Recent from talks
Nothing was collected or created yet.
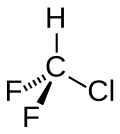
Chlorodifluoromethane
View on Wikipedia
| |||
 Liquefied chlorodifluoromethane boiling when exposed to ambient temperature and pressure.
| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
Chloro(difluoro)methane | |||
| Other names
Chlorodifluoromethane
Difluoromonochloromethane Monochlorodifluoromethane HCFC-22 R-22 Genetron 22 Freon 22 Arcton 4 Arcton 22 UN 1018 Difluorochloromethane Fluorocarbon-22 Refrigerant 22 | |||
| Identifiers | |||
3D model (JSmol)
|
|||
| ChEMBL | |||
| ChemSpider | |||
| ECHA InfoCard | 100.000.793 | ||
| EC Number |
| ||
| KEGG | |||
PubChem CID
|
|||
| RTECS number |
| ||
| UNII | |||
| UN number | 1018 | ||
CompTox Dashboard (EPA)
|
|||
| |||
| |||
| Properties | |||
| CHClF2 | |||
| Molar mass | 86.47 g/mol | ||
| Appearance | Colorless gas | ||
| Odor | Sweetish[1] | ||
| Density | 3.66 kg/m3 at 15 °C, gas | ||
| Melting point | −175.42 °C (−283.76 °F; 97.73 K) | ||
| Boiling point | −40.7 °C (−41.3 °F; 232.5 K) | ||
| 0.7799 vol/vol at 25 °C; 3.628 g/L | |||
| log P | 1.08 | ||
| Vapor pressure | 908 kPa at 20 °C | ||
Henry's law
constant (kH) |
0.033 mol⋅kg−1⋅bar−1 | ||
| −38.6·10−6 cm3/mol | |||
| Structure | |||
| Tetrahedral | |||
| Hazards | |||
| Occupational safety and health (OHS/OSH): | |||
Main hazards
|
Dangerous for the environment (N), Central nervous system depressant, Carc. Cat. 3 | ||
| GHS labelling: | |||

| |||
| Warning | |||
| H420 | |||
| P202, P262, P271, P403 | |||
| NFPA 704 (fire diamond) | |||
| Flash point | nonflammable[1] | ||
| 632 °C (1,170 °F; 905 K) | |||
| NIOSH (US health exposure limits): | |||
PEL (Permissible)
|
None[1] | ||
REL (Recommended)
|
TWA 1000 ppm (3500 mg/m3) ST 1250 ppm (4375 mg/m3)[1] | ||
IDLH (Immediate danger)
|
N.D.[1] | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||
Chlorodifluoromethane or difluoromonochloromethane is a hydrochlorofluorocarbon (HCFC). This colorless gas is better known as HCFC-22, or R-22, or CHClF
2. It was commonly used as a propellant and refrigerant. These applications were phased out under the Montreal Protocol in developed countries in 2020 due to the compound's ozone depletion potential (ODP) and high global warming potential (GWP), and in developing countries this process will be completed by 2030. R-22 is a versatile intermediate in industrial organofluorine chemistry, e.g. as a precursor to tetrafluoroethylene.
Production and current applications
[edit]Worldwide production of R-22 in 2008 was about 800 Gg per year, up from about 450 Gg per year in 1998, with most production in developing countries.[2] R-22 use is being phased out in developing countries, where it is largely used for air conditioning applications.
R-22 is prepared from chloroform:
- HCCl3 + 2 HF → HCF2Cl + 2 HCl
An important application of R-22 is as a precursor to tetrafluoroethylene. This conversion involves pyrolysis to give difluorocarbene, which dimerizes:[3]
- 2 CHClF2 → C2F4 + 2 HCl
The compound also yields difluorocarbene upon treatment with strong base and is used in the laboratory as a source of this reactive intermediate.
The pyrolysis of R-22 in the presence of chlorofluoromethane gives hexafluorobenzene.
Environmental effects
[edit]R-22 is often used as an alternative to the highly ozone-depleting CFC-11 and CFC-12, because of its relatively low ozone depletion potential of 0.055,[4] among the lowest for chlorine-containing haloalkanes. However, even this lower ozone depletion potential is no longer considered acceptable.
As an additional environmental concern, R-22 is a powerful greenhouse gas with a GWP equal to 1810 (which indicates 1810 times as powerful as carbon dioxide). Hydrofluorocarbons (HFCs) are often substituted for R-22 because of their lower ozone depletion potential, but these refrigerants often have a higher GWP. R-410A, for example, is often substituted, but has a GWP of 2088. Another substitute is R-404A with a GWP of 3900. Other substitute refrigerants are available with low GWP. Ammonia (R-717), with a GWP of <1, remains a popular substitute on fishing vessels and large industrial applications. Ammonia's toxicity in high concentrations limit its application in small-scale refrigeration applications.
Propane (R-290) is another example, and has a GWP of 3. Propane was the de facto refrigerant in systems smaller than industrial scale before the introduction of CFCs. The reputation of propane refrigerators as a fire hazard kept delivered ice and the ice box the overwhelming consumer choice despite its inconvenience and higher cost until safe CFC systems overcame the negative perceptions of refrigerators. Illegal to use as a refrigerant in the US for decades, propane is now permitted for use in limited mass suitable for small refrigerators. It is not lawful to use in air conditioners or larger refrigerators because of its flammability and potential for explosion.
-
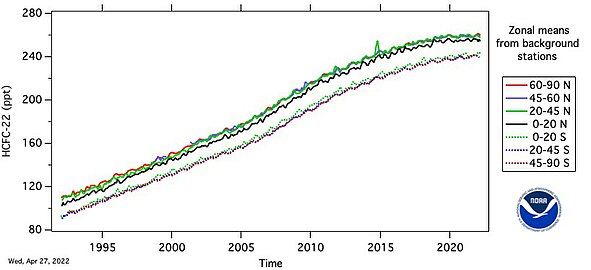
HCFC-22 measured by the Advanced Global Atmospheric Gases Experiment (AGAGE) in the lower atmosphere (troposphere) at stations around the world. Abundances are given as pollution free monthly mean mole fractions in parts-per-trillion.
-
Growth of R-22 (CFC-22) abundance in Earth's atmosphere since year 1992.[5]
Phaseout in the European Union
[edit]
Since 1 January 2010, it has been illegal to use newly manufactured HCFCs to service refrigeration and air-conditioning equipment; only reclaimed and recycled HCFCs may be used. In practice this means that the gas has to be removed from the equipment before servicing and replaced afterwards, rather than refilling with new gas.
Since 1 January 2015, it has been illegal to use any HCFCs to service refrigeration and air-conditioning equipment; broken equipment that used HCFC refrigerants must be replaced with equipment that does not use them.[6]
Phaseout in the United States
[edit]R-22 was mostly phased out in new equipment in the United States by regulatory action by the EPA under the Significant New Alternatives Program (SNAP) by rules 20 and 21 of the program,[7] due to its high global warming potential. The EPA program was consistent with the Montreal Accords, but international agreements must be ratified by the US Senate to have legal effect. A 2017 decision of the US Court of Appeals for the District of Columbia Circuit[8] held that the US EPA lacked authority to regulate the use of R-22 under SNAP. In essence the court ruled the EPA's statutory authority[9] was for ozone reduction, not global warming. The EPA subsequently issued guidance to the effect that the EPA would no longer regulate R-22. A 2018 ruling[10] by the same court held that the EPA failed to conform with required procedure when it issued its guidance pursuant to the 2017 ruling, voiding the guidance, but not the prior ruling that required it. The refrigeration and air conditioning industry had already discontinued production of new R-22 equipment. The practical effect of these rulings is to reduce the cost of imported R-22 to maintain aging equipment, extending its service life, while preventing the use of R-22 in new equipment.
R-22, retrofit using substitute refrigerants
[edit]This section may need to be rewritten to comply with Wikipedia's quality standards. (January 2025) |
The energy efficiency and system capacity of systems designed for R-22 is slightly greater using R-22 than the available substitutes.[11]
R-407A is for use in low- and medium-temp refrigeration. Uses a polyolester (POE) oil.
R-407C is for use in air conditioning. Uses a minimum of 20 percent POE oil.
R-407F and R-407H are for use in medium- and low-temperature refrigeration applications (supermarkets, cold storage, and process refrigeration); direct expansion system design only. They use a POE oil.
R-421A is for use in "air conditioning split systems, heat pumps, supermarket pak systems, dairy chillers, reach-in storage, bakery applications, refrigerated transport, self-contained display cabinets, and walk-in coolers". Uses mineral oil (MO), Alkylbenzene (AB), and POE.
R-422B is for use in low-, medium-, and high-temperature applications. It is not recommended for use in flooded applications.
R-422C is for use in medium- and low-temperature applications. The TXV power element will need to be changed to a 404A/507A element and critical seals (elastomers) may need to be replaced.
R-422D is for use in low-temp applications, and is mineral oil compatible.
R-424A is for use in air conditioning as well as medium-temp refrigeration temperature ranges of 20 to 50˚F. It works with MO, alkylbenzenes (AB), and POE oils.
R-427A is for use in air conditioning and refrigeration applications. It does not require all the mineral oil to be removed. It works with MO, AB, and POE oils.
R-434A is for use in water cooled and process chillers for air conditioning and medium- and low-temperature applications. It works with MO, AB, and POE oils.
R-438A (MO-99) is for use in low-, medium-, and high-temperature applications. It is compatible with all lubricants. [12]
R-458A is for use in air conditioning and refrigeration applications, without capacity or efficiency loss. Works with MO, AB, and POE oils.[13]
R-32 or HFC-32 (difluoromethane) is for use in air conditioning and refrigeration applications. It has zero ozone depletion potential (ODP) [2] and a global warming potential (GWP) index 675 times that of carbon dioxide.
Physical properties
[edit]| Property | Value |
|---|---|
| Density (ρ) at −69 °C (liquid) | 1.49 g⋅cm−3 |
| Density (ρ) at −41 °C (liquid) | 1.413 g⋅cm−3 |
| Density (ρ) at −41 °C (gas) | 4.706 kg⋅m−3 |
| Density (ρ) at 15 °C (gas) | 3.66 kg⋅m−3 |
| Specific gravity at 21 °C (gas) | 3.08 (air is 1) |
| Specific volume (ν) at 21 °C (gas) | 0.275 m3⋅kg−1 |
| Density (ρ) at 15 °C (gas) | 3.66 kg⋅m−3 |
| Triple point temperature (Tt) | −157.39 °C (115.76 K) |
| Critical temperature (Tc) | 96.2 °C (369.3 K) |
| Critical pressure (pc) | 4.936 MPa (49.36 bar) |
| Vapor pressure at 21.1 °C (pc) | 0.9384 MPa (9.384 bar)[14] |
| Critical density (ρc) | 6.1 mol⋅l−1 |
| Latent heat of vaporization (lv) at boiling point (−40.7 °C) | 233.95 kJ⋅kg−1 |
| Heat capacity at constant pressure (Cp) at 30 °C (86 °F) | 0.057 kJ.mol−1⋅K−1 |
| Heat capacity at constant volume (Cv) at 30 °C (86 °F) | 0.048 kJ⋅mol−1⋅K−1 |
| Heat capacity ratio (γ) at 30 °C (86 °F) | 1.178253 |
| Compressibility factor (Z) at 15 °C | 0.9831 |
| Acentric factor (ω) | 0.22082 |
| Molecular dipole moment | 1.458 D |
| Viscosity (η) at 0 °C | 12.56 μPa⋅s (0.1256 cP) |
| Ozone depletion potential (ODP) | 0.055 (CCl3F is 1) |
| Global warming potential (GWP) | 1810 (CO2 is 1) |
It has two allotropes: crystalline II below 59 K and crystalline I above 59 K and below 115.73 K.

| Temperature (K) | Density (kg/m^3) | Specific heat (kJ/kg K) | Dynamic viscosity (kg/m s) | Kinematic viscosity (m^2/s) | Conductivity (W/m K) | Thermal diffusivity (m^2/s) | Prandtl Number | Bulk modulus (K^-1) |
|---|---|---|---|---|---|---|---|---|
| 230 | 1416 | 1.087 | 3.56E-04 | 2.51E-07 | 0.1145 | 7.44E-08 | 3.4 | 0.00205 |
| 240 | 1386.6 | 1.1 | 3.15E-04 | 2.27E-07 | 0.1098 | 7.20E-08 | 3.2 | 0.00216 |
| 250 | 1356.3 | 1.117 | 2.80E-04 | 2.06E-07 | 0.1052 | 6.95E-08 | 3 | 0.00229 |
| 260 | 1324.9 | 1.137 | 2.50E-04 | 1.88E-07 | 0.1007 | 6.68E-08 | 2.8 | 0.00245 |
| 270 | 1292.1 | 1.161 | 2.24E-04 | 1.73E-07 | 0.0962 | 6.41E-08 | 2.7 | 0.00263 |
| 280 | 1257.9 | 1.189 | 2.01E-04 | 1.59E-07 | 0.0917 | 6.13E-08 | 2.6 | 0.00286 |
| 290 | 1221.7 | 1.223 | 1.80E-04 | 1.47E-07 | 0.0872 | 5.83E-08 | 2.5 | 0.00315 |
| 300 | 1183.4 | 1.265 | 1.61E-04 | 1.36E-07 | 0.0826 | 5.52E-08 | 2.5 | 0.00351 |
| 310 | 1142.2 | 1.319 | 1.44E-04 | 1.26E-07 | 0.0781 | 5.18E-08 | 2.4 | 0.004 |
| 320 | 1097.4 | 1.391 | 1.28E-04 | 1.17E-07 | 0.0734 | 4.81E-08 | 2.4 | 0.00469 |
| 330 | 1047.5 | 1.495 | 1.13E-04 | 1.08E-07 | 0.0686 | 4.38E-08 | 2.5 | 0.00575 |
| 340 | 990.1 | 1.665 | 9.80E-05 | 9.89E-08 | 0.0636 | 3.86E-08 | 2.6 | 0.00756 |
| 350 | 920.1 | 1.997 | 8.31E-05 | 9.04E-08 | 0.0583 | 3.17E-08 | 2.8 | 0.01135 |
| 360 | 823.4 | 3.001 | 6.68E-05 | 8.11E-08 | 0.0531 | 2.15E-08 | 3.8 | 0.02388 |
Price history and availability
[edit]
EPA's analysis indicated the amount of existing inventory was between 22,700t and 45,400t.[17][18][when?]
| Year | 2010 | 2011 | 2012 | 2013 | 2014 | 2015–2019 | 2020 |
|---|---|---|---|---|---|---|---|
| R-22 Virgin (t) | 49,900 | 45,400 | 25,100 | 25,600 | 20,200 | TBD | 0 |
| R-22 Recoupment (t) | -- | -- | -- | 2,950 | 2,950 | -- | -- |
| R-22 Total (t) | 49,900 | 45,400 | 25,100 | 28,600 | 23,100 | -- | -- |
In 2012 the EPA reduced the amount of R-22 by 45%, causing the price to rise by more than 300%. For 2013, the EPA has reduced the amount of R-22 by 29%.[19]
References
[edit]- ^ a b c d e NIOSH Pocket Guide to Chemical Hazards. "#0124". National Institute for Occupational Safety and Health (NIOSH).
- ^ Rosenthal, Elisabeth; Lehren, Andrew W. (20 June 2012). "Relief in Every Window, but Global Worry Too". The New York Times. Archived from the original on 21 June 2012. Retrieved 21 June 2012.
- ^ Siegemund, Günter; Schwertfeger, Werner; Feiring, Andrew; Sart, Bruce; Behr, Fred; Vogel, Herward; McKusick, Blaine (2002). "Fluorine Compounds, Organic". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a11_349. ISBN 978-3-527-30673-2.
- ^ The Montreal Protocol on Substances that Deplete the Ozone Layer. UNEP, 2000. ISBN 92-807-1888-6
- ^ "HCFC-22 (Chlorodifluoromethane)". NOAA Earth System Research Laboratories/Global Monitoring Division. Retrieved 12 February 2021.
- ^ "Guidance for Stationary Refrigeration & Air-Conditioning" (PDF). Department for Environment, Food and Rural Affairs. Archived (PDF) from the original on 10 March 2016. Retrieved 8 September 2015.
- ^ "SNAP Regulations". 4 November 2014. Archived from the original on 10 October 2015.
- ^ "Mexichem Fluor, Inc. v. EPA". Archived from the original on 17 August 2017.
- ^ "Ozone Protection under Title VI of the Clean Air Act". 14 July 2015. Archived from the original on 25 January 2016.
- ^ "Natural Resources Defense Council v. EPA". Archived from the original on 10 December 2020.
- ^ "THEORETICAL EVALUATION OF R22 AND R502 ALTERNATIVES" (PDF). Archived (PDF) from the original on 5 April 2015.
- ^ Retrofit Refrigerants Archived 24 June 2013 at archive.today
- ^ "Protection of Stratospheric Ozone: Determination 33 for Significant New Alternatives Policy Program". 21 July 2017.
- ^ "Frogen R-22 – Frogen UK: Refrigerant and Cooling Specialists". frogen.co.uk. Archived from the original on 25 January 2017. Retrieved 23 April 2018.
- ^ Holman, Jack P. (2002). Heat Transfer (9th ed.). New York, NY: McGraw-Hill Companies, Inc. pp. 600–606. ISBN 978-0-07-240655-9.
- ^ Incropera 1 Dewitt 2 Bergman 3 Lavigne 4, Frank P. 1 David P. 2 Theodore L. 3 Adrienne S. 4 (2007). Fundamentals of Heat and Mass Transfer (6th ed.). Hoboken, NJ: John Wiley and Sons, Inc. pp. 941–950. ISBN 978-0-471-45728-2.
{{cite book}}: CS1 maint: numeric names: authors list (link) - ^ "Protection of Stratospheric Ozone: Adjustments to the Allowance System for Controlling HCFC Production, Import, and Export". federalregister.gov. 3 April 2013. Archived from the original on 4 March 2016. Retrieved 23 April 2018.
- ^ "Protection of Stratospheric Ozone: Adjustments to the Allowance System for Controlling HCFC Production, Import, and Export". federalregister.gov. 3 April 2013. Archived from the original on 4 March 2016. Retrieved 23 April 2018.
- ^ Specialty Cooling and Heating (Blog) January 22, 2013 Archived 6 October 2013 at the Wayback Machine
External links
[edit]- MSDS from DuPont
- International Chemical Safety Card 0049
- Data at Integrated Risk Information System: IRIS 0657
- CDC – NIOSH Pocket Guide to Chemical Hazards – Chlorodifluoromethane
- Phase change data at webbook.nist.gov
- IR absorption spectra Archived 28 November 2007 at the Wayback Machine
- IARC summaries and evaluations: Vol. 41 (1986), Suppl. 7 (1987), Vol. 71 (1999)
Chlorodifluoromethane
View on GrokipediaChemical Properties
Molecular Structure and Nomenclature
Chlorodifluoromethane, with the molecular formula CHClF₂, consists of a central carbon atom covalently bonded to one hydrogen atom, one chlorine atom, and two fluorine atoms via single bonds.[1] The molecular geometry around the carbon is tetrahedral, with bond angles approximately 109.5°, characteristic of sp³ hybridized carbon atoms in saturated hydrocarbons and their derivatives.4/h1H) The C–H, C–Cl, and C–F bond lengths are typical for such halogenated methanes, with the two C–F bonds being shorter due to fluorine's high electronegativity.[6] The systematic IUPAC name is chlorodifluoromethane, reflecting the substitution of three hydrogen atoms in methane with one chlorine and two fluorine atoms, named in alphabetical order of prefixes.[1] Alternative systematic names include difluorochloromethane, though chlorodifluoromethane is preferred in modern nomenclature.4/h1H) Common industrial synonyms are HCFC-22 (hydrochlorofluorocarbon-22), R-22 (refrigerant designation), and Freon-22 (a historical trade name).[6] The InChI representation is InChI=1S/CHClF2/c2-1(3)4/h1H, standardizing its structural description for computational chemistry.[1]Physical and Thermodynamic Properties
Chlorodifluoromethane (CHClF₂) is a colorless, nonflammable gas under standard conditions, exhibiting a faint ethereal or sweetish odor. Its molecular weight is 86.47 g/mol, and it liquefies under moderate pressure, with a vapor density approximately 3 times that of air.[1][7][2] The compound has a boiling point of -40.8 °C at 1 atm and a melting point of -157.4 °C. Its critical temperature is 96 °C, and the critical pressure is 49.8 bar. Vapor pressure at 20 °C is 9.1 bar absolute, increasing to higher values with temperature, consistent with its use in pressurized systems.[8][9][10] Liquid density is 1.41 g/cm³ at -40 °F, while gas density is 3.66 kg/m³ at 15 °C and 1 atm. The specific gravity of the liquid exceeds that of water, causing it to sink in aqueous environments. Thermodynamic properties include a latent heat of vaporization of approximately 234 kJ/kg at the boiling point, supporting its refrigerant applications through efficient phase change energy transfer.[2]| Property | Value | Conditions |
|---|---|---|
| Boiling point | -40.8 °C | 1 atm |
| Melting point | -157.4 °C | - |
| Critical temperature | 96 °C | - |
| Critical pressure | 49.8 bar | - |
| Vapor pressure (20 °C) | 9.1 bar abs. | - |
| Liquid density | 1.41 g/cm³ | -40 °F |
| Gas density | 3.66 kg/m³ | 15 °C, 1 atm |
| Molecular weight | 86.47 g/mol | - |
Safety and Toxicity Profile
Chlorodifluoromethane exhibits low acute toxicity via inhalation, with LC50 values exceeding 307,000 ppm for 1 hour in mice and no lethality observed in rodents, rabbits, or dogs at concentrations up to 20% in air.[11][12] High-level exposures, however, can induce cardiac sensitization, leading to arrhythmias, unconsciousness, or death, particularly when combined with physical exertion or adrenaline release; symptoms include dizziness, loss of coordination, and central nervous system depression.[13][14] It acts primarily as an asphyxiant by displacing oxygen in confined spaces, with risks amplified by its use as a liquefied gas under pressure, which may rupture containers if heated.[9][13] Skin and eye contact with the liquid form causes frostbite or irritation due to rapid evaporation and cooling, but systemic absorption through intact skin is negligible.[13] Ingestion is not a relevant route given its gaseous nature at ambient temperatures.[15] Thermal decomposition during fires releases toxic products such as hydrogen fluoride, hydrogen chloride, and phosgene, necessitating respiratory protection and ventilation in incident scenarios.[16] Chronic exposure studies in animals, including rats and mice at up to 10,000 ppm for 90 days or longer, show no significant target organ toxicity, carcinogenicity, mutagenicity, or reproductive effects; developmental toxicity was absent in rabbits even at elevated concentrations.[15][8] Occupational exposure limits reflect this profile, with NIOSH recommending a 1,000 ppm 8-hour time-weighted average (TWA) and an immediately dangerous to life or health (IDLH) value of 50,000 ppm, while ACGIH lists a threshold limit value (TLV) of 1,000 ppm TWA.[17] Safe handling requires adequate ventilation to maintain exposures below these limits, use of self-contained breathing apparatus in confined spaces, and avoidance of mixing with air under pressure to prevent explosion risks.[18][14]Historical Development
Discovery and Early Synthesis
Chlorodifluoromethane (CHClF₂), also known as HCFC-22 or R-22, was synthesized through the controlled fluorination of chloroform (CHCl₃) with anhydrous hydrogen fluoride (HF) in the presence of a catalyst such as antimony pentachloride (SbCl₅). The reaction proceeds as CHCl₃ + 2 HF → CHClF₂ + 2 HCl, selectively replacing two chlorine atoms while preserving the hydrogen. This method built on earlier halogen exchange techniques but achieved the necessary selectivity for practical yields.[19] Early laboratory preparations occurred in the context of developing safe refrigerants, driven by the need to replace toxic and flammable options like ammonia and sulfur dioxide. In 1928, Thomas Midgley Jr., along with Albert L. Henne and Robert R. McNary at General Motors, initiated systematic synthesis of fluorinated hydrocarbons, initially focusing on fully halogenated chlorofluorocarbons (CFCs) such as dichlorodifluoromethane (R-12). By the mid-1930s, this effort extended to hydrochlorofluorocarbons, with CHClF₂ produced around 1935–1936 through optimized catalytic fluorination.[18][20] These syntheses marked the transition from academic curiosity to industrial viability, with DuPont commercializing the compound as Freon-22 by the late 1930s for refrigeration applications. The process required handling corrosive HF under pressure, often in lead-lined reactors to mitigate equipment degradation, and yielded byproduct HCl that necessitated efficient separation. Initial production scales were small, supporting testing in early air conditioning prototypes.[21]Commercial Production and Adoption
Chlorodifluoromethane, known as HCFC-22 or R-22, entered commercial production in 1936 as part of efforts to develop safer refrigerants following the invention of chlorofluorocarbons in the late 1920s.[20] Its synthesis involved reacting chloroform with hydrogen fluoride, building on earlier fluorocarbon chemistry pioneered by researchers like Thomas Midgley at General Motors and later scaled by companies such as DuPont under the Freon brand. Initial production focused on industrial refrigeration needs, where it offered advantages over toxic alternatives like ammonia and sulfur dioxide, including lower flammability and toxicity while maintaining effective cooling performance.[21] Adoption accelerated in the late 1950s, particularly in air conditioning systems, where R-22 replaced dichlorodifluoromethane (R-12) in many applications due to its higher efficiency, enabling smaller compressors and broader temperature ranges.[22] By the 1970s and 1980s, it had become the global standard for residential and commercial refrigeration, powering the expansion of centralized air conditioning in the United States and Europe amid post-World War II economic growth and rising demand for comfort cooling.[23] Its thermodynamic properties—such as a boiling point of -40.8°C and compatibility with mineral oils—facilitated widespread integration into hermetic compressors and systems designed for household use. Annual global production reached approximately 450 gigagrams by 1998, reflecting sustained industrial reliance before regulatory scrutiny intensified.[20]Production Processes
Industrial Manufacturing Methods
Chlorodifluoromethane (HCFC-22, CHClF₂) is primarily manufactured industrially through the liquid-phase fluorination of chloroform (CHCl₃) with anhydrous hydrogen fluoride (HF).[24] The reaction proceeds as CHCl₃ + 2HF → CHClF₂ + 2HCl, catalyzed by pentavalent antimony compounds such as antimony pentachloride (SbCl₅) or antimony pentafluoride (SbF₅).[25][1] This process operates under controlled temperature (typically 50–100°C) and pressure conditions to optimize yield and minimize byproducts like trifluoromethane (HFC-23).[24] The reactants are fed into a corrosion-resistant reactor lined with materials like Hastelloy or fluoropolymers to withstand the aggressive HF environment. The catalyst facilitates the stepwise substitution of chlorine atoms with fluorine, with the antimony species cycling between oxidation states (Sb(V)/Sb(III)) to regenerate activity. Reaction mixtures are distilled to separate the gaseous HCFC-22 product from HCl byproduct and unreacted materials, achieving selectivities above 90% under optimized conditions.[26] Minor variations include gas-phase processes or alternative catalysts like chromium oxides, but the antimony-catalyzed liquid-phase method dominates due to its established efficiency and scalability in commercial plants.[27] Byproduct management is critical, as trace HFC-23 formation (up to 1–2% of output) requires thermal incineration or capture to comply with emissions regulations. Global production historically peaked in the late 1990s, with facilities in the United States, Europe, and Asia employing this method until phaseout mandates reduced output post-2010.[24][25]Raw Materials and Byproducts
The primary raw materials for the industrial production of chlorodifluoromethane (HCFC-22) are chloroform (CHCl₃) and anhydrous hydrogen fluoride (HF), which react in a liquid-phase fluorination process typically catalyzed by antimony pentachloride (SbCl₅) or similar metal halides.[25][28] The stoichiometric reaction is CHCl₃ + 2 HF → CHClF₂ + 2 HCl, requiring precise control of temperature (around 50–100°C) and pressure to optimize yield and minimize side reactions.[25] Chloroform is derived from methane chlorination or other chlorocarbon processes, while HF is produced via fluorspar (CaF₂) reaction with sulfuric acid, reflecting the upstream supply chain's reliance on mineral and petrochemical feedstocks.[28] Key byproducts include hydrogen chloride (HCl), which forms in equimolar quantities to HCFC-22 and is recovered for reuse in other chemical syntheses, such as vinyl chloride production.[25] An undesirable byproduct is trifluoromethane (HFC-23, CHF₃), generated via over-fluorination (CHClF₂ + HF → CHF₃ + HCl), typically comprising 1–5% of the output depending on process efficiency and catalyst activity; HFC-23's high global warming potential has prompted regulatory requirements for its capture and destruction in modern facilities.[29][25] Minor byproducts may include unreacted chloroform, difluorochloromethane isomers, or chlorotrifluoromethane, which are separated via distillation and recycled or treated to reduce emissions.[24] Waste streams, including spent catalyst containing antimony fluorides, require specialized handling to prevent environmental release of heavy metals and fluorides.[30]Primary Applications
Use in Refrigeration and Air Conditioning Systems
Chlorodifluoromethane, designated as R-22, serves as a working fluid in vapor-compression refrigeration cycles for air conditioning and refrigeration systems, leveraging its favorable thermodynamic characteristics such as a boiling point of -40.8°C at atmospheric pressure, which enables efficient heat absorption at evaporator temperatures typical for cooling applications ranging from -10°C to 5°C.[7] Its high latent heat of vaporization and moderate critical temperature of 96°C support stable operation across a wide range of system pressures, contributing to effective cooling capacity in both residential and commercial units.[31] R-22's compatibility with conventional mineral oils used in compressors, combined with its non-flammability and relative chemical stability, made it a preferred refrigerant for hermetic systems in window air conditioners, split systems, and packaged units since its commercial adoption in the 1950s.[32] By the late 20th century, it dominated the market for residential air conditioning, powering over 80% of central AC systems in the United States before regulatory phaseouts began influencing replacements.[33] In commercial refrigeration, R-22 facilitated medium-temperature applications like supermarket display cases and walk-in coolers, where its pressure-temperature profile allowed for compact heat exchanger designs and reliable performance under varying loads.[34] The refrigerant's low toxicity and non-corrosive nature further enhanced its suitability for systems requiring safety in populated environments, though its ozone-depleting potential later prompted transitions to alternatives like R-410A.[35] Energy efficiency metrics, including a coefficient of performance comparable to other HCFCs, underscored R-22's role in minimizing operational costs in large-scale chillers for industrial processes until supply restrictions accelerated retrofits.[36]Other Industrial and Chemical Applications
Chlorodifluoromethane functions as a key chemical intermediate in organofluorine synthesis, notably serving as a precursor to tetrafluoroethylene, which is polymerized to produce polytetrafluoroethylene (PTFE) and other fluoropolymers essential for applications requiring high chemical resistance and low friction, such as coatings and seals.[37][5] This role leverages its reactivity to introduce fluorine atoms into complex molecular structures, with industrial processes often involving pyrolysis or catalytic reactions to generate the monomer.[37] Production data indicate that a portion of global HCFC-22 output—historically up to 10-20% in some facilities—has been directed toward such feedstocks, though exact figures vary by manufacturer and region due to phaseout pressures.[24] In solvent applications, chlorodifluoromethane has been utilized as a low-temperature solvent for extraction and cleaning tasks, particularly in degreasing metals and removing residues in precision industries, owing to its non-flammable nature and compatibility with sensitive equipment.[17] Its use in this capacity remains limited post-Montreal Protocol restrictions, primarily confined to legacy systems or exempted processes in developing nations, where alternatives like hydrocarbons pose flammability risks.[27] Historically, it served as an aerosol propellant in consumer products such as hairsprays and pharmaceuticals, valued for its stability and low toxicity profile compared to earlier chlorofluorocarbons, though this application has been largely discontinued in developed countries since the early 1990s due to ozone depletion concerns.[27] A smaller volume has also been employed as a blowing agent in polystyrene foam production, expanding the polymer matrix during extrusion to create lightweight insulation materials, with emissions controlled to minimize environmental release.[37][27] These non-refrigerant uses collectively represent a minor fraction of total consumption, overshadowed by refrigeration demands but critical in niche chemical manufacturing.[24]Environmental Considerations
Atmospheric Chemistry and Ozone Depletion Mechanism
Chlorodifluoromethane (HCFC-22) experiences significant removal in the troposphere via reaction with hydroxyl (OH) radicals, which abstract the hydrogen atom in the initial step: CHClF₂ + OH → H₂O + •CClF₂.[38] The •CClF₂ radical subsequently reacts with O₂ to form peroxy radicals, leading to degradation products such as carbonyl fluoride (COF₂) and hydrogen chloride (HCl), with HCl being scavenged by precipitation or further oxidized.[39] This process constitutes the dominant sink, yielding an atmospheric lifetime of 11.9 years and preventing most HCFC-22 from reaching the stratosphere intact, unlike fully halogenated chlorofluorocarbons (CFCs).[40] The fraction of HCFC-22 transported to the stratosphere—estimated at around 5-10% based on modeling of transport and loss rates—undergoes photolysis by short-wavelength ultraviolet radiation (λ < 220 nm): CHClF₂ + hν → Cl• + •CHF₂, with the •CHF₂ radical decomposing or reacting to release additional fluorine atoms but primarily the chlorine atom initiating depletion.[41] Stratospheric loss via O(¹D) reaction with HCFC-22 is minor compared to photolysis.[42] Released chlorine atoms (Cl•) participate in catalytic cycles depleting stratospheric ozone, including the primary null cycle: Cl• + O₃ → ClO + O₂, followed by ClO + O → Cl• + O₂, resulting in net O₃ destruction without net consumption of Cl• or O.[43] Auxiliary cycles involving ClO dimerization (2 ClO → Cl₂O₂ → 2 Cl•) or reactions with HO₂ further amplify loss, particularly in polar regions during spring when polar stratospheric clouds activate chlorine reservoirs like HCl and ClONO₂.[43] HCFC-22 contributes to stratospheric chlorine loading, measured at 320 ± 3 ppt in 2020, supporting ongoing but diminished ozone loss as total chlorine declines.[43] The ozone depletion potential (ODP) of HCFC-22, defined relative to CFC-11 (ODP = 1), is 0.055, accounting for its single chlorine atom and partial tropospheric removal efficiency in two-dimensional photochemical models validated against observations.[43] This value indicates HCFC-22 depletes ozone approximately 5.5% as effectively per unit mass as CFC-11, with chlorine release occurring efficiently once in the stratosphere but limited by atmospheric processing.[44]Greenhouse Gas Effects and Global Warming Potential
Chlorodifluoromethane (HCFC-22) functions as a greenhouse gas through its absorption of infrared radiation emitted from Earth's surface, particularly in the mid-infrared spectrum around 10.5–11.5 micrometers, where it overlaps with the atmospheric window and competes minimally with water vapor absorption.[45] This radiative efficiency, combined with its persistence in the troposphere, results in direct forcing of the climate system by trapping outgoing longwave radiation.[46] Unlike longer-lived gases such as CO₂, HCFC-22's atmospheric lifetime is estimated at 11.9 years, during which it undergoes photolysis and reaction with hydroxyl radicals, limiting its integrated climate impact but amplifying short-term warming per unit emission.[47] The 100-year global warming potential (GWP) of HCFC-22 is 1,760 relative to CO₂, as calculated in the IPCC Fifth Assessment Report using integrated radiative forcing over a 100-year horizon.[48] Alternative assessments, such as those by the California Air Resources Board, report a value of 1,810, reflecting minor variations in lifetime and spectral data inputs.[49] These metrics quantify HCFC-22's climate potency, indicating that one kilogram emitted equates to 1,760–1,810 kilograms of CO₂ in terms of sustained warming, driven primarily by its strong per-molecule forcing rather than longevity.[50] Atmospheric abundances of HCFC-22, measured via networks like AGAGE, reached monthly mean mole fractions exceeding 200 parts per trillion by the early 2010s, contributing to cumulative radiative forcing from hydrohalocarbons.[51] Phaseout efforts under the Montreal Protocol have slowed growth, with empirical data showing stabilization and decline post-2020 in compliant regions, thereby averting additional forcing estimated at 0.01–0.02 W/m² globally from unabated emissions.[52] While HCFC-22's greenhouse effects are overshadowed by CO₂ and methane in total forcing budgets, its high GWP underscores the climate co-benefits of ozone protection measures.[53] ![Growth of R-22 abundance in Earth's atmosphere since year 1992.][center]Empirical Evidence and Scientific Controversies
Atmospheric measurements from the Advanced Global Atmospheric Gases Experiment (AGAGE) and Cape Grim station indicate that chlorodifluoromethane (HCFC-22) mole fractions in the troposphere rose from negligible levels prior to the 1970s to approximately 250 parts per trillion (ppt) by the early 2020s, with annual growth rates peaking at 4-6 ppt per year during the 1990s and 2000s before slowing to near zero by 2020 due to production controls.[54][55] Global emission inventories derived from these observations estimate peak annual releases of around 400-500 gigagrams in the mid-2000s, predominantly from refrigeration and air conditioning sectors, with recent declines linked to Montreal Protocol compliance in developed nations.[51] Seasonal variations in concentrations, observed at multiple AGAGE sites, suggest influences from hemispheric transport and regional emission pulses, complicating bottom-up emission estimates.[51] Empirical links to stratospheric ozone depletion stem from laboratory-derived reaction kinetics showing HCFC-22 photolysis releases chlorine atoms in the stratosphere, contributing to catalytic ozone loss cycles, with an ozone depletion potential (ODP) quantified at 0.04-0.055 relative to CFC-11.[43] Observations from satellite and balloon-borne instruments correlate total stratospheric chlorine levels, including from HCFC-22, with Antarctic ozone minima, though HCFC-22's shorter atmospheric lifetime (approximately 12 years) limits its transport efficiency compared to longer-lived CFCs.[56] Ground-based and ozonesonde data from 1990-2020 show a partial recovery in total column ozone outside polar regions, attributable in models to declining ODS burdens, including HCFCs, with radiative forcing contributions from HCFC-22's global warming potential of 1760 over 100 years amplifying indirect climate feedbacks on ozone dynamics.[43][51] Scientific debates center on the precision of HCFC-22's ODP, with early 1990s analyses proposing values up to twice the consensus figure based on revised transport and reaction efficiencies, though subsequent assessments favor lower estimates supported by ensemble modeling.[56] Discrepancies between reported production under the Montreal Protocol and atmospheric growth rates, particularly in East Asia, imply unreported emissions 20-50% higher than inventories through the 2010s, raising questions about compliance verification and the role of byproduct gases like HFC-23 in offsetting phaseout benefits.[55][57] While mainstream assessments attribute observed ozone trends primarily to halogen loading, alternative interpretations highlight correlations with solar cycles and volcanic aerosols, suggesting models may overattribute depletion to anthropogenic HCFCs without isolating natural variability through long-term empirical baselines predating industrial emissions.[43]Regulatory Actions and Phaseouts
International Agreements Including the Montreal Protocol
The Montreal Protocol on Substances that Deplete the Ozone Layer, adopted on September 16, 1987, and entering into force on January 1, 1989, established a framework for phasing out the production and consumption of ozone-depleting substances, including hydrochlorofluorocarbons (HCFCs) such as chlorodifluoromethane (HCFC-22).[58] Initially focused on chlorofluorocarbons (CFCs), the protocol's amendments progressively incorporated HCFCs as transitional substitutes with lower ozone-depleting potential but still requiring elimination due to their contribution to stratospheric ozone loss.[59] The London Amendment of 1990 first controlled HCFCs by freezing their production and consumption levels for developed countries, while the Copenhagen Amendment of 1992 set specific reduction baselines and timelines, mandating a 65% reduction by 2005 and full phaseout by 2030 for non-Article 5 (developed) parties, with adjustments for essential uses.[60] For HCFC-22, which accounted for a significant portion of HCFC consumption due to its widespread use in refrigeration, the protocol differentiated schedules between developed and developing (Article 5) countries to account for economic disparities. Developed countries were required to reduce HCFC consumption by 35% from baseline by January 1, 2004, 65% by 2010, 90% by 2015, and 99.5% by 2020, with limited production allowances for servicing existing equipment until 2030.[61] Developing countries faced a baseline freeze in 2013 (using 2009-2010 averages or 65% of 2005-2007 averages), followed by reductions of 10% by 2015, 35% by 2020, 67.5% by 2025, and 85% by 2030, culminating in complete phaseout by January 1, 2030, except for limited essential uses.[58] The 2007 adjustment under the Montreal Protocol, formalized in the Beijing Amendment, accelerated these timelines for both groups, emphasizing HCFC-22's role in ongoing ozone depletion despite its shorter atmospheric lifetime compared to CFCs.[62] As of 2025, the protocol has achieved universal ratification by 197 parties, enabling global compliance monitoring through the UN Environment Programme's implementation agencies, which provide financial and technical assistance to developing nations via the Multilateral Fund established in 1991.[59] While HCFC-22 phaseout has progressed, with observed declines in atmospheric concentrations attributable to reduced emissions, enforcement relies on self-reported data and trade controls, with some reports of illegal production in non-compliant regions highlighting challenges in verification.[63] No other major international agreements specifically target HCFC-22 beyond the Montreal framework, though its greenhouse gas properties are addressed indirectly under the Kyoto Protocol's basket of fluorinated gases.[64]European Union Phaseout Timeline and Measures
The European Union accelerated the phase-out of hydrochlorofluorocarbons (HCFCs), including chlorodifluoromethane (HCFC-22), beyond the timelines set by the Montreal Protocol, primarily through Council Regulation (EC) No 2037/2000 and its successor, Regulation (EC) No 1005/2009.[65][66] These measures prohibited the production, import, export, and use of HCFCs in specified applications, with quotas applied to production and consumption calculated against 1997 baseline levels.[66] Key prohibitions on equipment and servicing were enacted earlier under Regulation (EC) No 2037/2000, banning the placement on the market of newly manufactured stationary refrigeration and air conditioning equipment containing virgin HCFCs effective 1 January 2004.[65] Regulation (EC) No 1005/2009 reinforced and extended these controls, prohibiting the supply of virgin HCFCs for servicing or maintenance of existing refrigeration, air conditioning, and heat pump equipment from 1 January 2010, while permitting limited use of reclaimed or recycled HCFCs until 31 December 2014 under strict recovery conditions.[66] Production and consumption quotas under Regulation (EC) No 1005/2009 followed a stepwise reduction schedule, as outlined below:| Period | Allowed Level (% of 1997 Baseline) | Reduction Achieved |
|---|---|---|
| 2010–2013 | 35% | 65% |
| 2014–2016 | 14% | 86% |
| 2017–2019 | 7% | 93% |
| From 1 January 2020 | 0% | 100% |
United States Phaseout and EPA Regulations
The United States Environmental Protection Agency (EPA) regulates chlorodifluoromethane (HCFC-22, also known as R-22) as a Class II ozone-depleting substance under Title VI of the Clean Air Act, as amended in 1990, to implement the phaseout commitments of the Montreal Protocol.[68] These regulations, codified in 40 CFR Part 82, Subpart A, control production, import, export, and consumption through an allowance system that progressively reduces quotas from a 1989 baseline level of approximately 110,000 metric tons annually for HCFC-22.[69] The EPA allocates production and consumption allowances to companies, which must surrender them for each metric ton produced or imported, ensuring compliance with international reduction schedules.[68] The phaseout proceeded in incremental steps aligned with Montreal Protocol milestones for developed countries. Production and consumption allowances for HCFC-22 were frozen at baseline levels effective January 1, 1996, followed by a 35% reduction by January 1, 2004, a 65% reduction by January 1, 2010, a 90% reduction by January 1, 2015, and a 99.5% reduction by January 1, 2020, leaving only trace allowances for minimal essential uses.[70] By January 1, 2020, new production and import of virgin HCFC-22 were effectively prohibited, with total annual quotas reduced to under 550 metric tons nationwide, primarily to facilitate the transition to alternatives.[68] Complete elimination of production and consumption allowances is mandated by January 1, 2030.[71] In addition to allowance reductions, EPA regulations prohibit the manufacture and import of new equipment containing or designed for HCFC-22 after specific dates to prevent lock-in of the substance in future systems. For comfort cooling appliances (e.g., residential and light commercial air conditioners), production using HCFC-22 was banned as of January 1, 2010; for industrial process refrigeration, the ban took effect January 1, 2010; and for other applications like chillers, it applied from January 1, 2015 or earlier.[68] These prohibitions, under 40 CFR 82.15 and 82.16, extend to servicing new equipment with virgin HCFC-22 post-ban dates, though reclaimed or recycled material remains permissible.[69] Servicing of existing HCFC-22 systems is governed by Section 608 of the Clean Air Act, which requires EPA-certified technicians to recover at least 80-90% of refrigerant during maintenance or disposal, prohibits intentional venting, and mandates use of recovery equipment meeting Society of Automotive Engineers (SAE) standards.[4] Reclaimed HCFC-22—reprocessed to ARI 700 purity standards (at least 99.5% purity with moisture and other contaminants limited)—can be used for servicing until supplies deplete, but post-2020 availability relies solely on recovery from existing stockpiles estimated at 50,000-100,000 metric tons in 2015.[68] Nonessential one-time uses, such as flushing solvents, were banned earlier, effective January 1, 1994.[69] Enforcement includes fines up to $44,539 per violation per day, with the EPA conducting audits and requiring annual reporting from allowance holders.[68]Status in Developing Nations and Global Compliance
Under the Montreal Protocol, developing countries classified as Article 5 parties receive extended timelines for phasing out hydrochlorofluorocarbons (HCFCs), including chlorodifluoromethane (HCFC-22), to accommodate economic development needs in refrigeration and air conditioning sectors.[72] The schedule mandates a freeze on HCFC consumption at 2011-2013 baseline levels starting in 2013, followed by reductions of 10% by 2015, 35% by 2020, 67.5% by 2025, and 85% by 2030, culminating in a complete phase-out by January 1, 2030.[58] This acceleration from an initial 2040 deadline was agreed in 2007 to balance ozone protection with the rapid growth in cooling demand in these nations.[73] As of 2025, many Article 5 countries are approaching or implementing the 67.5% reduction target, supported by HCFC Phase-out Management Plans (HPMPs) funded by the Protocol's Multilateral Fund, which has approved over $3 billion in assistance for technology transfer and alternatives adoption.[74] Major producers like China and India, accounting for the bulk of global HCFC-22 output, have reported progress through domestic quotas and enterprise-level conversions, though consumption in some regions persists due to legacy equipment and service needs.[75] Production for non-emissive feedstock uses, such as polytetrafluoroethylene manufacturing, remains permitted without phase-out obligations, representing nearly half of HCFC-22's global production mass.[76] Global compliance with HCFC phase-out commitments remains strong, with 198 parties ratifying the Protocol and over 98% adherence to prior CFC and halon schedules, but challenges persist in developing nations due to illegal trade and quota exceedances.[77] Reports indicate clandestine HCFC-22 production and smuggling, particularly from China post-2010, driven by black-market demand in regions with phase-out lags, undermining atmospheric decline efforts despite overall consumption drops.[78] Enforcement relies on national customs and the Protocol's Implementation Committee, which has addressed non-compliance cases through capacity-building rather than penalties, reflecting the treaty's emphasis on cooperative assistance over punitive measures.[79] Atmospheric monitoring shows HCFC-22 levels stabilizing or declining in some areas, but persistent emissions from developing countries highlight the need for vigilant verification to meet 2030 goals.[62]Alternatives and System Transitions
Direct Substitutes and Their Properties
Direct substitutes for chlorodifluoromethane (HCFC-22 or R-22) primarily consist of hydrofluorocarbon (HFC) and hydrofluoroolefin (HFO) blends designed for retrofit applications in existing refrigeration and air-conditioning systems, though true drop-in replacements without system modifications are limited due to differences in pressure, oil compatibility, and thermodynamic behavior.[80] Common options include R-407C, a zeotropic blend of difluoromethane (R-32), pentafluoroethane (R-125), and 1,1,1,2-tetrafluoroethane (R-134a) in a 23/25/52% mass ratio, which approximates R-22's performance in medium-temperature applications with minimal capacity loss (typically 5-10% lower cooling capacity) but exhibits a 6-7°C temperature glide requiring adjusted expansion devices.[81] R-407C has zero ozone depletion potential (ODP=0), a global warming potential (GWP) of approximately 1774 over 100 years, and operates at similar pressures to R-22 while necessitating polyol ester (POE) oil for lubricity, as it is immiscible with R-22's traditional mineral oil.[81][82] Newer HFO-containing blends like R-449A (Opteon XP40), composed of R-32 (24%), R-125 (25%), R-1234yf (25.7%), and R-134a (25.3%), serve as lower-GWP alternatives with a GWP of 1397 and ODP=0, offering near-equivalent capacity to R-22 in retrofits for commercial refrigeration while reducing flammability risks compared to pure hydrocarbons.[83] These substitutes generally require POE or polyalkylene glycol (PAG) lubricants and may involve leak checks or filter-drier replacements during transition, as HFCs/HFOs do not mix with mineral oils used in legacy R-22 systems.[80] Other near-drop-ins, such as R-438A (a blend of R-32, R-125, R-134a, R-600, and R-601a), provide closer pressure matching to R-22 with about 5% higher capacity and a GWP of 2260, suitable for residential air conditioning without compressor changes in many cases.[84]| Substitute | Composition | Normal Boiling Point (°C) | GWP (100-year) | ODP | Key Retrofit Notes |
|---|---|---|---|---|---|
| R-407C | R-32/R-125/R-134a (23/25/52%) | -43.6 | 1774 | 0 | Temperature glide ~7°C; similar pressures to R-22; requires POE oil.[85][81] |
| R-449A | R-32/R-125/R-1234yf/R-134a (24/25/25.7/25.3%) | -46 | 1397 | 0 | Lower GWP; mild flammability (A2L); capacity ~95% of R-22.[83] |
| R-438A | Proprietary HFC/HC blend | -42 | 2260 | 0 | Minimal charge adjustment; ~5% higher capacity.[84] |
Retrofit Strategies and Compatibility Issues
Retrofit strategies for chlorodifluoromethane (HCFC-22 or R-22) systems typically involve replacing the refrigerant with hydrofluorocarbon (HFC) blends such as R-407C, R-427A, or R-438A (MO99), while addressing compatibility challenges to minimize system modifications.[88] These approaches prioritize recovering the existing R-22 charge—permitted for reuse in compliant systems under EPA regulations—and then flushing the system to remove residual mineral oil, which is incompatible with the polyol ester (POE) oils required by most HFC substitutes.[89] Filter-driers must be replaced to prevent moisture and debris accumulation, and seals or gaskets swollen by mineral oil may require substitution to avoid leaks from material contraction with HFCs.[90] Oil compatibility remains a primary issue, as R-22's mineral oil does not mix adequately with HFC refrigerants, potentially leading to lubrication failure and compressor damage if not fully flushed.[91] For instance, R-407C, a near-azeotropic blend of R-32, R-125, and R-134a, demands POE oil and often necessitates thermal expansion valve (TXV) adjustments due to its 7-10% lower capacity and altered pressure-temperature profile compared to R-22.[88] Similarly, R-422D (an HFC blend including R-125 and R-600a) offers better glide compatibility for retrofits but can cause fractionation in non-azeotropic operation, exacerbating efficiency losses if the system lacks precise charge control.[92] No true "drop-in" replacements exist without risks, as HFC substitutes alter system dynamics, including reduced mass flow rates that strain capillary tube or fixed-orifice expansions, potentially requiring orifice enlargement or TXV replacement for stable superheat.[93] Compatibility testing, such as monitoring for leaks post-retrofit and verifying compressor oil return, is essential, with EPA guidelines emphasizing baseline performance checks before and after conversion to ensure at least 90% of original capacity.[94] In commercial applications, partial retrofits—replacing only refrigerant with minimal hardware changes—succeed in systems under 50 tons but often yield 5-15% efficiency drops, prompting hybrid strategies like combining retrofits with component upgrades for longer-term viability.[89] Systems with severe contamination or degraded components may necessitate full replacement over retrofitting due to cumulative incompatibility risks.[95]Performance and Efficiency Comparisons
R-407C, a ternary HFC blend (R-32/R-125/R-134a at 23%/25%/52%), delivers cooling capacity and coefficient of performance (COP) closely approximating those of chlorodifluoromethane (R-22) in retrofitted vapor compression systems, with deviations typically under 5% in capacity and efficiency under standard operating conditions.[96][97] In contrast, R-410A (R-32/R-125 at 50%/50%), optimized for new equipment, provides approximately 50% higher volumetric cooling capacity than R-22 at equivalent compressor speeds, enabling smaller system designs but necessitating components rated for 50% elevated discharge pressures (e.g., 2,800 kPa vs. 1,800 kPa for R-22).[96][98] However, R-410A's COP matches or slightly trails R-22's (e.g., 2.78 for R-22 baseline systems), with greater efficiency degradation at high ambient temperatures above 35°C, where capacity and COP decline more sharply due to thermodynamic properties.[99][100]| Refrigerant | Typical COP (Cooling, Evaporator -5°C/Condenser 50°C) | Volumetric Capacity Relative to R-22 | Notes on Efficiency |
|---|---|---|---|
| R-22 | 2.81 | 1.0 | Highest exergy efficiency (52.2%); stable at high ambients.[101] |
| R-407C | ~2.70–2.80 | ~0.95–1.05 | Similar to R-22; minor capacity glide affects heat transfer.[96][101] |
| R-410A | ~2.60–2.75 | ~1.5 | Higher capacity but increased compressor power; optimized new systems offset via design.[102][101] |