Recent from talks
Nothing was collected or created yet.
Pneumonic plague
View on Wikipedia
| Pneumonic plague | |
|---|---|
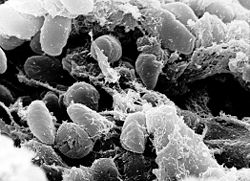 | |
| A scanning electron micrograph depicting a mass of Yersinia pestis bacteria | |
| Specialty | Infectious disease |
| Symptoms | Fever, headache, shortness of breath, cough, coughing up blood[1] |
| Usual onset | 3 to 7 days[2] |
| Causes | Yersinia pestis[3] |
| Risk factors | Rodents[3] |
| Diagnostic method | Sputum testing[1] |
| Treatment | Antibiotics[1] |
| Prognosis | Nearly 100% fatal if untreated[4] |
| Frequency | 3% of all Plague cases (CDC MMWR 2015) [5] |
Pneumonic plague is a severe lung infection caused by the bacterium Yersinia pestis.[3] Symptoms include fever, headache, shortness of breath, chest pain, coughing, and coughing up blood.[1] They typically start about three to seven days after exposure.[2] It is one of three forms of plague, the other two being septicemic plague and bubonic plague.[3]
The pneumonic form may occur following an initial bubonic or septicemic plague infection.[3] It may also result from breathing in airborne droplets from another person or animal infected with pneumonic plague.[1] The difference between the forms of plague is the location of infection; in pneumonic plague the infection is in the lungs, in bubonic plague the lymph nodes, and in septicemic plague within the blood.[3] Diagnosis is by testing the blood, sputum, or fluid from a lymph node.[1]
While vaccines are being developed, in most countries they are not yet commercially available.[1][3] Prevention is by avoiding contact with infected rodents, people, or cats.[1][3] It is recommended that those infected be isolated from others.[2] Treatment of pneumonic plague consists of antibiotics.[1]
Plague is present among rodents in Africa, the Americas, and Asia.[3] Pneumonic plague is more serious and less common than bubonic plague.[1] The total reported number of cases of all types of plague in 2013 was 783.[2] Left untreated, pneumonic plague is almost always fatal.[6] Some hypothesize that the pneumonic version of the plague was mainly responsible for the Black Death that resulted in approximately 25 million deaths in the 1300s.[2][7]
Signs and symptoms
[edit]The most apparent symptom of pneumonic plague is coughing, often with hemoptysis (coughing up blood). With pneumonic plague, the first signs of illness are fever, headache, weakness and rapidly developing pneumonia with shortness of breath, chest pain, cough and sometimes bloody or watery sputum.[8]
The pneumonia progresses for two to four days and may cause respiratory failure and shock. Patients will die without early treatment, some within 36 hours.[citation needed]
Initial pneumonic plague symptoms can often include the following:[citation needed]
Rapidly developing pneumonia with:[citation needed]
- Shortness of breath
- Chest pain
- Cough
- Bloody or watery sputum (saliva and discharge from respiratory passages).
Causes
[edit]Pneumonic plague can be caused in two ways: primary, which results from the inhalation of aerosolized plague bacteria, or secondary, when septicemic plague spreads into lung tissue from the bloodstream. Pneumonic plague is not exclusively vector-borne like bubonic plague; instead, it can be spread from person to person. There have been cases of pneumonic plague resulting from the dissection or handling of contaminated animal tissue. This is one of the types of plague formerly known as the Black Death.[9]
Treatment
[edit]Pneumonic plague is a very aggressive infection requiring early treatment, which must be given within 24 hours of first symptoms to reduce the risk of death.[8] Streptomycin, gentamicin, tetracyclines and chloramphenicol are all able to kill the causative bacterium.[citation needed]
Antibiotic treatment for seven days will protect people who have had direct, close contact with infected patients. Wearing a close-fitting surgical mask also protects against infection.[8]
The mortality rate from untreated pneumonic plague approaches 100% although victims of the Black Death who vomited blood occasionally survived, such as the chronicler Marcha di Marco Battagli.[10][11]
Modern outbreaks
[edit]Since 2002, the World Health Organization (WHO) has reported seven plague outbreaks, though some may go unreported because they often happen in remote areas. Between 1998 and 2009, nearly 24,000 cases were reported, including about 2,000 deaths, in Africa, Asia, the Americas, and Eastern Europe. Ninety-eight percent of the world's cases occur in Africa.
Democratic Republic of the Congo
[edit]Two outbreaks occurred in the Democratic Republic of the Congo in 2005 and 2006.[12] The outbreak in 2005 was only detected by looking back at blood samples.[12] The total death toll was 111.
India
[edit]In September 1994, India experienced an outbreak of plague that killed 50 and caused travel to New Delhi by air to be suspended until the outbreak was brought under control.[13] The outbreak was feared to be much worse because the plague superficially resembles other common diseases such as influenza and bronchitis; over 200 people who had been quarantined were released when they did not test positive for the plague.[14] All but three of the deaths occurred around the city of Surat.[15]
China
[edit]A major outbreak of the pneumonic plague occurred in Manchuria from 1910 to 1911, in what became known as the Manchurian plague, killing around 60,000 people.[16] The Qing court dispatched Wu Lien-teh, a doctor educated at Cambridge University, to oversee disease control and treatment efforts. He made the novel observation that the disease was transmitted by air, and developed prototypical respirators to help prevent its spread.[17] A second, less deadly outbreak occurred in 1920–21, killing approximately 9,300 people.[16]
The People's Republic of China has eradicated pneumonic plague from most parts of the country, but still reports occasional cases in remote western areas, where the disease is carried by rats and the marmots that live across the Himalayan plateau. Outbreaks can be caused when a person eats an infected marmot or comes into contact with fleas carried by rats. A 2006 WHO report from an international meeting on plague cited a Chinese government disease expert as saying that most cases of the plague in China's northwest occur when hunters are contaminated while skinning infected animals.[18]
The expert said at the time that, due to the region's remoteness, the disease killed more than half the infected people. The report also said that since the 1990s, there was a rise in plague cases in humans, from fewer than 10 in the 1980s to nearly 100 cases in 1996 and 254 in 2000.[19] In September 2008, two people in East Tibet died of pneumonic plague.[20]
An outbreak of the disease in China began in August 2009 in Ziketan Town located in Qinghai Province. The town was sealed off, and several people died as a result of the disease.[18][21] According to spokesperson Vivian Tan of the WHO office in Beijing, "In cases like this [in August 2009], we encourage the authorities to identify cases, to investigate any suspicious symptoms among close contacts, and to treat confirmed cases as soon as possible. So far, they have done exactly that. There have been sporadic cases reported around the country in the last few years so the authorities do have the experience to deal with this."[22]
In September 2010, five cases of pneumonic plague were reported in Tibet.[23]
In July 2014, Chinese media reported one case found in Gansu.[24]
On 12 November 2019, it was announced that two people from the Chinese province of Inner Mongolia were diagnosed with pneumonic plague. They received treatment in Chaoyang District, Beijing, and authorities implemented preventative control measures.[25] Later in November, a third case of plague was confirmed. A 55-year-old man was diagnosed with bubonic plague after eating wild rabbit in Inner Mongolia. The region's health commission says it has no evidence to suggest that this case is linked to the previous two.[26] By the end of November, a fourth case was confirmed. Chinese health authorities reported a fresh case of bubonic plague in the country's northern Inner Mongolia region, bringing the total number of reported plague cases originating from Inner Mongolia to four.[27]
Peru
[edit]In August 2010, Peru's health minister, Oscar Ugarte, announced that an outbreak of plague had killed a 14-year-old boy and had infected at least 31 people in a northern coastal province. The boy died of bubonic plague on 26 July 2010. Ugarte stated that authorities were screening sugar and fish meal exports from Ascope Province, located about 325 miles (520 km) northwest of Lima, not far from the popular Chicama beach. Most of the infections in Peru were bubonic plague, with four cases of pneumonic plague.[28]
The first recorded plague outbreak in Peru was in 1903. Before the above case, the last known outbreak was in 1994, killing 35 people.[29]
Madagascar
[edit]
In November 2013, an outbreak of plague occurred in the African island nation of Madagascar.[30] As of 16 December, at least 89 people were infected, with 39 deaths[31] with at least two cases involving pneumonic plague. However, as many as 90% of cases were later reported to have involved pneumonic plague.[32]
From 23 August to 30 September 2017, a total of 73 suspected, probable, and confirmed cases of pneumonic plague, including 17 deaths, were reported in Madagascar.[33] The diagnosis was confirmed by the Institut Pasteur de Madagascar by a polymerase chain reaction test, while field health workers used a Rapid Diagnostic Test. The WHO and Institut Pasteur de Madagascar were both involved in administering antibiotic compounds and attempting to stop the spread of the disease. By mid-October, there were an estimated 684 confirmed cases of plague with 474 pneumonic, 156 bubonic and one septicemic. The remainder were not classified. At least 74 deaths have been ascribed to pneumonic plague.[34]
The outbreak officially ended on 26 November 2017 with 2,348 cases and 202 deaths officially reported.[35]
United States
[edit]In the fall of 1924, an outbreak occurred in Los Angeles[36] that killed 30 people.
On 2 November 2007, wildlife biologist Eric York died of pneumonic plague in Grand Canyon National Park. York was exposed to the bacteria while conducting a necropsy on a cougar carcass.[37]
In 2014, the Colorado Department of Public Health and Environment confirmed that a Colorado man had been diagnosed with pneumonic plague, the first confirmed human case in Colorado in more than 10 years, and one of only 60 cases since 1957. The man was found to have the disease after the family dog died unexpectedly, and a necropsy revealed that the disease was the cause.[38][39] Three additional pneumonic plague cases were confirmed in Colorado before the outbreak ended.[40]
A person near Flagstaff, Arizona died of pneumonic plague in July 2025.[41]
References
[edit]- ^ a b c d e f g h i j "FAQ Plague". www.cdc.gov. Archived from the original on 14 March 2017. Retrieved 13 March 2017.
- ^ a b c d e "Plague". World Health Organization. September 2016. Archived from the original on 24 April 2015. Retrieved 14 March 2017.
- ^ a b c d e f g h i "Plague". www.who.int. Archived from the original on 19 March 2017. Retrieved 14 March 2017.
- ^ "Plague" (PDF). www.cfsph.iastate.edu. Archived from the original (PDF) on 31 January 2017.
- ^ "Human Plague — United States, 2015". www.cdc.gov. 28 August 2015. Archived from the original on 11 February 2025. Retrieved 14 March 2025.
- ^ "Plague" (PDF). Archived from the original (PDF) on 17 September 2004.
- ^ McCoy, Terrence (31 March 2014). "Everything you know about the Black Death is wrong". Washington Post. The Washington Post. Archived from the original on 27 August 2016. Retrieved 14 March 2017.
- ^ a b c Facts about Pneumonic Plague (Center for Disease Control, 2004) Archived 2 August 2013 at the Wayback Machine
- ^ Benedictow, Ole Jørgen (2004). The Black Death, 1346–1353: The Complete History. Boydell & Brewer. pp. 27–28. ISBN 0-85115-943-5.
- ^ Plague Archived 31 January 2017 at the Wayback Machine. (Iowa State University, 2004)
- ^ Samuel Cohn's The Black Death Transformed: Disease and Culture in Early Renaissance Europe, pg 93.
- ^ a b Bertherat, Eric; Thullier, Philippe; Shako, Jean Christophe; England, Kathleen; Koné, Mamadou-Lamine; Arntzen, Lorraine; Tomaso, Herbert; Koyange, Louis; Formenty, Pierre; Ekwanzala, Florent; Crestani, Rosa; Ciglenecki, Isa; Rahalison, Lila (2011). "Lessons Learned about Pneumonic Plague Diagnosis from 2 Outbreaks, Democratic Republic of the Congo". Emerging Infectious Diseases. 17 (5): 778–84. doi:10.3201/eid1705.100029. PMC 3321750. PMID 21529384.
- ^ Plague Fears Cancel Flights. The Daily Gleaner, 1 October 1994.
- ^ Plague Fears Beginning to Recede. The Daily Gleaner, 2 October 1994.
- ^ Mavalankar, D. V. (October 1995). "Indian 'plague' epidemic: unanswered questions and key lessons". Journal of the Royal Society of Medicine. 88 (10): 547–551. PMC 1295353. PMID 8537942.
- ^ a b Welford, Mark R.; Bossak, Brian H.; Carter, Dee A. (22 December 2009). "Validation of Inverse Seasonal Peak Mortality in Medieval Plagues, Including the Black Death, in Comparison to Modern Yersinia pestis-Variant Diseases". PLOS ONE. 4 (12) e8401. Bibcode:2009PLoSO...4.8401W. doi:10.1371/journal.pone.0008401. PMC 2791870. PMID 20027294.
- ^ Wilson, Mark (24 March 2020). "The untold origin story of the N95 mask". Fast Company.
- ^ a b "China disinfects town where plague killed 3rd man". Associated Press. 4 August 2009. Archived from the original on 8 August 2009.
- ^ Macartney, Jane (3 August 2009). Entire town in quarantine after two die from pneumonic plague in China Archived 29 April 2011 at the Wayback Machine. The Times (London).
- ^ "Canada Tibet Committee | Library | WTN". www.tibet.ca. Archived from the original on 14 November 2009.
- ^ "4th plague patient near death in NW China province". Xinhua. 5 August 2009. Archived from the original on 8 August 2009.
- ^ Alert over China plague outbreak Archived 3 August 2009 at the Wayback Machine. Al Jazeera. 3 August 2009.
- ^ Dawa, W; Pan, W. J.; Gu, X. Y.; Zhang, S. Q.; Dawa, C; Yi, X; Ciwang, Z; Wang, Y; Li, S. Y.; Jiang, R. M. (2011). "Clinical features, diagnosis and treatment of 5 cases of primary pneumonic plague in Tibet in 2010". Zhonghua jie he he hu xi za zhi. 34 (6): 404–08. PMID 21781509.
- ^ "151 quarantined in Gansu after plague death – China". Chinadaily.com.cn. 18 July 2014. Archived from the original on 18 July 2014. Retrieved 30 January 2018.
{{cite web}}: CS1 maint: bot: original URL status unknown (link) - ^ Yeung, Jessie. "Two people got the plague in China. Why is it still a thing?". CNN. Retrieved 14 November 2019.
- ^ Samuel, Sigal (14 November 2019). "We never really got rid of the plague. 3 people in China just caught it". Vox. Retrieved 25 July 2020.
- ^ "China reports fourth case of plague this month". Reuters. 28 November 2019. Retrieved 25 July 2020 – via www.reuters.com.
- ^ Peru suffers deadly outbreak of bubonic and pneumonic plague Archived 4 February 2013 at the Wayback Machine, The Telegraph, 3 August 2010. Accessed 4 November 2014.
- ^ "Peru's health minister says plague outbreak has killed teenager, infected at least 31 people". Archived from the original on 17 August 2010. Retrieved 3 August 2010.
- ^ "Plague kills 39 in Madagascar". www.theaustralian.com.au. 12 December 2013.
- ^ "Madagascar hit by 'deadlier plague'". BBC News. 11 December 2013. Archived from the original on 16 December 2013. Retrieved 16 December 2013.
- ^ "Bubonic plague 'worse than Black Death' kills 39 in Madagascar" Archived 16 December 2013 at the Wayback Machine, South China Morning Post, 12 December 2013. Accessed 4 November 2014.
- ^ "Plague – Madagascar Disease outbreak news". Archived from the original on 2 October 2017. Retrieved 5 October 2017.
- ^ Beaumont, Peter (19 October 2017). "'It is a dangerous moment': Madagascar plague death toll reaches 74". The Guardian.
- ^ "WHO | Plague – Madagascar". WHO. Archived from the original on 28 November 2017. Retrieved 7 August 2020.
- ^ Donnelly, Thomas M.; Bergin, Ingrid; Ihrig, Melanie (2015). "Biology and Diseases of Other Rodents". Laboratory Animal Medicine. pp. 285–349. doi:10.1016/B978-0-12-409527-4.00007-9. ISBN 978-0-12-409527-4.
- ^ "Plague emerges in Grand Canyon, kills biologist". USAToday.com. Archived from the original on 19 April 2012.
- ^ Coffman, Keith. Man diagnosed with rare pneumonic plague in Colorado, Reuters (9 July 2014).
- ^ Kaplan, Sarah. "A Colorado pit bull infected humans with the plague". Washington Post. ISSN 0190-8286. Retrieved 21 September 2020.
- ^ Basak, Sonali; Oldham, Jennifer. Four Cases of Life-Threatening Plague Found in Colorado Archived 14 January 2015 at the Wayback Machine, Bloomberg, 19 July 2014. Accessed 4 November 2014.
- ^ "Plague kills Arizona resident, marking first recorded death since 2007, health officials say". CBS News. 12 July 2025. Retrieved 12 July 2025.
External links
[edit] Media related to Pneumonic plague at Wikimedia Commons
Media related to Pneumonic plague at Wikimedia Commons- Frequently Asked Questions | Plague | CDC. Centers for Disease Control and Prevention.

