viable populations.[30] Endemism is particularly high in the Mediterranean, with distinct clades restricted to the Iberian Peninsula and Ibero-Maghrebian regions, such as N. astreptophora.[30]Habitat Preferences
Natrix snakes, as a genus, exhibit semi-aquatic to terrestrial lifestyles, with a strong dependence on proximity to permanent or semi-permanent water bodies such as rivers, streams, lakes, ponds, marshes, swamps, and coastal wetlands for thermoregulation, foraging, and shelter. These habitats provide essential moisture and opportunities for basking on emergent vegetation or banks, while surrounding terrestrial areas support movement and refuge.[4] In terms of vegetation, the genus favors diverse landscapes including temperate forests, grasslands, mixed woodlands, dense scrublands, and riverine corridors, often selecting sunny, open microhabitats with ample cover such as reed beds, fallen logs, dense undergrowth, or rocky outcrops for concealment and ambush hunting. Species show a marked preference for ecotones and habitat edges over dense interiors like closed-canopy forests or intensively cropped fields.[33][4] Climatically, Natrix species are adapted to temperate zones characterized by mild winters and moderate precipitation, typically avoiding arid deserts or extreme cold beyond their range; hibernation occurs during colder months (November to February) in sheltered sites like rodent burrows, rock fissures, cellars, or even underwater in milder conditions, with activity resuming in spring (March to October).[4][27] The altitudinal distribution extends from sea level up to 2,000–3,200 meters, encompassing lowlands to montane regions but excluding high-altitude extremes or hyper-arid environments.[4] Natrix tolerates certain human-modified habitats, such as agricultural farmlands, irrigation ditches, fish ponds, and humid meadows, where they can exploit altered water sources; however, they are sensitive to habitat degradation from drainage schemes, water pollution, and urbanization, which reduce suitable wetland availability and affect body condition.[34][4] Species within the genus display some variation in habitat use, with more aquatic preferences in species like N. maura compared to the semi-aquatic N. natrix.[35]Behavior
Activity Patterns
Species of the genus Natrix are primarily diurnal, foraging and basking during daylight hours to regulate body temperature and capture prey, with activity peaking in the morning and afternoon in temperate regions.[36] They frequently bask in open sunlit areas to achieve preferred body temperatures around 30–34°C, which optimize locomotion and digestion.[36] In hotter climates, some species shift toward crepuscular or nocturnal patterns to avoid midday heat, as evidenced in N. tessellata, and recent citizen science observations (as of 2024) indicate occasional nocturnal activity in N. natrix under certain conditions such as warmer nights or urban environments.[37][38] In temperate zones, Natrix species follow a distinct seasonal cycle, emerging from brumation sites in early spring (typically March–April) and remaining active through autumn (September–October), with inactivity during winter lasting approximately 5–7 months when temperatures drop below 10°C.[25] Brumation occurs in communal hibernacula such as rodent burrows or rock crevices, where metabolic rates decrease significantly to conserve energy.[39] Activity levels are highest from May to August, aligning with warmer weather that supports foraging and growth.[25] Movement patterns in Natrix are generally territorial yet flexible, with individuals maintaining home ranges of 0.3–5 hectares, though linear distances traveled can reach up to 500 meters daily during active periods.[40] Seasonal migrations occur, particularly to aquatic habitats for breeding, but snakes return to established ranges afterward. On land, locomotion involves lateral undulation for efficient travel over vegetation and soil, while in water, they employ side-to-side undulating motions for swimming; climbing on low vegetation is occasional but not a primary mode.[41] Optimal activity temperatures range from 25–35°C, within which foraging and movement efficiency peak; below 18°C or above 35°C, snakes reduce activity, seeking refuge by burrowing into soil or submerging in water to avoid thermal stress.[36] For instance, N. tessellata may exhibit increased nocturnal activity in warmer southern ranges to mitigate daytime overheating.[37]Defense Mechanisms
Species in the genus Natrix employ a suite of anti-predator strategies that emphasize evasion, deception, and chemical deterrence, reflecting their non-venomous nature and reliance on behavioral adaptations for survival. Primary defenses include the release of foul-smelling musk from cloacal glands, thanatosis (feigning death), and bluffing displays that mimic more dangerous species. These tactics are particularly effective against avian and mammalian predators, allowing many individuals to escape unharmed, though they offer limited protection from human activities such as road traffic.[42][43] A key chemical defense is the cloacal discharge of malodorous musk produced by anal glands, which serves to repel close-range threats by creating an unpleasant odor and sticky coating on the predator. This behavior is widespread across Natrix species; for instance, the dice snake (N. tessellata) frequently everts its cloaca and smears musk during capture, combining it with other displays for enhanced deterrence. In grass snakes (N. natrix and N. helvetica), musking often accompanies handling or restraint, providing a non-lethal barrier against mammalian predators like foxes or birds of prey. The secretion's insecticidal properties may also prevent secondary infections from scavengers post-release.[44][42][45] Thanatosis represents a passive yet dramatic defense, where individuals feign death to convince predators that the prey is unpalatable or already deceased. In N. natrix, this involves inverting the body to expose the bright yellow ventral side, gaping the mouth with the tongue protruded, and remaining immobile for minutes to hours, often after initial escape attempts fail. The dice snake (N. tessellata) exhibits an elaborate version, incorporating cloacal musk and even cloacal bleeding (autohaemorrhaging) to simulate decay, which has been shown to reduce predator interest in experimental settings. This strategy is more common in adults than hatchlings and correlates with lower attack persistence from predators like birds. Studies suggest that death feigning can increase survival in simulated predatory encounters.[46][43][47] Physical evasion prioritizes flight over confrontation, with Natrix species leveraging their agility for rapid escape. Semi-aquatic forms like the dice snake and viperine snake (N. maura) excel at swift swimming to reach water refuges, while terrestrial species such as the barred grass snake climb vegetation or burrow into cover. Tail thrashing accompanies these efforts, distracting attackers and aiding disentanglement without true autotomy, as Natrix lack the regenerative caudal structures seen in some lizards. These maneuvers are most effective in open habitats, allowing evasion from many initial predatory encounters in field observations.[42][48] Bluff displays further enhance survival by intimidating potential threats through morphological exaggeration. Hissing and neck flattening, observed in N. helvetica and N. maura, mimic the hooding of venomous vipers, potentially serving as Batesian mimicry to deter visually oriented predators. Mouth gaping reveals enlarged teeth associated with mildly toxic Duvernoy's gland secretions, bluffing venomous capability despite the saliva's low potency. These displays are size-dependent, with larger individuals more likely to employ them aggressively. Coloration patterns, which integrate camouflage for initial hiding, support these tactics by breaking the outline during static threats (see Coloration and Patterns section).[49][50][42] Overall, these mechanisms contribute to moderate to high efficacy against natural predators. However, anthropogenic threats like vehicle collisions bypass these defenses, leading to significant mortality in populated areas.[43][46]Ecology
Diet and Predation
Species of the genus Natrix are opportunistic carnivores, with diets dominated by amphibians and fish, supplemented by small mammals and invertebrates depending on availability and habitat. In N. natrix (grass snake), anurans account for approximately 63% of prey items, small mammals 25%, fish 10%, and birds 1%, reflecting a generalist feeding strategy influenced by local prey abundance.[51] Conversely, N. tessellata (dice snake) is more specialized, with fish comprising over 80% of its diet across 87 taxa, while amphibians and other vertebrates make up the remaining 20%, particularly in arid or montane regions where fish are scarce.[52] N. maura (viperine snake) shows a similar pattern, with amphibians constituting 86.6% of consumed prey.[53] Prey selection varies seasonally; for instance, N. natrix targets fish during spring spawning, shifts to newts in summer, and focuses on frogs and toads from July onward.[3] Hunting occurs via ambush tactics or active pursuit in both aquatic and terrestrial settings, leveraging the snakes' semiaquatic lifestyle for access to prey. Natrix species typically swallow prey alive but may employ constriction for larger items or mild envenomation from the Duvernoy's gland—a posterior oral gland producing toxins that aid in immobilization without rapid lethality.[3] Prey size generally reaches up to 50% of the snake's body length, with larger individuals consuming bigger items while smaller snakes (<400 mm snout-vent length) focus on juveniles or tadpoles.[51] As predators, Natrix snakes face threats from birds of prey such as hawks and herons, mammals including foxes, and larger reptiles, with juveniles experiencing particularly high mortality rates due to their vulnerability.[3][54] Ecologically, they play a key role in controlling amphibian and fish populations in wetland and riparian zones, helping maintain balance in aquatic food webs; their dependence on healthy amphibian communities also positions them as bioindicators of wetland integrity.[55][56]Reproduction
Natrix species exhibit a polygynous mating system, in which males compete for access to females during the spring breeding season, typically from April to June. Male-male competition often involves ritualized combat behaviors, such as body twining and wrestling, where larger males tend to achieve greater mating success within aggregations known as mating balls. These interactions align with the snakes' seasonal activity patterns, peaking shortly after emergence from hibernation. All species in the genus Natrix are oviparous, with females producing a single clutch of eggs annually.[57] Clutch sizes typically range from 10 to 40 eggs, though this varies with female body size and environmental conditions, with larger females laying more eggs.[57] Eggs are laid in June or July, measuring 2-3 cm in length with soft, leathery shells, and are deposited in communal nesting sites such as rotting vegetation, manure heaps, or compost piles that provide suitable humidity and heat.[58] Incubation occurs externally in these warm, humid microhabitats, with optimal temperatures around 25-30°C facilitating embryonic development.[58] Hatching takes 4-10 weeks, depending on temperature, with higher temperatures accelerating the process but potentially affecting hatchling morphology if extremes are reached.[20] Females provide no parental care after oviposition, leaving eggs vulnerable to high predation rates by mammals, birds, and other reptiles.[57] Sexual maturity is reached at 3-5 years of age, corresponding to a snout-vent length of approximately 50-70 cm, after which individuals breed annually.[59] In the wild, Natrix snakes have a lifespan of 10-15 years, though captives can live up to 25 years under optimal conditions.[60]Species
Extant Species
The genus Natrix comprises five extant species of non-venomous colubrid snakes in the subfamily Natricinae, primarily distributed across Europe, western Asia, and northwestern Africa.[61] These species exhibit varying degrees of semi-aquatic habits, with distinct morphological and ecological adaptations. Natrix astreptophora, known as the Iberian grass snake or red-eyed grass snake, is characterized by a reddish iris, reduced ventral scale counts (typically 150-170), and a dorsal pattern of alternating dark oval spots or bold bands on an olive-gray background; it inhabits wetlands and grasslands in the Iberian Peninsula, southern France, and northwestern Africa (Morocco, Algeria, Tunisia), reaching lengths up to 100 cm.[62][63] Natrix helvetica, the barred grass snake, features a distinctive barred or zigzag dorsal pattern, yellow collar, and black dorsal spots; it occurs in western and central Europe including the UK, France, Germany west of the Rhine, Italy, and northern Spain, with adults typically measuring 90-150 cm.[64][65] Natrix maura, the viperine water snake, has a slender body, viper-like head shape, and often uniform grayish or olive dorsal coloration with faint bands; it is the most aquatic species, favoring rivers and lakes in the Iberian Peninsula, southern France, Italy (Sardinia), and northwestern Africa, growing to about 80-100 cm and specializing in a fish-based diet.[16][66] Natrix natrix, the common grass snake, displays a variable olive-green to brown dorsum with a prominent yellow or white collar and dark vertebral stripe; it ranges widely from Scandinavia and western Europe (east of the Rhine) across central and eastern Europe to western Asia (including parts of Russia, the Middle East, and northwestern China), attaining lengths of 100-150 cm (up to 205 cm maximum) and primarily preying on amphibians.[11][67] Natrix tessellata, the dice snake, exhibits a checkered or tessellated dorsal pattern of dark spots on a grayish to yellowish background, adapted for camouflage in aquatic environments; it inhabits rivers, lakes, and coasts from central Europe through eastern Europe and western Asia to northeastern Egypt and northwestern China, reaching 100-130 cm and being predominantly piscivorous.[68][69] Key differences among the species include habitat affinity and trophic specialization: N. maura and N. tessellata are highly aquatic and fish-oriented, while N. natrix and N. helvetica are more terrestrial with amphibian-focused diets; N. astreptophora shows intermediate traits with bolder patterning for open habitats.[70] The following table summarizes comparative traits:| Species | Maximum Size (cm) | Dorsal Pattern | Primary Diet | Geographic Range |
|---|---|---|---|---|
| N. astreptophora | 100 | Bold bands or oval spots | Amphibians, fish | Iberia, S France, NW Africa |
| N. helvetica | 150 | Barred or zigzag | Amphibians | W/C Europe, UK, N Spain |
| N. maura | 100 | Faint bands, uniform olive | Fish | Iberia, S France, NW Africa, Sardinia |
| N. natrix | 150 (max 205) | Yellow collar, vertebral stripe | Amphibians | Europe (east of Rhine) to W Asia |
| N. tessellata | 130 | Checkered/tessellated spots | Fish | C/E Europe to W Asia, NE Egypt |
Extinct Species
The fossil record of the genus Natrix spans from the Early Miocene to the Pleistocene, documenting approximately 10-15 named extinct species primarily from European deposits, with the temporal range extending from about 20 million years ago to 10,000 years ago.[72] These fossils reveal an early diversification of natricine snakes in aquatic and semi-aquatic environments, with key occurrences in central and western Europe, including sites in the Czech Republic, Poland, France, and Hungary.[73] Asian records, such as Pliocene fossils from Moldova, indicate a broader historical distribution before the Pleistocene.[10] Notable extinct species include Natrix merkurensis Ivanov, 2002, from the Early Miocene (MN 3a) Merkur-North locality in the Czech Republic, representing one of the earliest records and characterized by vertebrae with moderately developed subcentral ridges.[72] Natrix sansaniensis (Lartet, 1851), known from Middle Miocene sites like Sansan in France, features typical Natrix-like vertebral morphology, including a bulky centrum and low neural arch, and marks an early appearance of the genus in western Europe.[72] Natrix rudabanyaensis Szyndlar, 2005, from the Late Miocene (MN 9) of Rudabánya, Hungary, is a small-sized form with rounded haemal keels, suggesting adaptation to forested wetland habitats.[74] Further examples encompass Natrix longivertebrata Szyndlar, 1984, which has one of the longest stratigraphic ranges among fossil colubrids, from the Late Miocene to Upper Pliocene across sites in Poland, France, and Moldova, with elongated vertebrae (centrum length 4.27–5.58 mm) indicating a transitional form toward extant N. natrix.[73] Natrix parva Szyndlar, 1984, from Miocene deposits in Poland (possibly extending to Late Pleistocene), is distinguished by its small size and straight hypapophyses, though its taxonomic status remains debated due to limited material.[73] These species often co-occur with fossils of amphibians and fish in fluvial and lacustrine sediments, pointing to paleoenvironments dominated by ancient wetlands and riparian forests.[72] Evolutionary trends in the Natrix fossil record show a progression toward greater body size and enhanced aquatic specialization, with vertebral features like deepened prezygapophyseal accessory processes evolving from Miocene ancestors.[10] The extinction of many pre-Pleistocene forms, including N. longivertebrata and N. rudabanyaensis, correlates with post-Pleistocene climate cooling and habitat fragmentation, reducing suitable wetland areas across Europe.[10] This paleontological evidence underscores the genus's resilience, as surviving lineages adapted to changing conditions leading to modern diversity.[75]Conservation
Status Across Species
The genus Natrix comprises species that are predominantly assessed as Least Concern (LC) on the IUCN Red List as of the latest assessments (e.g., 2021), reflecting their wide distributions across Eurasia and North Africa, though many populations exhibit fragmentation and local declines due to habitat alterations.[76] For instance, N. natrix (now often split into N. helvetica in western Europe, recognized as a distinct species since 2017) is classified as LC globally and in Europe, but regional assessments indicate vulnerability in isolated populations, such as in the United Kingdom where it is considered a priority species under the UK Biodiversity Action Plan with evidence of population declines.[77] Recent European Red List updates (2025) confirm stable LC status for N. helvetica. Species-specific statuses vary, with most remaining stable or LC but certain taxa facing heightened risks. N. helvetica, the barred grass snake of western Europe, is LC overall, but the subspecies N. h. cetti is classified as Endangered due to its restricted range and ongoing habitat loss in Sardinia.[78] N. maura, the viperine snake, is LC across its Iberian and North African range, with stable populations in core wetland habitats. N. tessellata, the dice snake, is also LC but with a decreasing trend noted in peripheral populations, such as in the Czech Republic where habitat degradation has led to local extirpations and a national status of Critically Endangered.[79] N. astreptophora, the Iberian grass snake, lacks a global IUCN assessment but is regarded as LC in Spain and Portugal, though vulnerable in parts of Iberia due to agricultural intensification affecting riparian zones.[62] The subspecies N. n. cypriaca (Cyprus grass snake) stands out as Critically Endangered, with sparse populations confined to ephemeral wetlands on Cyprus and recent evidence of local extinctions, prompting calls for its formal inclusion on the IUCN Red List.[80] Population trends across the genus show stability in core Eurasian ranges but declines in peripheral and fragmented areas where wetland loss has reduced suitable habitats over recent decades.[79] These declines are most pronounced in isolated populations, such as those of N. helvetica in the UK and N. tessellata in central Europe, while central Asian and eastern European core areas remain relatively secure.[81][82] Several Natrix species receive legal protections under European frameworks, including Annex V of the EU Habitats Directive, which mandates strict regulation of exploitation for N. helvetica, N. tessellata, N. maura, and N. astreptophora to maintain favorable conservation status in designated Natura 2000 sites.[83][84] Additionally, they are listed under Appendices II and III of the Bern Convention, affording protection against deliberate killing or trade in signatory countries.[85] Conservation monitoring relies on citizen science initiatives and genetic studies to assess subspecies viability, such as ongoing surveys by the Amphibian and Reptile Groups of the UK (ARG UK) for N. helvetica and targeted population censuses for N. n. cypriaca using environmental DNA and field observations to track genetic diversity in small, isolated groups.[86][87]Threats and Protection
Natrix species face significant anthropogenic threats that contribute to population declines across their range. Habitat destruction, primarily through wetland drainage and agricultural expansion, is a leading risk, fragmenting essential aquatic and riparian environments critical for foraging and hibernation. Road mortality exacerbates this issue, with vehicles causing high rates of fatalities, particularly among juveniles dispersing from natal sites; studies on Natrix natrix have recorded mortality peaks exceeding 200 individuals per kilometer annually on suburban roads. Persecution driven by misconceptions of venomousness leads to illegal killings, as these non-venomous colubrids are often targeted out of fear in rural and urban fringes. Secondary threats include climate change, which disrupts hibernation cycles and breeding phenology; long-term data from northern populations of N. natrix indicate shifts in survival rates and body size linked to warming temperatures. Pollution from pesticides diminishes prey availability, such as amphibians, while invasive species introduce competition for resources in altered habitats. These factors compound habitat fragmentation, reducing overall population viability. Conservation efforts emphasize habitat restoration through initiatives like the EU LIFE projects, which have targeted wetland recovery and landscape connectivity in regions such as Cyprus to benefit Natrix populations. Education campaigns aim to dispel myths about snake danger, reducing persecution, while captive breeding programs for endangered subspecies, including the Cyprus grass snake (N. natrix cypriaca), have been established since around 2010 to bolster genetic stocks and support reintroductions. In protected areas like the Danube Delta Biosphere Reserve, enhanced management has contributed to favorable conservation statuses for N. tessellata through population stabilization and habitat safeguards. Successes include observed recoveries in isolated populations within restored wetlands, such as those for N. tessellata in the Danube Delta, where protected zones have facilitated increased abundances amid broader declines elsewhere. Looking ahead, creating wildlife corridors is essential to enhance connectivity between fragmented habitats, while ongoing research into genetic diversity will inform targeted interventions to maintain adaptive potential in the face of escalating environmental pressures.References
- https://en.wiktionary.org/wiki/natrix

