Recent from talks
Nothing was collected or created yet.
Atmosphere of Earth
View on Wikipedia

The atmosphere of Earth consists of a layer of mixed gas (commonly referred to as air) that is retained by gravity, surrounding the Earth's surface. It contains variable quantities of suspended aerosols and particulates that create weather features such as clouds and hazes. The atmosphere serves as a protective buffer between the Earth's surface and outer space. It shields the surface from most meteoroids and ultraviolet solar radiation, reduces diurnal temperature variation – the temperature extremes between day and night, and keeps it warm through heat retention via the greenhouse effect. The atmosphere redistributes heat and moisture among different regions via air currents, and provides the chemical and climate conditions that allow life to exist and evolve on Earth.
By mole fraction (i.e., by quantity of molecules), dry air contains 78.08% nitrogen, 20.95% oxygen, 0.93% argon, 0.04% carbon dioxide, and small amounts of other trace gases (see Composition below for more detail). Air also contains a variable amount of water vapor, on average around 1% at sea level, and 0.4% over the entire atmosphere.
Earth's primordial atmosphere consisted of gases accreted from the solar nebula, but the composition changed significantly over time, affected by many factors such as volcanism, outgassing, impact events, weathering and the evolution of life (particularly the photoautotrophs). In the present day, human activity has contributed to atmospheric changes, such as climate change (mainly through deforestation and fossil-fuel–related global warming), ozone depletion and acid deposition.
The atmosphere has a mass of about 5.15×1018 kg,[2] three quarters of which is within about 11 km (6.8 mi; 36,000 ft) of the surface. The atmosphere becomes thinner with increasing altitude, with no definite boundary between the atmosphere and outer space. The Kármán line at 100 km (62 mi) is often used as a conventional definition of the edge of space. Several layers can be distinguished in the atmosphere based on characteristics such as temperature and composition, namely the troposphere, stratosphere, mesosphere, thermosphere (formally the ionosphere), and exosphere. Air composition, temperature and atmospheric pressure vary with altitude. Air suitable for use in photosynthesis by terrestrial plants and respiration of terrestrial animals is found within the troposphere.[3]
The study of Earth's atmosphere and its processes is called atmospheric science (aerology), and includes multiple subfields, such as climatology and atmospheric physics. Early pioneers in the field include Léon Teisserenc de Bort and Richard Assmann.[4] The study of the historic atmosphere is called paleoclimatology.
Composition
[edit]
The three major constituents of Earth's atmosphere are nitrogen, oxygen, and argon. Water vapor accounts for roughly 0.25% of the atmosphere by mass. In the lower atmosphere, the concentration of water vapor (a greenhouse gas) varies significantly from around 10 ppm by mole fraction in the coldest portions of the atmosphere to as much as 5% by mole fraction in hot, humid air masses, and concentrations of other atmospheric gases are typically quoted in terms of dry air (without water vapor).[8]: 8 The remaining gases are often referred to as trace gases,[9] among which are other greenhouse gases, principally carbon dioxide, methane, nitrous oxide, and ozone. Besides argon, other noble gases, neon, helium, krypton, and xenon are also present. Filtered air includes trace amounts of many other chemical compounds.[10]
Many substances of natural origin may be present in locally and seasonally variable small amounts as aerosols in an unfiltered air sample, including dust of mineral and organic composition, pollen and spores, sea spray, and volcanic ash.[11] Various industrial pollutants also may be present as gases or aerosols, such as chlorine (elemental or in compounds),[12] fluorine compounds,[13] and elemental mercury vapor.[14] Sulfur compounds such as hydrogen sulfide and sulfur dioxide (SO2) may be derived from natural sources or from industrial air pollution.[11][15]

| Dry air | |||||
|---|---|---|---|---|---|
| Gas | Volume fraction(A) | Mass fraction | |||
| Name | Formula | in ppm(B) | in % | in ppm | in % |
| Nitrogen | N2 | 780,800 | 78.08 | 755,200 | 75.52 |
| Oxygen | O2 | 209,500 | 20.95 | 231,400 | 23.14 |
| Argon | Ar | 9,340 | 0.9340 | 12,900 | 1.29 |
| Carbon dioxide[6] | CO2 | 412 | 0.0412 | 626 | 0.063 |
| Neon | Ne | 18.2 | 0.00182 | 12.7 | 0.00127 |
| Helium | He | 5.24 | 0.000524 | 0.724 | 0.0000724 |
| Methane[7] | CH4 | 1.79 | 0.000179 | 0.99 | 0.000099 |
| Krypton | Kr | 1.14 | 0.000114 | 3.3 | 0.00033 |
| If air is not dry: | |||||
| Water vapor(D) | H2O | 0–30,000(D) | 0–3%(E) | ||
|
The total ppm above adds up to more than 1 million (currently 83.43 above it) due to experimental error. | |||||
The average molecular weight of dry air, which can be used to calculate densities or to convert between mole fraction and mass fraction, is about 28.946[17] or 28.964[18][5]: 257 g/mol. This is decreased when the air is humid.
Up to an altitude of around 100 km (62 mi), atmospheric turbulence mixes the component gases so that their relative concentrations remain the same. There exists a transition zone from roughly 80 to 120 km (50 to 75 mi) where this turbulent mixing gradually yields to molecular diffusion. The latter process forms the heterosphere where the relative concentration of lighter gases increase with altitude.[19]
Stratification
[edit]
In general, air pressure and density decrease with altitude in the atmosphere. However, temperature has a more complicated profile with altitude and may remain relatively constant or even increase with altitude in some regions (see the temperature section).[21] Because the general pattern of the temperature/altitude profile, or lapse rate, is constant and measurable by means of instrumented balloon soundings, the temperature behavior provides a useful metric to distinguish atmospheric layers. This atmospheric stratification divides the Earth's atmosphere into five main layers with these typical altitude ranges:[22][23]
- Exosphere: 700–10,000 km (435–6,214 mi)[24]
- Thermosphere: 80–700 km (50–435 mi)[25]
- Mesosphere: 50–80 km (31–50 mi)
- Stratosphere: 12–50 km (7–31 mi)
- Troposphere: 0–12 km (0–7 mi)[26]
Exosphere
[edit]The exosphere is the outermost layer of Earth's atmosphere (though it is so tenuous that some scientists consider it to be part of interplanetary space rather than part of the atmosphere). It extends from the thermopause (also known as the "exobase") at the top of the thermosphere to a poorly defined boundary with the solar wind and interplanetary medium. The altitude of the exobase varies from about 500 kilometres (310 mi; 1,600,000 ft) to about 1,000 kilometres (620 mi) in times of higher incoming solar radiation.[27]
The upper limit varies depending on the definition. Various authorities consider it to end at about 10,000 kilometres (6,200 mi)[28] or about 190,000 kilometres (120,000 mi)—about halfway to the moon, where the influence of Earth's gravity is about the same as radiation pressure from sunlight.[27] The geocorona visible in the far ultraviolet (caused by neutral hydrogen) extends to at least 100,000 kilometres (62,000 mi).[27]
This layer is mainly composed of extremely low densities of hydrogen, with limited amounts of helium, carbon dioxide, and nascent oxygen closer to the exobase.[29] The atoms and molecules are so far apart that they can travel hundreds of kilometres without colliding with one another.[21]: 14–4 Thus, the exosphere no longer behaves like a gas, and the particles constantly escape into space. These free-moving particles follow ballistic trajectories and may migrate in and out of the magnetosphere or the solar wind. Every second, the Earth loses about 3 kg of hydrogen, 50 g of helium, and much smaller amounts of other constituents.[30]
The exosphere is too far above Earth for meteorological phenomena to be possible. The exosphere contains many of the artificial satellites that orbit Earth.[31]
Thermosphere
[edit]The thermosphere is the second-highest layer of Earth's atmosphere. It extends from the mesopause (which separates it from the mesosphere) at an altitude of about 80 km (50 mi) up to the thermopause at an altitude range of 500–1000 km (310–620 mi). The height of the thermopause varies considerably due to changes in solar activity.[25] The passage of the dusk and dawn solar terminator creates background density perturbations up to a factor of two through this layer, forming a dominant feature in this region.[32] Because the thermopause lies at the lower boundary of the exosphere, it is also referred to as the exobase. Overlapping the thermosphere, from 50 to 600 kilometres (31 to 373 mi) above Earth's surface, is the ionosphere – a region of enhanced plasma density.[33][34]
The temperature of the thermosphere gradually increases with height and can rise as high as 1500 °C (2700 °F), though the gas molecules are so far apart that its temperature in the usual sense is not very meaningful. This temperature increase is caused by absorption of ionizing UV and X-ray emission from the Sun.[34][35] The air is so rarefied that an individual molecule (of oxygen, for example) travels an average of 1 kilometre (0.62 mi; 3300 ft) between collisions with other molecules.[36] Although the thermosphere has a high proportion of molecules with high energy, it would not feel hot to a human in direct contact, because its density is too low to conduct a significant amount of energy to or from the skin.[34]
This layer is completely cloudless and free of water vapor. However, non-hydrometeorological phenomena such as the aurora borealis and aurora australis are occasionally seen in the thermosphere at an altitude of around 100 km (62 mi).[37] The colors of the aurora are linked to the properties of the atmosphere at the altitude they occur. The most common is the green aurora, which comes from atomic oxygen in the 1S state, and occurs at altitudes from 120 to 400 km (75 to 250 mi).[38] The International Space Station orbits in the thermosphere, between 370 and 460 km (230 and 290 mi).[39] It is this layer where many of the satellites orbiting the Earth are present.[31]
Mesosphere
[edit]
The mesosphere is the third highest layer of Earth's atmosphere, occupying the region above the stratosphere and below the thermosphere. It extends from the stratopause at an altitude of about 50 km (31 mi) to the mesopause at 80–85 km (50–53 mi) above sea level.[34] Temperatures drop with increasing altitude to the mesopause that marks the top of this middle layer of the atmosphere. It is the coldest place on Earth and has an average temperature around −85 °C (−120 °F; 190 K).[40][41] Because the atmosphere absorbs sound waves at a rate that is proportional to the square of the frequency, audible sounds from the ground do not reach the mesosphere. Infrasonic waves can reach this altitude, but they are difficult to emit at a high power level.[42]
Just below the mesopause, the air is so cold that even the very scarce water vapor at this altitude can condense into polar-mesospheric noctilucent clouds of ice particles. These are the highest clouds in the atmosphere and may be visible to the naked eye if sunlight reflects off them about an hour or two after sunset or similarly before sunrise. They are most readily visible when the Sun is around 4 to 16 degrees below the horizon.[43]
Lightning-induced discharges known as transient luminous events (TLEs) occasionally form in the mesosphere above tropospheric thunderclouds.[44] The mesosphere is also the layer where most meteors and satellites burn up upon atmospheric entrance.[34][45] It is too high above Earth to be accessible to jet-powered aircraft and balloons, and too low to permit orbital spacecraft. The mesosphere is mainly accessed by sounding rockets and rocket-powered aircraft.[46]
Stratosphere
[edit]
The stratosphere is the second-lowest layer of Earth's atmosphere. It lies above the troposphere and is separated from it by the tropopause. This layer extends from the top of the troposphere at roughly 12 km (7.5 mi) above Earth's surface to the stratopause at an altitude of about 50 to 55 km (31 to 34 mi).[22] 99% of the total mass of the atmosphere lies below 30 km (19 mi),[47] and the atmospheric pressure at the top of the stratosphere is roughly 1/1000 the pressure at sea level.[48] It contains the ozone layer, which is the part of Earth's atmosphere that contains relatively high concentrations of that gas.[49]
The stratosphere defines a layer in which temperatures rise with increasing altitude. This rise in temperature is caused by the absorption of ultraviolet radiation (UV) from the Sun by the ozone layer, which restricts turbulence and mixing. Although the temperature may be −80 °C (−110 °F; 190 K) at the tropopause, the top of the stratosphere is much warmer, and may be just below 0 °C.[50][49] This layer is unique to the Earth; neither Mars nor Venus have a stratosphere because of low abundances of oxygen in their atmospheres.[51]
The stratospheric temperature profile creates very stable atmospheric conditions, so the stratosphere lacks the weather-producing air turbulence that is so prevalent in the troposphere. Consequently, the stratosphere is almost completely free of clouds and other forms of weather.[49] However, polar stratospheric or nacreous clouds are occasionally seen in the lower part of this layer of the atmosphere where the air is coldest.[52] The stratosphere is the highest layer that can be accessed by jet-powered aircraft.[53]
Troposphere
[edit]
The troposphere is the lowest layer of Earth's atmosphere. It extends from Earth's surface to an average height of about 12 km (7.5 mi), although this altitude varies from about 9 km (5.6 mi) at the geographic poles to 17 km (11 mi) at the Equator,[26] with some variation due to weather. The troposphere is bounded above by the tropopause, a boundary marked in most places by a temperature inversion (i.e. a layer of relatively warm air above a colder one), and in others by a zone that is isothermal with height.[54][55]
Although variations do occur, the temperature usually declines with increasing altitude in the troposphere because the troposphere is mostly heated through energy transfer from the surface. Thus, the lowest part of the troposphere (i.e. Earth's surface) is typically the warmest section of the troposphere. This promotes vertical mixing (hence, the origin of its name in the Greek word τρόπος, tropos, meaning "turn").[56] The troposphere contains roughly 80% of the mass of Earth's atmosphere.[57] The troposphere is denser than all its overlying layers because a larger atmospheric weight sits on top of the troposphere and causes it to be more severely compressed. Fifty percent of the total mass of the atmosphere is located in the lower 5.5 km (3.4 mi) of the troposphere.[47]
Nearly all atmospheric water vapor or moisture is found in the troposphere, so it is the layer where most of Earth's weather takes place. The ability of the atmosphere to retain water decreases as the temperature declines, so 90% of the water vapor is held in the lower part of the troposphere.[58] It has basically all the weather-associated cloud genus types generated by active wind circulation, although very tall cumulonimbus thunder clouds can penetrate the tropopause from below and rise into the lower part of the stratosphere.[59] Most conventional aviation activity takes place in the troposphere, and it is the only layer accessible by propeller-driven aircraft.[53] Contrails are formed from jet engine water emission at altitudes where the atmospheric temperature is about −53 °C (−63 °F); typically around 7.7 km (4.8 mi) for modern engines.[60]
Other layers
[edit]Within the five principal layers above, which are largely determined by temperature, several secondary layers may be distinguished by other properties:
- The ozone layer is contained within the stratosphere. In this layer ozone reaches a peak concentration of 15 parts per million at an altitude of 32 km (20 mi), which is much higher than in the lower atmosphere but still very small compared to the main components of the atmosphere.[61] It is mainly located in the lower portion of the stratosphere from about 15–35 km (9.3–21.7 mi),[5]: 260 though the thickness varies seasonally and geographically. About 90% of the ozone in Earth's atmosphere is contained in the stratosphere.[62]
- The ionosphere is a region of the atmosphere that is ionized by solar radiation. It plays a significant role in auroras, airglow, and space weather phenomenon.[63][64] During daytime hours, it stretches from 50 to 1,000 km (31 to 621 mi) and includes the mesosphere, thermosphere, and parts of the exosphere. However, ionization in the mesosphere largely ceases during the night.[65] The ionosphere forms the inner edge of the plasmasphere – the inner magnetosphere.[66] It has practical importance because it influences, for example, radio propagation on Earth.[67]
- The homosphere and heterosphere are defined by whether the atmospheric gases are well mixed. The surface-based homosphere includes the troposphere, stratosphere, mesosphere, and the lowest part of the thermosphere, where the chemical composition of the atmosphere does not depend on molecular weight because the gases are mixed by turbulence.[68] This relatively homogeneous layer ends at the turbopause found at about 100 km (62 mi; 330,000 ft),[19] the very edge of space itself as accepted by the FAI, which places it about 20 km (12 mi; 66,000 ft) above the mesopause.
- Above this altitude lies the heterosphere, which includes the exosphere and most of the thermosphere. Here, the chemical composition varies with altitude. This is because the distance that particles can move without colliding with one another is large compared with the size of motions that cause mixing. This allows the gases to stratify by molecular weight,[19] with the heavier ones, such as oxygen and nitrogen, present only near the bottom of the heterosphere. The upper part of the heterosphere is composed almost completely of hydrogen, the lightest element.[69]
- The planetary boundary layer is the part of the troposphere that is closest to Earth's surface and is directly affected by it, mainly through turbulent diffusion. During the day the planetary boundary layer usually is well-mixed, whereas at night it becomes stably stratified with weak or intermittent mixing. The depth of the planetary boundary layer ranges from as little as about 100 metres (330 ft) on clear, calm nights to 1,000–1,500 m (3,300–4,900 ft) or more during the afternoon.[70]
- The barosphere is the region of the atmosphere where the barometric law applies. It ranges from the ground to the thermopause. Above this altitude, the velocity distribution is non-Maxwellian due to high velocity atoms and molecules being able to escape the atmosphere.[71]
The average temperature of the atmosphere at Earth's surface is 14 °C (57 °F; 287 K)[72] or 15 °C (59 °F; 288 K),[73] depending on the reference.[74][75][76]
Physical properties
[edit]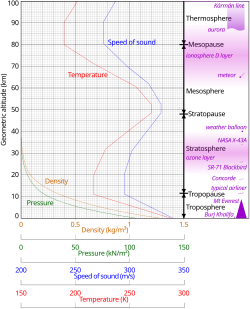
Pressure and thickness
[edit]The average atmospheric pressure at sea level is defined by the International Standard Atmosphere as 101325 pascals (760.00 Torr; 14.6959 psi; 760.00 mmHg).[5]: 257 This is sometimes referred to as a unit of standard atmospheres (atm). Total atmospheric mass is 5.1480×1018 kg (1.13494×1019 lb),[78] about 2.5% less than would be inferred from the average sea-level pressure and Earth's area of 51007.2 megahectares,[5]: 240 this portion being displaced by Earth's mountainous terrain. Atmospheric pressure is the total weight of the air above unit area at the point where the pressure is measured. Thus air pressure varies with location and weather.
Air pressure decreases exponentially with altitude at a rate that depends on the air temperature. The rate of decrease is determined by a temperature-dependent parameter called the scale height: for each increase in altitude by this height, the pressure decreases by a factor of e (the base of natural logarithms, approximately 2.718). For Earth, this value is typically 5.5 to 6 km for altitudes up to around 80 km (50 mi).[79] However, the atmosphere is more accurately modeled with a customized equation for each layer that takes gradients of temperature, molecular composition, solar radiation and gravity into account. At heights over 100 km, the atmosphere is not well mixed, so each chemical species has its own scale height. At altitudes of 200 to 300 km, the combined scale height is 20 to 30 km.[79]
The mass of Earth's atmosphere is distributed approximately as follows:[80]
- 50% is below 5.6 km (18,000 ft)
- 90% is below 16 km (52,000 ft)
- 99.99997% is below 100 km (62 mi; 330,000 ft), the Kármán line. By international convention, this marks the beginning of space where human travelers are considered astronauts.
By comparison, the summit of Mount Everest is at 8,848 m (29,029 ft); commercial airliners typically cruise between 9 and 12 km (30,000 and 38,000 ft),[81] where the lower density and temperature of the air improve fuel economy; weather balloons reach about 35 km (115,000 ft);[82] and the highest X-15 flight in 1963 reached 108.0 km (354,300 ft).
Even above the Kármán line, significant atmospheric effects such as auroras still occur.[37] Meteors begin to glow in this region,[34] though the larger ones may not burn up until they penetrate more deeply. The various layers of Earth's ionosphere, important to HF radio propagation, begin below 100 km and extend beyond 500 km. By comparison, the International Space Station typically orbit at 370–460 km,[39] within the F-layer of the ionosphere,[5]: 271 where they encounter enough atmospheric drag to require reboosts every few months, otherwise orbital decay will occur, resulting in a return to Earth.[39] Depending on solar activity, satellites can experience noticeable atmospheric drag at altitudes as high as 600–800 km.[83]
Temperature
[edit]
Starting at sea level, the temperature decreases with altitude until reaching the stratosphere at around 11 km. Above, the temperature stabilizes over a large vertical distance. Starting above about 20 km, the temperature increases with height, due to heating within the ozone layer caused by the capture of significant ultraviolet radiation from the Sun by the molecular oxygen and ozone gas in this region. A second region of increasing temperature with altitude occurs at very high altitudes, in the aptly-named thermosphere above 90 km.[34]
During the night, the ground radiates more energy than it gains from the atmosphere. As energy is conducted from the nearby atmosphere to the cooler ground, it creates a temperature inversion where the local temperature increases with altitude up to around 1,000 m.[84]
Speed of sound
[edit]Because in an ideal gas of constant composition the speed of sound depends only on temperature and not on pressure or density, the speed of sound in the atmosphere with altitude takes on the form of the complicated temperature profile (see illustration to the right), and does not mirror altitudinal changes in density or pressure.[85] For example, at sea level the speed of sound is 340 m/s. At the average temperature of the stratosphere, –60°C, the speed of sound decreases to 290 m/s.[86]
Density and mass
[edit]
The density of air at sea level is about 1.29 kg/m3 (1.29 g/L, 0.00129 g/cm3).[5]: 257 Density is not measured directly but is calculated from measurements of temperature, pressure and humidity using the equation of state for air (a form of the ideal gas law). Atmospheric density decreases as the altitude increases. This variation can be approximately modeled using the barometric formula.[87] More sophisticated models are used to predict the orbital decay of satellites.[88]
The average mass of the atmosphere is about 5 quadrillion (5×1015) tonnes or 1/1,200,000 the mass of Earth. According to the American National Center for Atmospheric Research, "The total mean mass of the atmosphere is 5.1480×1018 kg with an annual range due to water vapor of 1.2 or 1.5×1015 kg, depending on whether surface pressure or water vapor data are used; somewhat smaller than the previous estimate. The mean mass of water vapor is estimated as 1.27×1016 kg and the dry air mass as 5.1352 ±0.0003×1018 kg."[89]
Optical properties
[edit]
Solar radiation (or sunlight) is the energy Earth receives from the Sun. Earth also emits radiation back into space, but at longer wavelengths that humans cannot see. As energy propagates through the atmosphere, it is impacted by the process of radiative transfer. That is, some of the incoming and emitted radiation is subject to absorption, emission, and scattering by the atmosphere. Another portion of the incident energy is reflected,[90][91] with the two most important atmospheric reflectors being dust and clouds. Depending on the properties of the aerosol, clouds can reflect up to 70% of the incident radiation. Globally, clouds reflect 20% of the incoming energy, contributing two thirds of the planet's total albedo.[92] In May 2017, glints of light, seen as twinkling from an orbiting satellite a million miles away, were found to be reflected light from ice crystals in the troposphere.[93][94]
Scattering
[edit]When light passes through Earth's atmosphere, photons interact with it through scattering. If the light does not interact with the atmosphere, it is called direct radiation and is what you see if you were to look directly at the Sun. Indirect radiation is light that has been scattered in the atmosphere. For example, on an overcast day when you cannot see your shadow, there is no direct radiation reaching you, it has all been scattered. As another example, due to a phenomenon called Rayleigh scattering, shorter (blue) wavelengths scatter more easily than longer (red) wavelengths. This is why the sky looks blue; you are seeing scattered blue light. This is also why sunsets are red. Because the Sun is close to the horizon, the Sun's rays pass through more atmosphere than normal before reaching your eye. Much of the blue light has been scattered out, leaving the red light in a sunset.[95]
Absorption
[edit]
Different molecules absorb different wavelengths of radiation. For example, O2 and O3 absorb almost all radiation with wavelengths shorter than 300 nanometres.[96] Water (H2O) absorbs at many wavelengths above 700 nm.[97] When a molecule absorbs a photon, it increases the energy of the molecule. This heats the atmosphere, but the atmosphere also cools by emitting radiation, as discussed below. In astronomical spectroscopy, the absorption of specific frequencies by the atmosphere is referred to as telluric contamination.[98]
The combined absorption spectra of the gases in the atmosphere leave "windows" of low opacity, allowing the transmission of only certain bands of light. The optical window runs from around 300 nm (ultraviolet-C) up into the range humans can see, the visible spectrum (commonly called light), at roughly 400–700 nm and continues to the infrared to around 1100 nm. There are also infrared and radio windows that transmit some infrared and radio waves at longer wavelengths. For example, the radio window runs from about one centimetre to about eleven-metre waves.[99]
Emission
[edit]Emission is the opposite of absorption, it is when an object emits radiation. Objects tend to emit amounts and wavelengths of radiation depending on their "black body" emission curves, therefore hotter objects tend to emit more radiation, with shorter wavelengths. Colder objects emit less radiation, with longer wavelengths. For example, the Sun is approximately 6,000 K (5,730 °C; 10,340 °F), its radiation peaks near 500 nm, and is visible to the human eye. Earth is approximately 290 K (17 °C; 62 °F), so its radiation peaks near 10,000 nm, and is much too long to be visible to humans.[100]
Because of its temperature, the atmosphere emits infrared radiation. For example, on clear nights Earth's surface cools down faster than on cloudy nights. This is because clouds (H2O) are strong absorbers and emitters of infrared radiation.[101] This is also why it becomes colder at night at higher elevations.
The greenhouse effect is directly related to this absorption and emission effect. Some gases in the atmosphere absorb and emit infrared radiation, but do not interact in this manner with sunlight in the visible spectrum. Common examples of these are CO2 and H2O.[102] Without greenhouse gases in the atmosphere, the average temperature of Earth's surface would be a frozen −18 °C (0 °F), rather than the present comfortable average of 15 °C (59 °F).[103]
Refractive index
[edit]
The refractive index of air is close to, but just greater than, 1.[104] Systematic variations in the refractive index can lead to the bending of light rays over long optical paths. One example is that, under some circumstances, observers on board ships can see other vessels just over the horizon because light is refracted in the same direction as the curvature of Earth's surface.[105]
The refractive index of air depends on temperature,[106] giving rise to refraction effects when the temperature gradient is large. An example of such effects is the mirage.[107]
Circulation
[edit]
Atmospheric circulation is the large-scale movement of air through the troposphere, and the means (with ocean circulation) by which heat is distributed around Earth. The large-scale structure of the atmospheric circulation varies from year to year, but the basic structure remains fairly constant because it is determined by Earth's rotation rate and the difference in solar radiation between the equator and poles. The axial tilt of the planet means the location of maximum heat is continually changing, resulting in seasonal variations. The uneven distribution of land and water further breaks up the flow of air.[108]
The flow of air around the planet is divided into three main convection cells by latitude. Around the equator, the Hadley cell is driven by the rising flow of air along the equator. In the upper atmosphere, this air flows toward the poles. At mid latitudes, this circulation is reversed, with ground air flowing toward the poles with the Ferrel cell. Finally, in the high latitudes is the Polar cell, where air again rises and flows toward the poles.[108]
The interface between these cells is responsible for jet streams. These are narrow, fast moving bands that flow from west to east and typically form at an elevation of around 9,100 m (30,000 ft). Jet streams can shift around depending on conditions. They are strongest in winter, when the boundaries between hot and cold air are the most pronounced.[109] In the middle latitudes, it is instabilities in the jet streams that are responsible for moving weather systems.[110]
As with the oceans, the Earth's atmosphere is subject to waves and tidal forces. These are triggered by non-uniform heating by the Sun, and by the daily solar cycle, respectively. Wave-like behavior can occur on a variety of scales, from smaller gravity waves that transfer momentum into the higher atmospheric layers, to much larger planetary waves, or Rossby waves. Atmospheric tides are periodic oscillations of the troposphere and stratosphere that transport energy to the upper atmosphere.[111]
Evolution of Earth's atmosphere
[edit]Earliest atmosphere
[edit]The first atmosphere, during the Early Earth's Hadean eon, consisted of gases in the solar nebula, primarily hydrogen, and probably simple hydrides such as those now found in the gas giants (Jupiter and Saturn), notably water vapor, methane and ammonia. During this earliest era, the Moon-forming collision and numerous impacts with large meteorites heated the atmosphere, driving off the most volatile gases. The collision with Theia, in particular, melted and ejected large portions of Earth's mantle and crust and outgassed significant amounts of steam which eventually cooled and condensed to contribute to ocean water at the end of the Hadean.[112]: 10
Second atmosphere
[edit]The increasing solidification of Earth's crust at the end of the Hadean closed off most of the advective heat transfer to the surface, causing the atmosphere to cool, which condensed most of the water vapor out of the air precipitating into a superocean. Further outgassing from volcanism, supplemented by gases introduced by huge asteroids during the Late Heavy Bombardment, created the subsequent Archean atmosphere, which consisted largely of nitrogen plus carbon dioxide, methane and inert gases.[112] A major part of carbon dioxide emissions dissolved in water and reacted with metals such as calcium and magnesium during weathering of crustal rocks to form carbonates that were deposited as sediments. Water-related sediments have been found that date from as early as 3.8 billion years ago.[113]
About 3.4 billion years ago, nitrogen formed the major component of the then-stable "second atmosphere". The influence of the evolution of life has to be taken into account rather soon in the history of the atmosphere because hints of earliest life forms appeared as early as 3.5 billion years ago.[114] How Earth at that time maintained a climate warm enough for liquid water and life, if the early Sun put out 30% lower solar radiance than today, is a puzzle known as the "faint young Sun paradox".[115]
The geological record however shows a continuous relatively warm surface during the complete early temperature record of Earth – with the exception of one cold glacial phase about 2.4 billion years ago. In the late Neoarchean, an oxygen-containing atmosphere began to develop, apparently due to a billion years of cyanobacterial photosynthesis (known as the Great Oxygenation Event),[116] which have been found as stromatolite fossils from 2.7 billion years ago. The early basic carbon isotopy (isotope ratio proportions) strongly suggests conditions similar to the current, and that the fundamental features of the carbon cycle became established as early as 4 billion years ago.[117]
Ancient sediments in the Gabon dating from between about 2.15 and 2.08 billion years ago provide a record of Earth's dynamic oxygenation evolution. These fluctuations in oxygenation were likely driven by the Lomagundi-Jatuli Carbon Isotope Excursion.[118]
Third atmosphere
[edit]The constant re-arrangement of continents by plate tectonics influences the long-term evolution of the atmosphere by transferring carbon dioxide to and from large continental carbonate stores. Free oxygen did not exist in the atmosphere until about 2.4 billion years ago during the Great Oxygenation Event[119] and its appearance is indicated by the end of banded iron formations (which signals the depletion of substrates that can react with oxygen to produce ferric deposits) during the early Proterozoic eon.[120]
Before this time, any oxygen produced by cyanobacterial photosynthesis would be readily removed by the oxidation of reducing substances on the Earth's surface, notably ferrous iron, sulfur and atmospheric methane. Free oxygen molecules did not start to accumulate in the atmosphere until the rate of production of oxygen began to exceed the availability of reductant materials that removed oxygen. This point signifies a shift from a reducing atmosphere to an oxidizing atmosphere.[121] O2 showed major variations during the Proterozoic, including a billion-year period of euxinia, until reaching a steady state of more than 15% by the end of the Precambrian.[122]
The rise of the more robust eukaryotic photoautotrophs (green and red algae) injected further oxygenation into the air, especially after the end of the Cryogenian global glaciation, which was followed by an evolutionary radiation event during the Ediacaran period known as the Avalon explosion, where complex metazoan life forms (including the earliest cnidarians, placozoans and bilaterians) first proliferated. The following time span from 539 million years ago to the present day is the Phanerozoic eon, during the earliest period of which, the Cambrian, more actively moving metazoan life began to appear and rapidly diversify in another radiation event called the Cambrian explosion, whose locomotive metabolism was fuelled by the rising oxygen level.[123]

The amount of oxygen in the atmosphere has fluctuated over the last 600 million years, reaching a peak of about 35% around 280 million years ago during the Carboniferous period, significantly higher than today's 21%.[126] Two main processes govern changes in the atmosphere: the evolution of plants and their increasing role in carbon fixation, and the consumption of oxygen by rapidly diversifying animal faunae and also by plants for photorespiration and their own metabolic needs at night. Breakdown of pyrite and volcanic eruptions release sulfur into the atmosphere, which reacts and hence reduces oxygen in the atmosphere.[127] However, volcanic eruptions also release carbon dioxide,[128] which can fuel oxygenic photosynthesis by terrestrial and aquatic plants. The cause of the variation of the amount of oxygen in the atmosphere is not precisely understood. Periods with more oxygen in the atmosphere were often associated with more rapid development of animals.[119]
Air pollution
[edit]Air pollution is the introduction of airborne chemicals, particulate matter or biological materials that cause harm or discomfort to organisms.[129] The population growth, industrialization and motorization of human societies have significantly increased the amount of airborne pollutants in the Earth's atmosphere, causing noticeable problems such as smogs, acid rains and pollution-related diseases. The depletion of the stratospheric ozone layer, which shields the surface from harmful ionizing ultraviolet radiations, is also caused by air pollution, chiefly from chlorofluorocarbons and other ozone-depleting substances.[130]
Since 1750, human activity, especially after the Industrial Revolution, has increased the concentrations of various greenhouse gases, most importantly carbon dioxide, methane and nitrous oxide. Greenhouse gas emissions, coupled with deforestation and destruction of wetlands via logging and land developments, have caused an observed rise in global temperatures, with the global average surface temperatures being 1.1 °C higher in the 2011–2020 decade than they were in 1850.[131] It has raised concerns of man-made climate change, which can have significant environmental impacts such as sea level rise, ocean acidification, glacial retreat (which threatens water security), increasing extreme weather events and wildfires, ecological collapse and mass dying of wildlife.[132]
See also
[edit]- Aerial perspective
- Air (classical element)
- Airshed
- Atmospheric dispersion modeling
- Atmospheric electricity
- Biosphere
- Climate system
- COSPAR International Reference Atmosphere (CIRA)
- Environmental impact of aviation
- Global dimming
- Global surface temperature
- Hydrosphere
- Lithosphere
- Reference atmospheric model
References
[edit]- ^ "Gateway to Astronaut Photos of Earth". NASA. Retrieved 2018-01-29.
- ^ Lide, David R., ed. (May 28, 1996). Handbook of Chemistry and Physics (77th ed.). Boca Raton, FL: CRC Press. pp. 14–17. ISBN 978-0-8493-0477-4.
- ^ "What Is... Earth's Atmosphere? - NASA". 2024-05-13. Retrieved 2024-06-18.
- ^ Vázquez, M.; Hanslmeier, A. (2006). "Historical Introduction". Ultraviolet Radiation in the Solar System. Astrophysics and Space Science Library. Vol. 331. Springer Science & Business Media. p. 17. Bibcode:2005ASSL..331.....V. doi:10.1007/1-4020-3730-9_1. ISBN 978-1-4020-3730-6.
- ^ a b c d e f g h Cox, Arthur N., ed. (2002). "11. Earth". Allen's Astrophysical Quantities (4th ed.). New York, NY: Springer New York. doi:10.1007/978-1-4612-1186-0. ISBN 978-1-4612-7037-9.
- ^ a b "Trends in Atmospheric Carbon Dioxide", Global Greenhouse Gas Reference Network, NOAA, 2019, retrieved 2019-05-31
- ^ a b "Trends in Atmospheric Methane", Global Greenhouse Gas Reference Network, NOAA, 2019, retrieved 2019-05-31
- ^ a b Wallace, John M.; Hobbs, Peter V. (2006). "Chapter 1. Introduction and Overview". Atmospheric Science: An Introductory Survey (PDF) (Second ed.). Elsevier. pp. 1–21. ISBN 978-0-12-732951-2. Archived from the original (PDF) on 2018-07-28. Retrieved 2018-07-28.
- ^ "Trace Gases". Encyclopedia of the Atmospheric Environment. Archived from the original on 9 October 2010. Retrieved 2010-10-16.
- ^ Graedel, T. E.; et al. (2012). Atmospheric Chemical Compounds: Sources, Occurrence and Bioassay. Elsevier. pp. v–ix. ISBN 978-0-08-091842-6.
- ^ a b Colbeck, Ian; Lazaridis, Mihalis (February 2010). "Aerosols and environmental pollution". Naturwissenschaften. 97 (2): 117–131. Bibcode:2010NW.....97..117C. doi:10.1007/s00114-009-0594-x. PMID 19727639.
- ^ Wang, Hao; et al. (2017). "Mixed Chloride Aerosols and their Atmospheric Implications: A Review". Aerosol and Air Quality Research. 17 (4). Taiwan Association for Aerosol Research: 878–887. Bibcode:2017AAQR...17..878W. doi:10.4209/aaqr.2016.09.0383. hdl:10397/103030.
- ^ Faust, J. A. (February 22, 2023). "PFAS on atmospheric aerosol particles: a review". Environmental Science: Processes & Impacts. 25 (2): 133–150. doi:10.1039/d2em00002d. PMID 35416231.
- ^ Pacyna, Jozef M.; et al. (October 2016). "Current and future levels of mercury atmospheric pollution on a global scale". Atmospheric Chemistry and Physics. 16 (19): 12495–12511. Bibcode:2016ACP....1612495P. doi:10.5194/acp-16-12495-2016. hdl:11250/2452800.
- ^ Kumar, Manoj; Francisco, Joseph S. (January 2017). "Elemental sulfur aerosol-forming mechanism". Proceedings of the National Academy of Sciences. 114 (5): 864–869. Bibcode:2017PNAS..114..864K. doi:10.1073/pnas.1620870114. PMC 5293086. PMID 28096368.
- ^ Jacob, Daniel J. (1999). Introduction to atmospheric chemistry (Online-Ausg ed.). Princeton, N.J.: Princeton University Press. ISBN 978-1-4008-4154-7.
- ^ Möller, Detlev (2003). Luft: Chemie, Physik, Biologie, Reinhaltung, Recht. Walter de Gruyter. p. 173. ISBN 3-11-016431-0.
- ^ Çengel, Yunus (2013). Termodinamica e trasmissione del calore (4th ed.). McGraw-Hill Education. ISBN 978-88-386-6511-0.
- ^ a b c Schlatter, Thomas W. (2009). "Atmospheric Composition and Vertical Structure". Environmental Impact and Manufacturing. Vol. 6. pp. 1–54. Retrieved 2025-07-20. See p. 6.
- ^ Brekke, Asgeir (2013). Physics of the Upper Polar Atmosphere. Springer Atmospheric Sciences. Berlin, Heidelberg: Springer Berlin Heidelberg. doi:10.1007/978-3-642-27401-5. ISBN 978-3-642-27400-8.
- ^ a b Champion, K. S. W.; et al. (1985). "Standard and reference atmospheres". In Jursa, Adolf S. (ed.). Handbook of geophysics and the space environment (PDF). Air Force Geophysics Library. Retrieved 2025-07-20.
- ^ a b Buis, Alan (October 22, 2024). "Earth's Atmosphere: A Multi-layered Cake". NASA. Retrieved 2025-07-21.
- ^ Zell, Holly (March 2, 2015). "Earth's Upper Atmosphere". NASA. Retrieved 2017-02-20.
- ^ "Exosphere - overview". University Corporation for Atmospheric Research. 2011. Archived from the original on 17 May 2017. Retrieved April 19, 2015.
- ^ a b Russell, Randy (2008). "The Thermosphere". National Earth Science Teachers Association (NESTA). Retrieved 2013-10-18.
- ^ a b Geerts, B.; Linacre, E. (November 1997). "The height of the tropopause". Department of Atmospheric Science, University of Wyoming. Archived from the original on February 22, 2001. Retrieved 2012-04-18.
- ^ a b c "Exosphere - overview". University Corporation for Atmospheric Research. 2011. Archived from the original on 17 May 2017. Retrieved April 19, 2015.
- ^ "Earth's Atmospheric Layers". NASA. January 22, 2013.
- ^ Singh, Vir (2020). Environmental Plant Physiology: Botanical Strategies for a Climate Smart Planet. CRC Press. ISBN 978-1-000-02486-9.
- ^ Catling, David C.; Zahnle, Kevin J. (May 2009). "The Planetary Air Leak" (PDF). Scientific American. 300 (5): 36–43. Bibcode:2009SciAm.300e..36C. doi:10.1038/scientificamerican0509-36 (inactive 21 July 2025). PMID 19438047. Retrieved 25 July 2012.
{{cite journal}}: CS1 maint: DOI inactive as of July 2025 (link) - ^ a b Liou, J.-C.; Johnson, N. L. (2008). "Instability of the present LEO satellite populations". Advances in Space Research. 41 (7): 1046–1053. Bibcode:2008AdSpR..41.1046L. doi:10.1016/j.asr.2007.04.081. hdl:2060/20060024585.
- ^ Fitzpatrick, D. J.; et al. (June 16, 2025). "Solar Terminator Waves Revealed as Dominant Features of Upper Thermospheric Density". Geophysical Research Letters. 52 (11) e2025GL115612: 1. Bibcode:2025GeoRL..5215612F. doi:10.1029/2025GL115612.
- ^ Blaunstein, Nathan; Plohotniuc, Eugeniu (May 13, 2008). Ionosphere and Applied Aspects of Radio Communication and Radar. CRC Press. p. 1. ISBN 978-1-4200-5517-7.
- ^ a b c d e f g "Layers of the Atmosphere". National Oceanic and Atmospheric Administration. August 20, 2024. Retrieved 2025-07-21.
- ^ Flock, Warren L. (1987). Propagation effects on satellite systems at frequencies below 10 GHz – a handbook for satellite system design. NASA Reference Publication. Vol. 1108 (2nd ed.). National Aeronautics and Space Administration. pp. 1–19 to 1–22.
- ^ Ahrens, C. Donald (2005). Essentials of Meteorology (4th ed.). Thomson Brooks/Cole. ISBN 978-0-534-40679-0.
- ^ a b Lodders, Katharina; Fegley, Jr, Bruce (2015). Chemistry of the Solar System. Royal Society of Chemistry. ISBN 978-1-78262-601-5.
- ^ "Aurora Tutorial". Space Weather Prediction Center, NOAA. Retrieved 2025-07-26.
- ^ a b c "International Space Station". NASA. May 23, 2023. Retrieved 2025-07-23.
- ^ States, Robert J.; Gardner, Chester S. (January 2000). "Thermal Structure of the Mesopause Region (80–105 km) at 40°N Latitude. Part I: Seasonal Variations". Journal of the Atmospheric Sciences. 57 (1): 66–77. Bibcode:2000JAtS...57...66S. doi:10.1175/1520-0469(2000)057<0066:TSOTMR>2.0.CO;2.
- ^ Buchdahl, Joe. "Atmosphere, Climate & Environment Information Programme". Encyclopedia of the Atmospheric Environment. Department for Environment, Food and Rural Affairs. Archived from the original on 2010-07-01. Retrieved 2012-04-18.
- ^ Yang, Xunren (2016). Atmospheric Acoustics. Walter de Gruyter GmbH & Co KG. ISBN 978-3-11-038302-7.
- ^ Gadsden, Michael; Parvianinen, Pekka (2006). "Observing noctilucent clouds". International Association of Geomagnetism & Aeronomy. Retrieved 2025-07-21.
- ^ Sato, M.; et al. (May 2015). "Overview and early results of the Global Lightning and Sprite Measurements mission". Journal of Geophysical Research: Atmospheres. 120 (9): 3822–3851. Bibcode:2015JGRD..120.3822S. doi:10.1002/2014JD022428.
- ^ Karahan, B.; et al. (April 2025). Lemmens, S.; et al. (eds.). Statistical analysis of destructive satellite re-entry uncertainties (PDF). Procedings of the 9th European Conference on Space Debris, Bonn, Germany, 1–4 April 2025. ESA Space Debris Office. Retrieved 2025-07-22.
- ^ Heatwole, Scott E. (September 2024). "Current usage of sounding rockets to study the upper atmosphere". Proceedings of the National Academy of Sciences. 121 (40) e2413285121. id. e2413285121. Bibcode:2024PNAS..12113285H. doi:10.1073/pnas.2413285121. PMC 11459162. PMID 39302994.
- ^ a b Holloway, Ann M.; Wayne, Richard P. (2015). Atmospheric Chemistry. Royal Society of Chemistry. ISBN 978-1-78262-593-3.
- ^ Schmunk, Robert B. (April 3, 2025). "Introduction to Clouds". NASA. Retrieved 2025-07-22.
- ^ a b c Saha, Pijushkanti (2012). Modern Climatology. Allied Publishers. p. 21. ISBN 978-81-8424-756-5.
- ^ Journal of the Atmospheric Sciences (1993). "stratopause". Archived from the original on 2013-10-19. Retrieved 2013-10-18.
- ^ de Pater, Imke; Lissauer, Jack J. (2015). Planetary Sciences (2 ed.). Cambridge University Press. pp. 81–82. ISBN 978-1-316-19569-7.
- ^ Salby, Murry L. (1996). Fundamentals of Atmospheric Physics. International Geophysics. Vol. 61. Elsevier. pp. 283–285. ISBN 978-0-08-053215-8.
- ^ a b Filippone, Antonio (2012). Advanced Aircraft Flight Performance. Cambridge Aerospace Series. Vol. 34. Cambridge University Press. ISBN 978-1-107-02400-7.
- ^ Barry, R. G.; Chorley, R. J. (1971). Atmosphere, Weather and Climate. London: Menthuen & Co Ltd. p. 65. ISBN 978-0-416-07940-1.
- ^ Tyson, P. D.; Preston-Whyte, R. A. (2000). The Weather and Climate of Southern Africa (2nd ed.). Oxford: Oxford University Press. p. 4. ISBN 0-19-571806-2.
- ^ Frederick, John E. (2008). Principles of Atmospheric Science. Jones & Bartlett Learning. pp. 15–17. ISBN 978-0-7637-4089-4.
- ^ "Troposphere". Concise Encyclopedia of Science & Technology. McGraw-Hill. 1984. ISBN 0-07-045482-5.
It contains about four-fifths of the mass of the whole atmosphere.
- ^ Singh, P.; Singh, Vijay P. (2001). Snow and Glacier Hydrology. Springer Netherlands. p. 56. ISBN 0-7923-6767-7.
- ^ Wang, Pao K.; et al. (November 2009). "Further evidences of deep convective vertical transport of water vapor through the tropopause". Atmospheric Research. 94 (3): 400–408. Bibcode:2009AtmRe..94..400W. doi:10.1016/j.atmosres.2009.06.018.
- ^ Tiwary, Abhishek; Williams, Ian (2018). Air Pollution: Measurement, Modelling and Mitigation (Fourth ed.). CRC Press. p. 218. ISBN 978-1-4987-1946-9.
- ^ "NASA Ozone Watch". NASA Goddard Spaceflight Center. Retrieved 2025-07-22.
- ^ "Science: Ozone Basics". Stratospheric Ozone. National Oceanic and Atmospheric Association. Retrieved 2025-07-22.
- ^ Newell, Patrick T.; et al. (May 2001). "The role of the ionosphere in aurora and space weather". Reviews of Geophysics. 39 (2): 137–149. Bibcode:2001RvGeo..39..137N. doi:10.1029/1999RG000077.
- ^ Basavaiah, Nathani (2012). Geomagnetism: Solid Earth and Upper Atmosphere Perspectives. Springer Science & Business Media. ISBN 978-94-007-0403-9.
- ^ "The Ionosphere". University Corporation for Atmospheric Research. Retrieved 2025-07-23.
- ^ Gallagher, D. L. (April 26, 2023). "The Earth's Plasmasphere". NASA. Retrieved 2025-07-23.
- ^ Kirby, S. S.; et al. (2006). "Studies of the ionosphere and their application to radio transmission" (PDF). Proceedings of the Institute of Radio Engineers. 22 (4): 481–521. doi:10.1109/JRPROC.1934.225867. Retrieved 2025-07-23.
- ^ "homosphere – AMS Glossary". Amsglossary.allenpress.com. Archived from the original on 14 September 2010. Retrieved 2010-10-16.
- ^ Helmenstine, Anne Marie (June 16, 2018). "The 4 Most Abundant Gases in Earth's Atmosphere". Retrieved 2025-07-21.
- ^ Haby, Jeff. "The Planetary Boundary Layer". National Weather Service. Retrieved 2025-07-23.
- ^ Bauer, Siegfried; Lammer, Helmut (2013). Planetary Aeronomy: Atmosphere Environments in Planetary Systems. Physics of Earth and Space Environments. Springer Science & Business Media. pp. 4–5. ISBN 978-3-662-09362-7.
- ^ "Earth's Atmosphere". Archived from the original on 2009-06-14.
- ^ "NASA – Earth Fact Sheet". Nssdc.gsfc.nasa.gov. Archived from the original on 30 October 2010. Retrieved 2010-10-16.
- ^ "Global Surface Temperature Anomalies". Archived from the original on 2009-03-03.
- ^ "Earth's Radiation Balance and Oceanic Heat Fluxes". Archived from the original on 2005-03-03.
- ^ "Coupled Model Intercomparison Project Control Run" (PDF). Archived from the original (PDF) on 2008-05-28.
- ^ Geometric altitude vs. temperature, pressure, density, and the speed of sound derived from the 1962 U.S. Standard Atmosphere.
- ^ Trenberth, Kevin E.; Smith, Lesley (January 1970). "The Mass of the Atmosphere: A Constraint on Global Analyses". Journal of Climate. 18 (6): 864. Bibcode:2005JCli...18..864T. CiteSeerX 10.1.1.727.6573. doi:10.1175/JCLI-3299.1. S2CID 16754900.
- ^ a b Daniel, R. R. (2002). Concepts in Space Science. Universities Press. pp. 70–72. ISBN 978-81-7371-410-8.
- ^ Lutgens, Frederick K.; Tarbuck, Edward J. (1995). The Atmosphere (6th ed.). Prentice Hall. pp. 14–17. ISBN 0-13-350612-6.
- ^ Sforza, Pasquale (2014). "Fuselage Design". Commercial Airplane Design Principles. pp. 47–79. doi:10.1016/B978-0-12-419953-8.00003-6. ISBN 978-0-12-419953-8.
- ^ Kräuchi, A.; et al. (2016). "Controlled weather balloon ascents and descents for atmospheric research and climate monitoring". Atmospheric Measurement Techniques. 9 (3): 929–938. Bibcode:2016AMT.....9..929K. doi:10.5194/amt-9-929-2016. PMC 5734649. PMID 29263765.
- ^ Anderson, Brian J.; Mitchell, Donald G. (2005). "The Space Environment". In Pisacane, Vincent L. (ed.). Fundamentals of Space Systems. Oxford University Press. p. 56. ISBN 978-0-19-516205-9.
- ^ "Atmospheric controllers of local nighttime temperature". Pennsylvania State University. 2004. Retrieved 2025-07-24.
- ^ Benson, Tom. "Speed of sound". NASA Glenn Research Center. Retrieved 2025-07-24.
- ^ Wang, Hongwei (2023). A Guide to Fluid Mechanics. Cambridge University Press. ISBN 978-1-108-49883-8.
- ^ Hall, Nancy, ed. (May 13, 2021). "Earth Atmosphere Model". NASA Glenn Research Center. Retrieved 2025-07-24.
- ^ Kumar, R.; et al. (March 2022). "Simulation of the orbital decay of a spacecraft in low Earth orbit due to aerodynamic drag". The Aeronautical Journal. 126 (1297): 565–583. doi:10.1017/aer.2021.83.
- ^ Trenberth, Kevin E.; Smith, Lesley (March 15, 2005). "The Mass of the Atmosphere: A Constraint on Global Analyses". Journal of Climate. 18 (6): 864–875. Bibcode:2005JCli...18..864T. doi:10.1175/JCLI-3299.1. JSTOR 26253433.
- ^ "Absorption / reflection of sunlight". Understanding Global Change. Retrieved 2023-06-13.
- ^ "The Atmospheric Window". National Oceanic and Atmospheric Administration. Retrieved 2023-06-13.
- ^ Sirvatka, Paul. "Radiation and the Earth-atmosphere system: 'Why is the sky blue?'". Introduction to meteorology. College of DuPage. Retrieved 2025-07-25.
- ^ St. Fleur, Nicholas (May 19, 2017). "Spotting Mysterious Twinkles on Earth From a Million Miles Away". The New York Times. Retrieved 20 May 2017.
- ^ Marshak, Alexander; et al. (May 15, 2017). "Terrestrial glint seen from deep space: oriented ice crystals detected from the Lagrangian point". Geophysical Research Letters. 44 (10): 5197. Bibcode:2017GeoRL..44.5197M. doi:10.1002/2017GL073248. hdl:11603/13118. S2CID 109930589.
- ^ Bloomfield, Louis A. (2007). How Everything Works: Making Physics Out of the Ordinary. John Wiley & Sons. p. 456. ISBN 978-0-470-17066-3.
- ^ Ondoh, Tadanori; Marubashi, Katsuhide, eds. (2001). Science of Space Environment. Wave summit course. IOS Press. p. 8. ISBN 978-4-274-90384-7.
- ^ Collins, William D.; et al. (September 2006). "Effects of increased near-infrared absorption by water vapor on the climate system". Journal of Geophysical Research: Atmospheres. 111 (D18). ID D18109. Bibcode:2006JGRD..11118109C. doi:10.1029/2005JD006796.
- ^ Wang, Sharon Xuesong; et al. (November 2022). "Characterizing and Mitigating the Impact of Telluric Absorption in Precise Radial Velocities". The Astronomical Journal. 164 (5). id. 211. arXiv:2206.07287. Bibcode:2022AJ....164..211W. doi:10.3847/1538-3881/ac947a.
- ^ McLean, Ian S. (2008). Electronic Imaging in Astronomy: Detectors and Instrumentation. Astronomy and Planetary Sciences (2nd ed.). Springer Science & Business Media. pp. 38–40. ISBN 978-3-540-76582-0.
- ^ Shelton, Marlyn L. (2009). Hydroclimatology: Perspectives and Applications. Cambridge University Press. pp. 35–37. ISBN 978-0-521-84888-6.
- ^ Bohren, Craig F.; Clothiaux, Eugene E. (2006). Fundamentals of Atmospheric Radiation. John Wiley & Sons. pp. 26–29. ISBN 978-3-527-60837-9.
- ^ Wrigglesworth, John (1997). Energy And Life. Lifelines Series. CRC Press. p. 155. ISBN 978-1-4822-7275-8.
- ^ Ma, Qiancheng (March 1998). "Greenhouse Gases: Refining the Role of Carbon Dioxide". NASA Goddard Institute for Space Studies. Retrieved 2025-07-24.
- ^ Voronin, A.; Zheltikov, A. (2017). "The generalized Sellmeier equation for air". Scientific Reports. 7 46111. Bibcode:2017NatSR...746111V. doi:10.1038/srep46111. PMC 5569311. PMID 28836624. 46111. Figure 1 gives a refractive index of 1.000273 at 23 C.
- ^ Basey, David (March 2, 2019). "Atmospheric Refraction". British Astronomical Association. Retrieved 2025-07-24.
- ^ Edlén, Bengt (1966). "The refractive index of air". Metrologia. 2 (2): 71–80. Bibcode:1966Metro...2...71E. doi:10.1088/0026-1394/2/2/002.
- ^ Young, Andrew T. (2025). "An Introduction to Mirages". San Diego State University. Retrieved 2025-07-25.
- ^ a b "Global Atmospheric Circulations". National Oceanic and Atmospheric Administration. October 3, 2023. Retrieved 2025-07-25.
- ^ "The Jet Stream". National Oceanic and Atmospheric Administration. December 9, 2024. Retrieved 2025-07-25.
- ^ Shuckburgh, Emily (2011). "Weather and Climate". In Moffatt, H. Keith; Shuckburgh, Emily (eds.). Environmental Hazards: The Fluid Dynamics And Geophysics Of Extreme Events. Lecture Notes Series, Institute For Mathematical Sciences, National University Of Singapore. Vol. 21. World Scientific. p. 87. ISBN 978-981-4464-67-3.
- ^ Volland, Hans (2012). Atmospheric Tidal and Planetary Waves. Atmospheric and Oceanographic Sciences Library. Vol. 12. Springer Science & Business Media. pp. 1–5. ISBN 978-94-009-2861-9.
- ^ a b Zahnle, K.; et al. (2010). "Earth's Earliest Atmospheres". Cold Spring Harbor Perspectives in Biology. 2 (10) a004895. doi:10.1101/cshperspect.a004895. PMC 2944365. PMID 20573713.
- ^ Windley, B. (1984). The Evolving Continents. New York: Wiley Press.
- ^ Schopf, J. (1983). Earth's Earliest Biosphere: Its Origin and Evolution. Princeton, N.J.: Princeton University Press.
- ^ Feulner, Georg (2012). "The faint young Sun problem". Reviews of Geophysics. 50 (2) 2011RG000375: RG2006. arXiv:1204.4449. Bibcode:2012RvGeo..50.2006F. doi:10.1029/2011RG000375. S2CID 119248267.
- ^ Lyons, Timothy W.; et al. (February 2014). "The rise of oxygen in Earth's early ocean and atmosphere". Nature. 506 (7488): 307–315. Bibcode:2014Natur.506..307L. doi:10.1038/nature13068. PMID 24553238. S2CID 4443958.
- ^ Hayes, John M.; Waldbauer, Jacob R. (June 29, 2006). "The Carbon Cycle and Associated Redox Processes through Time". Philosophical Transactions of the Royal Society B: Biological Sciences. 361 (1470): 931–950. doi:10.1098/rstb.2006.1840. JSTOR 20209694. PMC 1578725. PMID 16754608.
- ^ Lyons, Timothy W.; et al. (2014). "Atmospheric oxygenation three billion years ago". Nature. 506 (7488): 307–15. Bibcode:2014Natur.506..307L. doi:10.1038/nature13068. PMID 24553238. S2CID 4443958.
- ^ a b Cordeiro, Ingrid Rosenburg; Tanaka, Mikiko (September 2020). "Environmental Oxygen is a Key Modulator of Development and Evolution: From Molecules to Ecology". BioEssays. 42 (9). 2000025. doi:10.1002/bies.202000025. PMID 32656788.
- ^ Lantink, Margriet L.; et al. (February 2018). "Fe isotopes of a 2.4 Ga hematite-rich IF constrain marine redox conditions around the GOE". Precambrian Research. 305: 218–235. Bibcode:2018PreR..305..218L. doi:10.1016/j.precamres.2017.12.025. hdl:1874/362652.
- ^ Laakso, T. A.; Schrag, D. P. (May 2017). "A theory of atmospheric oxygen". Geobiology. 15 (3): 366–384. Bibcode:2017Gbio...15..366L. doi:10.1111/gbi.12230. PMID 28378894.
- ^ Scotese, Christopher R. (2010). "Back to Earth History: Summary Chart for the Precambrian". Paleomar Project. Retrieved 2025-07-21.
- ^ Towe, K. M. (April 1970). "Oxygen-Collagen Priority and the Early Metazoan Fossil Record". Proceedings of the National Academy of Sciences of the United States of America. 65 (4): 781–788. Bibcode:1970PNAS...65..781T. doi:10.1073/pnas.65.4.781. PMC 282983. PMID 5266150.
- ^ Martin, Daniel; et al. (2016). "The human physiological impact of global deoxygenation". The Journal of Physiological Sciences. 67 (1): 97–106. doi:10.1007/s12576-016-0501-0. ISSN 1880-6546. PMC 5138252. PMID 27848144.
- ^ Riding, R. (October 2009). "An atmospheric stimulus for cyanobacterial-bioinduced calcification ca. 350 million years ago?". PALAIOS. 24 (10): 685–696. Bibcode:2009Palai..24..685R. doi:10.2110/palo.2009.p09-033r. ISSN 0883-1351.
- ^ a b Berner, R. A. (March 2001). "Modeling atmospheric O2 over Phanerozoic time". Geochimica et Cosmochimica Acta. 65 (5): 685–694. doi:10.1016/S0016-7037(00)00572-X. ISSN 0016-7037.
- ^ Calvo-Flores, Francisco G. (2025). Understanding the Chemistry of the Environment. John Wiley & Sons. ISBN 978-1-119-56863-6.
- ^ Gerlach, Terry (June 2011). "Volcanic versus anthropogenic carbon dioxide". Eos, Transactions American Geophysical Union. 92 (24): 201–202. Bibcode:2011EOSTr..92..201G. doi:10.1029/2011EO240001.
- ^ "Pollution". Merriam-Webster. Retrieved 2025-07-25.
- ^ Harrop, Owen (2003). Air Quality Assessment and Management: A Practical Guide. Clay's Library of Health and the Environment. CRC Press. pp. 30–49. ISBN 978-0-203-30263-7.
- ^ IPCC (2021). "Summary for Policymakers" (PDF). IPCC AR6 WG1. pp. 4–5. Archived from the original (PDF) on 2021-08-11. Retrieved 2021-11-20.
- ^ Managing Climate Risks, Facing up to Losses and Damages. Paris: OECD Publishing. November 2021. Bibcode:2021mcrf.book.....O. doi:10.1787/55ea1cc9-en. ISBN 978-92-64-43966-5.
External links
[edit]- Buchan, Alexander (1878). . Encyclopædia Britannica. Vol. III (9th ed.). pp. 28–36.
- Interactive global map of current atmospheric and ocean surface conditions.
Atmosphere of Earth
View on GrokipediaChemical Composition
Major Gases
The dry atmosphere of Earth consists predominantly of nitrogen (N₂), which constitutes 78.08% by volume, oxygen (O₂) at 20.95%, and argon (Ar) at 0.934%.[2] These three gases account for approximately 99.964% of the total dry air composition.[2] Nitrogen (N₂), a diatomic molecule that is colorless and odorless at standard temperature and pressure (STP: 0°C, 1 atm), has a melting point of -210°C, boiling point of -196°C, density of 1.25 g/L, and low water solubility (~0.02 g/L at 20°C). It is highly inert due to the strong N≡N triple bond, does not support combustion, is essential for life but requires fixation for biological use, and plays a key role in diluting reactive gases. Oxygen (O₂), a diatomic molecule that is colorless, odorless, and paramagnetic, has a melting point of -219°C, boiling point of -183°C, density of 1.43 g/L, and slight water solubility (~0.03 g/L at 20°C). It is a strong oxidizing agent that supports combustion and respiration essential for aerobic life. Argon (Ar), a monatomic noble gas that is colorless and odorless, has a melting point of -189°C, boiling point of -186°C, density of 1.78 g/L, and very low water solubility. Chemically inert with no stable compounds under normal conditions, it originates primarily from the radioactive decay of potassium-40 in the Earth's crust.[1] Carbon dioxide (CO₂), a linear triatomic molecule that is colorless and odorless at STP, sublimes at -78.5°C (no liquid phase at 1 atm), has a density of 1.98 g/L, and moderate water solubility (~1.45 g/L at 25°C). Chemically, it is non-flammable, forms carbonic acid in water (a weak acid), is involved in photosynthesis, respiration, and the greenhouse effect, and reacts with bases and some metals. Though present in trace amounts at about 430 parts per million (0.043%) as of June 2025, it is considered among the major variable components due to its influence on climate and its increasing concentration from anthropogenic emissions.[8] This level represents a rise from pre-industrial values of around 280 ppm, driven by fossil fuel combustion and land-use changes.[9] Water vapor, varying from near 0% to 4% depending on temperature and location, is excluded from dry air measurements but is the most abundant greenhouse gas by impact.[1]| Gas | Chemical Formula | Volume Percentage (Dry Air) |
|---|---|---|
| Nitrogen | N₂ | 78.08 |
| Oxygen | O₂ | 20.95 |
| Argon | Ar | 0.934 |
| Carbon Dioxide | CO₂ | 0.043 (430 ppm, 2025) |
Trace Gases and Variable Components
Trace gases in Earth's atmosphere, defined as those with volume mixing ratios below approximately 1% excluding nitrogen, oxygen, and argon, include carbon dioxide (CO₂), methane (CH₄), nitrous oxide (N₂O), and ozone (O₃), along with minor noble gases such as neon and helium. These gases collectively account for less than 0.1% of dry air by volume, yet play critical roles in radiative forcing, chemical reactions, and atmospheric dynamics.[11] [12] Carbon dioxide, the most abundant anthropogenic trace gas, reached a global average concentration of 425.43 parts per million (ppm) in October 2025, as measured at observatories including Mauna Loa. This represents an increase of about 3.5 ppm from 2024 levels, driven primarily by fossil fuel combustion, deforestation, and cement production, with natural sinks absorbing roughly half of emissions.[13] [14] Methane concentrations average approximately 1,950 parts per billion (ppb), sourced from biogenic processes like wetlands and enteric fermentation, as well as fossil fuel extraction; annual increases have averaged 10-13 ppb in recent years. Nitrous oxide stands at around 336 ppb, mainly from agricultural fertilizers and industrial activities.[15] [16] Ozone concentrations vary greatly by altitude and location, with tropospheric levels typically 20-100 ppb and stratospheric peaks exceeding 10 ppm, formed via photochemical reactions involving oxygen and nitrogen oxides. Other trace constituents, such as neon (18.18 ppm), helium (5.24 ppm), and krypton (1.14 ppm), originate from primordial atmospheric retention and radioactive decay, remaining stable over human timescales.[17] Variable components, primarily water vapor, fluctuate widely and are excluded from standard dry air compositions. Water vapor content ranges from near 0% in arid, cold conditions to 4% by volume in saturated tropical air near the surface, exerting dominant control over local humidity and contributing substantially to the natural greenhouse effect through its high concentration and heat capacity. Aerosols—solid or liquid particles like sulfates, sea salt, and mineral dust—vary temporally with emissions, weather, and seasonal cycles, typically numbering 10-1,000 per cubic centimeter and modulating radiation balance by scattering sunlight and serving as cloud condensation nuclei.[18] [19] [20]| Trace Gas | Approximate Concentration (dry air, ppmv unless noted) | Primary Sources |
|---|---|---|
| CO₂ | 425 | Fossil fuels, respiration, oxidation |
| CH₄ | 1.95 (ppb) | Wetlands, agriculture, leaks |
| N₂O | 0.336 (ppb) | Soils, fertilizers |
| O₃ | 0.3-0.7 (total column average) | Photochemical production |
| Ne | 18.18 | Primordial |
| He | 5.24 | Radioactive decay, solar wind |
Spatial and Temporal Variations
The mixing ratios of the major atmospheric constituents—nitrogen, oxygen, and argon—remain nearly constant with altitude up to the turbopause at approximately 100 km, where diffusive separation begins to dominate over turbulent mixing, leading to gradual fractionation by molecular weight in the heterosphere above.[21] In the lower atmosphere (troposphere and stratosphere), these gases exhibit minimal horizontal spatial variations, typically less than 0.1% deviation from global means, due to their long atmospheric lifetimes exceeding years and efficient global mixing by winds.[5] Temporal fluctuations in their concentrations are similarly negligible on diurnal, seasonal, or even decadal scales, except for anthropogenic influences on oxygen via fossil fuel combustion, which cause a measurable but small decline of about 4 per meg per year globally.[22] Trace gases display more pronounced spatial and temporal variations driven by their sources, sinks, and shorter lifetimes. Carbon dioxide (CO₂), with a global mean mixing ratio of around 420 ppm as of 2023, shows well-mixed background levels but exhibits a seasonal cycle amplitude that increases with latitude in the Northern Hemisphere, ranging from 2-5 ppm near the equator to over 15 ppm at 60°N, primarily due to terrestrial photosynthesis and respiration imbalances over extensive boreal forests and croplands.[23] [24] This cycle peaks in May (Northern Hemisphere winter) and troughs in September, with interhemispheric asymmetry arising from greater landmass and vegetation in the north compared to the ocean-dominated south.[25] Local spatial enhancements occur near emission hotspots like urban areas or biomass burning regions, though atmospheric transport homogenizes these on scales beyond 1000 km within weeks.[26] Water vapor, the most variable constituent, constitutes 0-4% by volume in the troposphere, with spatial maxima in the warm tropics (up to 3-4% near the surface) decreasing poleward to under 0.2% in polar regions due to the Clausius-Clapeyron relation linking saturation vapor pressure to temperature.[27] Temporally, it fluctuates diurnally by 10-20% over land due to evapotranspiration and condensation cycles, and seasonally by up to 50% in monsoon-influenced areas, reflecting precipitation-evaporation dynamics and storm tracks.[28] Unlike longer-lived gases, its short residence time of days to weeks confines variations to regional scales, with negligible presence above the tropopause.[5] Ozone (O₃), a key trace gas, varies sharply with altitude, peaking at 10 ppm in the stratospheric ozone layer around 25-30 km before dropping to tropospheric levels of 20-100 ppb, influenced by photochemical production and transport.[29] In the troposphere, spatial patterns show higher concentrations in the Northern Hemisphere (annual mean ~40 ppb) versus the south (~25 ppb), driven by industrial NOx emissions and continental pollution export.[30] Temporal cycles include summer maxima from enhanced photochemistry and biomass fire seasons, with diurnal peaks in the afternoon; trends indicate increases of 0.5-1 ppb per decade in polluted regions since 1990, though satellite data reveal heterogeneous global patterns.[31] [32] Methane (CH₄) and nitrous oxide (N₂O), well-mixed greenhouse gases, show subdued variations: CH₄ at ~1900 ppb has minor seasonal amplitudes (<10 ppb) from wetland emissions peaking in summer hemispheres, with spatial gradients near sources like rice paddies or oil fields dissipating within months.[5] N₂O at 330 ppb varies even less, with lifetime-driven uniformity except for stratospheric depletion.[33] These patterns underscore how atmospheric lifetime governs variability scale, with short-lived species like reactive hydrocarbons (e.g., isoprene) confined to biogenic hotspots and diurnal cycles.[34]Vertical Stratification
Troposphere
The troposphere constitutes the lowest layer of Earth's atmosphere, bounded above by the tropopause and extending from the surface upward. It encompasses the region where nearly all weather phenomena occur due to intense vertical mixing driven by solar heating of the ground and subsequent convection. This layer holds about 75% of the total mass of the atmosphere and virtually all water vapor, enabling processes like cloud formation and precipitation.[35][36] The thickness of the troposphere varies geographically and seasonally, averaging around 12 kilometers but ranging from approximately 7 kilometers over the poles during winter to 18-20 kilometers near the equator. This variation arises from differences in surface temperature and convection strength, with warmer equatorial air expanding the layer and colder polar air contracting it. The tropopause, marking the upper boundary, is defined as the level where the temperature lapse rate drops below 2 K per kilometer, transitioning to the more stable stratosphere.[37][38][39] Within the troposphere, temperature decreases with altitude at an average environmental lapse rate of about 6.5 °C per kilometer, though this can vary locally due to moisture content and stability. Dry adiabatic lapse rates reach 9.8 °C per kilometer in unsaturated air, while moist processes reduce it to around 5-6 °C per kilometer, influencing thunderstorm development and atmospheric stability. Near the surface, air pressure is highest, decreasing exponentially with height, and the layer supports aviation up to the cruising altitudes of commercial jets, typically below 12 kilometers.[40] The composition mirrors the overall atmosphere—roughly 78% nitrogen, 21% oxygen, and 1% argon and other inert gases—but includes higher concentrations of variable components like water vapor (up to 4% in humid tropics), aerosols, and pollutants near the surface. Convection currents mix these constituents thoroughly, distributing heat, moisture, and trace gases, which play critical roles in the hydrological cycle and short-term climate regulation.[35][41]Stratosphere
The stratosphere is the atmospheric layer extending from the tropopause, typically at altitudes of approximately 12 to 20 kilometers above Earth's surface, up to about 50 kilometers.[36] Its lower boundary varies latitudinally, reaching as low as 7-10 kilometers near the poles and as high as 17-20 kilometers near the equator.[42] The upper boundary, known as the stratopause, marks a transition to the mesosphere at around 50 kilometers.[43] Unlike the troposphere below, the stratosphere exhibits a temperature inversion, with temperatures increasing with altitude from about -60°C at the base to near 0°C at the top.[44] This warming results from the absorption of ultraviolet radiation by ozone molecules concentrated in the ozone layer, which spans roughly 15 to 35 kilometers altitude.[45] The ozone layer, comprising a peak concentration of about 10 parts per million by volume, effectively shields Earth's surface from harmful solar UV radiation shorter than 290 nanometers.[46] Compositionally, the stratosphere resembles the troposphere in major gases—nitrogen (78%) and oxygen (21%)—but features significantly lower water vapor content, rendering it extremely dry and inhibiting cloud formation except for polar stratospheric clouds (PSCs) in cold polar regions during winter.[44] Ozone constitutes a trace but critical component, accounting for the layer's radiative heating. The region holds about 20% of the atmosphere's total mass despite its thin vertical extent.[47] Vertical stability arises from the density gradient and temperature profile, minimizing turbulence and convection, which confines most weather phenomena to the troposphere.[44] Dynamically, the stratosphere hosts strong zonal winds, including stratospheric jet streams and the polar vortex—a cyclonic circulation of westerly winds encircling the poles at 15-50 kilometers altitude, peaking in winter with speeds exceeding 100 meters per second.[48] The polar vortex influences tropospheric weather patterns by modulating the position of the polar jet stream, with disruptions such as sudden stratospheric warmings potentially leading to cold outbreaks at the surface.[49] Aircraft routinely operate in the lower stratosphere for efficient high-altitude flight, benefiting from reduced drag and stable conditions.[4]Mesosphere
The mesosphere constitutes the atmospheric layer extending from approximately 50 km to 85 km altitude above Earth's surface, positioned between the stratopause and mesopause boundaries.[4] This region features a temperature lapse rate where thermal profiles decline with increasing elevation, culminating in the coldest temperatures of the atmosphere near the mesopause at around -90°C (183 K) or lower during summer conditions.[50] [51] Atmospheric density in the mesosphere diminishes rapidly, with pressures ranging from about 1 mbar at its base to 0.001 mbar at the upper limit, rendering it sparsely populated by molecular nitrogen (N₂) and oxygen (O₂) that comprise over 99% of the gaseous mix, alongside trace water vapor insufficient for significant cloud formation except under specialized conditions.[4] The layer's low density and residual frictional interactions cause most incoming meteoroids to ablate and vaporize upon entry, producing visible meteors primarily within this altitude band due to the balance of sufficient particle collisions for heating without overwhelming ionization seen higher up.[51] Dynamic features include strong zonal winds exceeding 100 m/s in the summer hemisphere and turbulent mixing, contributing to the formation of noctilucent clouds—thin, icy formations at 80-85 km altitude visible at high latitudes during summer twilight, nucleated on meteor-derived dust particles when temperatures drop below -120°C.[36] [52] These polar mesospheric clouds serve as indicators of upper atmospheric cooling trends, with observations linking their increased frequency and southward extension to stratospheric temperature declines.[36] Exploration of the mesosphere remains challenging, as conventional balloons and aircraft cannot access it, relying instead on sounding rockets and remote sensing via lidar or satellite instruments to measure parameters like wind velocities and trace gas distributions, which reveal seasonal variations in composition driven by photodissociation and vertical transport.[51] The upper mesosphere hosts the D-layer of the ionosphere during daylight, where solar radiation ionizes nitric oxide, influencing radio wave propagation but dissipating rapidly at night.[4]Thermosphere
The thermosphere extends from the top of the mesosphere at approximately 85 to 90 km altitude up to about 600 km, where it transitions into the exosphere.[50][53] This layer is characterized by a marked increase in temperature with altitude, driven by the absorption of intense ultraviolet and x-ray solar radiation by sparse atmospheric constituents, resulting in exospheric temperatures that can exceed 2000 K during periods of high solar activity. Despite these elevated kinetic temperatures, the extremely low particle density—on the order of 10^{-9} to 10^{-12} kg/m³—means that molecular collisions are rare, so objects within the thermosphere do not experience significant heating from conduction.[54] The composition of the thermosphere shifts with height: atomic oxygen (O) dominates below 200 km, giving way to molecular nitrogen (N₂) and nitric oxide (NO) lower down, while helium (He) and atomic hydrogen (H) increase above 300 km.[54] Ultraviolet radiation causes photodissociation of molecular species into atoms and photoionization, producing free electrons and ions that form the bulk of the ionosphere, which overlaps primarily with the thermosphere's lower and middle regions.[55] This ionization supports phenomena such as auroras, where charged particles from solar wind precipitate into the upper thermosphere (typically 100–400 km), exciting neutral atoms that emit light upon recombination.[56] Density in this layer is highly variable, with total neutral mass density scaling exponentially with solar extreme ultraviolet (EUV) flux; during solar maximum, the thermosphere expands upward by hundreds of kilometers, elevating densities at satellite altitudes (e.g., 400 km) by factors of 2–10 and increasing orbital drag on low Earth orbit objects like the International Space Station.[57] Solar activity profoundly influences thermospheric dynamics: geomagnetic storms from coronal mass ejections can heat the layer rapidly, causing vertical expansions and global density enhancements of up to 300% at 400 km, while long-term cooling trends from rising CO₂ concentrations counteract some solar forcing by enhancing infrared radiative losses.[58][59] Models like the Drag Temperature Model (DTM) empirically parameterize these variations using satellite drag data, revealing that thermospheric scale heights increase with exospheric temperature, which correlates with the 10.7 cm solar radio flux index (F10.7) as a proxy for EUV output.[60] Reentry vehicles and high-altitude research balloons probe this region, confirming that frictional heating during descent becomes significant above 120 km due to residual densities despite the overall sparsity.[61]Exosphere
The exosphere constitutes the outermost region of Earth's atmosphere, commencing at an altitude of approximately 600 kilometers above the surface and extending outward to around 10,000 kilometers, beyond which it imperceptibly transitions into the vacuum of interplanetary space.[4] This layer lacks a sharply defined upper boundary, as atmospheric particles become sufficiently sparse that their distribution follows ballistic trajectories influenced primarily by Earth's gravity rather than frequent collisions.[62] The base of the exosphere, known as the exobase, typically lies between 500 and 1,000 kilometers altitude, varying with solar activity and geomagnetic conditions that affect the underlying thermosphere's expansion. In this regime, the mean free path of particles exceeds the atmospheric scale height, rendering collisional interactions negligible and marking the effective cessation of hydrodynamic atmospheric behavior.[63] Compositionally, the exosphere consists predominantly of light atomic species, including hydrogen and helium, with minor contributions from atomic oxygen, nitrogen, and trace heavier elements such as carbon dioxide dissociates.[64] These constituents derive from upward diffusion and photo-dissociation of molecules from lower layers, resulting in number densities on the order of 10^4 to 10^7 particles per cubic centimeter at the exobase, diminishing exponentially with height.[63] Helium arises mainly from alpha decay in the Earth's crust and subsequent upward transport, while hydrogen originates from water vapor photodissociation in the mesosphere and stratosphere.[64] The low density—approaching interstellar vacuum levels at upper extents—implies that satellite orbits in this region experience minimal aerodynamic drag, though occasional neutral particle interactions influence long-term decay for high-altitude spacecraft.[63] Temperatures in the exosphere range from 1,000 to 2,000 Kelvin or higher, driven by solar ultraviolet radiation and particle precipitation rather than conductive or convective heat transfer, given the rarity of collisions.[36] This thermal regime exhibits extreme diurnal and solar cycle variations, with densities and effective temperatures correlating inversely during solar minima and maxima due to enhanced heating and expansion of underlying layers.[65] Particles here possess kinetic energies sufficient for some to achieve escape velocity, leading to gradual atmospheric loss via thermal Jeans escape, predominantly of hydrogen, at rates estimated around 3 kilograms per second under average conditions.[64] Such dynamics contribute to the geocorona, a faint hydrogen halo observable in ultraviolet wavelengths, extending Earth's atmospheric influence into the magnetosphere.[66]Ionosphere and Other Transitional Features
The ionosphere is the region of Earth's upper atmosphere characterized by substantial ionization, extending from roughly 60 km to beyond 1000 km altitude, where solar extreme ultraviolet and X-ray radiation dissociates and ionizes neutral constituents such as molecular nitrogen, molecular oxygen, and atomic oxygen, producing free electrons and positive ions that form a partially ionized plasma.[67][68] Electron densities in this plasma vary from 10^2 to 10^6 electrons per cubic centimeter, peaking in the F region during daytime, and enable key geophysical effects including the reflection and refraction of radio waves for long-distance communication, as well as the generation of auroral displays through interactions with charged particles from the magnetosphere.[69][70] The ionosphere's extent and ionization levels fluctuate with solar zenith angle, geomagnetic activity, season, and the 11-year solar cycle, with reduced densities at night due to electron-ion recombination in the absence of photoionizing radiation.[68] Structurally, the ionosphere features distinct layers defined by maxima in electron density: the D layer (60–90 km), which exists primarily during daylight, attenuates high-frequency radio signals through absorption by neutral collisions, and largely dissipates after sunset; the E layer (90–150 km), which supports reflection of medium- to high-frequency radio waves; and the F layer (150–500 km or higher), the most persistent and densest, subdividing diurnally into the lower F1 sublayer (150–220 km) and upper F2 sublayer (220 km and above), where the principal daytime peak density occurs and persists into the night as a single layer.[71][70] These layers lack rigid boundaries, blending gradually, and their behavior stems from the balance of photoionization production rates, which decrease with altitude due to diminishing neutral densities and increasing solar radiation penetration, against loss mechanisms like radiative recombination and charge exchange.[68] Beyond the ionosphere, transitional features delineate shifts in atmospheric dynamics and composition. The turbopause, situated at 90–110 km, separates the lower homosphere—where eddy diffusion from turbulence maintains uniform mixing of gases regardless of molecular weight—from the upper heterosphere, where molecular diffusion prevails, causing lighter species like atomic hydrogen and helium to concentrate at higher altitudes.[72] The homopause, typically aligning closely with the turbopause near 100 km, similarly signifies the altitude where vertical eddy diffusion coefficients fall below molecular diffusion rates (around 10^5–10^6 cm²/s), enabling gravitational separation and compositional stratification.[73] At greater heights, the exobase (500–1000 km) marks the effective upper limit of significant particle collisions, defined where the atmospheric mean free path approximates the local scale height (∼50–100 km), transitioning the medium from collisional to ballistic particle trajectories that can lead to atmospheric escape.[74] These interfaces critically govern trace gas distributions, ionospheric coupling to the neutral atmosphere, and the flux of material into interplanetary space.[75]Physical Properties
Pressure and Thickness Profiles
Atmospheric pressure at sea level averages 101,325 Pa (1,013.25 hPa), defined as the standard value in models like the U.S. Standard Atmosphere of 1976.[76] This pressure arises from the weight of the overlying air column under hydrostatic equilibrium, where the pressure gradient balances gravitational force: , with as air density and as gravitational acceleration.[77] Substituting the ideal gas law , where is the specific gas constant and is temperature, yields an exponential pressure decrease for constant temperature: , with scale height km near the surface.[78][79] In practice, temperature variations across atmospheric layers cause deviations from a single exponential profile, requiring piecewise models.[77] In the troposphere, pressure halves approximately every 5.5 km due to the combined effects of cooling lapse rate and density reduction.[80] The U.S. Standard Atmosphere tabulates values, showing pressure dropping to about 26,500 Pa at 10 km, 5,500 Pa at 20 km, and below 1 Pa above 100 km.[76] Higher layers like the stratosphere exhibit slower decreases initially due to warming, while the mesosphere and above see sharper falls as molecular diffusion dominates.[76] The atmosphere's thickness lacks a discrete boundary, thinning gradually into space; practical definitions place 99% of mass below roughly 100 km, with the exosphere extending to 10,000 km where pressure approaches solar wind levels.[81] About 75% of total atmospheric mass resides within 11 km of the surface, reflecting the exponential concentration near the ground.[82] This profile influences aviation limits, spaceflight, and planetary escape, with the Kármán line at 100 km marking the transition where aerodynamic lift fails for conventional vehicles.[81]| Altitude (km) | Pressure (hPa) | Notes |
|---|---|---|
| 0 | 1013.25 | Sea level standard[76] |
| 10 | 264.99 | Troposphere top approximation[76] |
| 20 | 55.29 | Stratosphere entry[76] |
| 50 | 1.20 | Mesosphere[76] |
| 100 | 0.0003 | Thermosphere, near vacuum[76] |
Temperature Gradients
The vertical temperature profile of Earth's atmosphere exhibits distinct gradients across its layers, primarily driven by radiative absorption, convection, and adiabatic processes. In the troposphere, from sea level to about 11 km altitude, temperature decreases with height at an average environmental lapse rate of 6.5 K/km, as defined in standard atmospheric models.[77] This rate results from the expansion and cooling of rising air parcels, moderated by latent heat release from condensation in moist conditions, which reduces the gradient below the dry adiabatic lapse rate of 9.8 K/km derived from the gravitational acceleration and specific heat capacity of dry air (Γ_d = g / c_p, where g ≈ 9.8 m/s² and c_p ≈ 1004 J/kg·K).[83] Above the tropopause, the stratosphere (approximately 11–50 km) features a temperature inversion, where temperature increases with altitude at rates up to +2.8 K/km in upper portions, reaching a maximum of about 270 K at the stratopause.[84] This positive gradient (negative lapse rate) arises from the absorption of ultraviolet radiation by stratospheric ozone, which heats the layer and suppresses convection. The U.S. Standard Atmosphere 1976 delineates sub-layers: isothermal from 11–20 km at 216.65 K, followed by gradual warming to 32 km and beyond.[84] In the mesosphere (50–85 km), temperature declines sharply with altitude, achieving lapse rates around 2–3 K/km, culminating in the coldest temperatures near the mesopause at approximately 180–190 K.[36] This cooling stems from diminished radiative heating and increased infrared emission to space in the thin, low-density air, with minimal molecular absorption of solar radiation. The thermosphere (above 85 km) then shows a steep positive temperature gradient, with temperatures rising to 500–2000 K or higher, contingent on solar activity levels that modulate extreme ultraviolet absorption by atomic oxygen and other species.[36] These upper gradients are highly variable, influenced by geomagnetic activity and diurnal cycles, contrasting the more stable lower-atmosphere profiles.| Layer | Approximate Altitude (km) | Average Temperature Gradient (K/km) | Key Driver |
|---|---|---|---|
| Troposphere | 0–11 | -6.5 | Convection and adiabatic cooling |
| Stratosphere | 11–50 | +0 to +2.8 | Ozone UV absorption |
| Mesosphere | 50–85 | -2 to -3 | Radiative cooling |
| Thermosphere | 85+ | + (steep, variable) | EUV/X-ray absorption |
Density and Total Mass
The total mass of Earth's atmosphere is approximately 5.15 × 10¹⁸ kg, equivalent to about 0.000086% of the planet's total mass of 5.97 × 10²⁴ kg.[85][86] This value is derived from integrating the atmospheric column mass, calculated as surface pressure divided by gravitational acceleration (yielding ~10,000 kg/m² per unit area), multiplied by Earth's surface area of ~5.1 × 10¹⁴ m².[87][88] Roughly three-quarters of this mass resides within the troposphere below 11 km altitude, with the remainder distributed across higher layers where density drops sharply.[89] Atmospheric density at sea level, under standard conditions of 15°C and 101.325 kPa pressure, is 1.225 kg/m³ for dry air.[76][90] This density arises from the ideal gas law, ρ = P / (R_specific T), where R_specific is the specific gas constant for air (~287 J/kg·K), reflecting the mixture's mean molecular weight dominated by nitrogen (78%) and oxygen (21%).[77] Variations occur with temperature, humidity, and local pressure; for instance, pure dry air at 0°C and standard pressure has a density of ~1.293 kg/m³.[3] Density decreases with altitude primarily due to the exponential decline in pressure under hydrostatic equilibrium, where dP/dh = -ρg, leading to the barometric formula approximation ρ(h) ≈ ρ₀ exp(-h/H) with scale height H ~8.4 km in the troposphere.[77][91] In the U.S. Standard Atmosphere model, density falls to ~0.66 kg/m³ at 5 km, ~0.43 kg/m³ at 10 km, and ~10⁻⁶ kg/m³ near 100 km, transitioning to near-vacuum conditions in the exosphere.[76][92] This profile underscores the atmosphere's thinness, with 99% of mass below ~50 km despite extending hundreds of kilometers upward.[93]Acoustic and Mechanical Properties
The speed of sound in Earth's atmosphere, a key acoustic property, is determined by the medium's temperature, composition, and pressure, following the relation , where is the adiabatic index (approximately 1.4 for diatomic air), is the gas constant, is absolute temperature, and is molar mass.[94] At sea level under standard conditions (15°C, 101.3 kPa), this yields about 340 m/s, increasing to 343.5 m/s at 21°C and 358 m/s at 40°C due to enhanced molecular kinetic energy facilitating faster pressure wave propagation.[94] Altitude introduces variability: in the troposphere, decreasing temperature reduces speed to around 295 m/s at high altitudes, while stratospheric inversions can cause refraction, bending rays upward in stable layers or downward in inversions, affecting long-range propagation like infrasound from explosions.[95][96] Atmospheric attenuation of sound arises from classical viscosity, thermal conduction, and molecular relaxation (rotational and vibrational in N₂ and O₂), with absorption converting acoustic energy to heat; this effect scales strongly with frequency (e.g., higher for >1 kHz), temperature, and humidity, as water vapor enhances relaxation processes.[97][98] For instance, at 1 kHz and 20°C with 50% relative humidity, attenuation is roughly 1-2 dB/km, but rises in drier or colder conditions due to reduced rotational relaxation.[99] Wind shear and turbulence further scatter waves, introducing excess attenuation beyond spherical spreading, particularly for low-frequency signals that propagate farther via ducting in temperature gradients.[100][101] Mechanically, Earth's atmosphere behaves as a compressible Newtonian fluid with low shear strength, characterized by dynamic viscosity (resistance to shear flow) and kinematic viscosity (shear diffusivity). At sea-level standard conditions (15°C, 1.225 kg/m³ density), is approximately 1.789 × 10^{-5} Pa·s, increasing mildly with temperature (e.g., 1.86 × 10^{-5} Pa·s at 25°C) via enhanced molecular collisions, while decreasing with altitude due to lower density despite similar .[102][103] Kinematic viscosity rises from about 1.46 × 10^{-5} m²/s at sea level to higher values aloft, influencing turbulence scales via the Reynolds number , where low near the surface promotes instability in flows like boundary-layer winds.[104] Elasticity manifests in the bulk modulus , quantifying resistance to uniform compression; for air at sea level, Pa, linking directly to acoustic speed as .[76] The atmosphere's near-zero shear modulus underscores its fluid nature, enabling viscous dissipation in gradients but negligible rigidity, with compressibility ( Pa^{-1}) far exceeding solids, facilitating pressure equalization over scales larger than the scale height (~8 km).[92] These properties underpin mechanical responses like drag on objects (proportional to modulated by viscosity in boundary layers) and wave damping in atmospheric tides.[105]Optical and Radiative Properties
Scattering and Visibility
Rayleigh scattering by atmospheric molecules, primarily nitrogen (78%) and oxygen (21%), dominates in clear conditions and is proportional to the inverse fourth power of wavelength (1/λ⁴), scattering shorter blue-violet light more efficiently than longer red wavelengths and producing the blue daytime sky.[106][107] At sunrise and sunset, the increased optical path length scatters away shorter wavelengths, transmitting predominantly red light and yielding orange-red hues.[107] Mie scattering arises from larger particles such as aerosols (e.g., dust, smoke, sea spray) and cloud droplets comparable in size to visible wavelengths (0.1–10 μm), with forward-directed and less wavelength-selective scattering that appears white or gray, as seen in clouds and haze layers.[107][108] This process attenuates direct sunlight and enhances forward scatter under low solar angles, further degrading contrast for distant objects.[109] Atmospheric visibility, the horizontal distance at which a dark object of 2% contrast threshold becomes indistinguishable against the horizon sky, follows Koschmieder's law: meteorological range V = 3.912 / β, where β (in Mm⁻¹) is the total extinction coefficient summing molecular (Rayleigh) scattering, aerosol (Mie) scattering, and minor absorption.[109][108] In pristine conditions, Rayleigh scattering alone yields β ≈ 10 Mm⁻¹, limiting V to ~390 km; typical aerosol-laden atmospheres raise β to 100–1000 Mm⁻¹, reducing V to 4–40 km.[109][108] Aerosol particles (0.1–2.5 μm diameter) drive most impairments via high scattering efficiency (e.g., 3 m²/g for sulfates, dominant in eastern U.S. at 1.5 μg/m³ concentrations), with hygroscopic species like sulfates and nitrates swelling in humid air (RH >70%), amplifying β by factors of 2–4 at 80–90% RH due to increased size and refractive index.[109] Gases such as NO₂ add selective absorption (peaking in blue), imparting haze a brownish tint, while meteorological factors like inversions trap particulates, exacerbating uniform haze over layered plumes.[109] Natural baselines show V of 100–180 km in western U.S. versus 90–130 km in the east, underscoring regional aerosol influences.[109]Absorption and Transmission
The Earth's atmosphere selectively absorbs and transmits electromagnetic radiation across various wavelengths, influencing the planet's energy balance and habitability. Short-wavelength ultraviolet (UV) radiation is strongly absorbed by molecular oxygen (O₂) and ozone (O₃) in the upper atmosphere, preventing most harmful UV from reaching the surface. Specifically, the ozone layer absorbs nearly all UVC (100-280 nm) and about 90-97% of UVB (280-315 nm), while UVA (315-400 nm) is largely transmitted.[110][111] In contrast, visible light (approximately 400-700 nm) experiences high transmission, with minimal absorption except for minor contributions from ozone and oxygen, enabling solar energy to drive photosynthesis and surface heating.[112] In the infrared (IR) spectrum, absorption dominates due to greenhouse gases such as water vapor (H₂O), carbon dioxide (CO₂), and methane (CH₄), which capture outgoing thermal radiation from Earth's surface. CO₂, for instance, strongly absorbs IR at around 15 micrometers, while H₂O absorbs across broader bands in the mid- and far-IR. This absorption re-emits radiation in all directions, including downward, contributing to the greenhouse effect that raises surface temperatures by approximately 33°C compared to a non-greenhouse atmosphere. Transmission occurs in specific "atmospheric windows," such as the 8-14 micrometer band (thermal IR window), where ozone and other gases absorb less, allowing some heat escape to space and enabling remote sensing applications.[113][114][112] Longer wavelengths, including microwaves and radio waves, are mostly transmitted through the atmosphere with low absorption, except in specific molecular resonance bands, facilitating satellite communications and radar. X-rays and gamma rays are completely absorbed high in the atmosphere by interactions with neutral atoms and ions, posing no surface risk. These selective interactions define atmospheric transmission windows—regions of relative transparency like the optical (0.3-1.1 μm), near-IR, mid-IR (8-14 μm), and radio windows—while absorption bands by O₂, O₃, CO₂, and H₂O block other spectra, shaping radiative forcing and protecting life from ionizing radiation.[115][112]Emission and Radiative Balance
The radiative balance of Earth's atmosphere maintains equilibrium between absorbed shortwave solar radiation and emitted longwave infrared radiation at the top of the atmosphere (TOA). This balance determines the planet's effective temperature, approximately 255 K, which would prevail without atmospheric effects. Observations indicate a global mean incoming solar flux of 340 W/m² at TOA, with about 240 W/m² absorbed by the surface and atmosphere after reflection of 99 W/m² (29%) by clouds, aerosols, and the surface.[116] The outgoing longwave radiation (OLR) similarly averages 240 W/m², achieving near balance, though satellite measurements from NASA's CERES instruments reveal a small positive imbalance of about 0.8 W/m², signifying net energy accumulation in the Earth system.[116][114] Emission primarily occurs as thermal infrared radiation from the warmed surface and atmosphere. The surface, at an average temperature of 288 K, emits approximately 396 W/m² of longwave radiation based on blackbody calculations, but greenhouse gases such as water vapor, carbon dioxide, and methane absorb roughly 75-90% of this upward flux.[117] The atmosphere then re-emits this energy isotropically: downward to the surface (contributing to the greenhouse warming effect) and upward toward space. Direct escape of surface-emitted infrared to space accounts for only about 40-50 W/m², while the bulk of OLR—around 195 W/m²—originates from atmospheric layers, particularly clouds and upper tropospheric regions.[116] This selective emission spectrum, peaking in the infrared, contrasts with the shortwave solar input, enabling the atmosphere's role in redistributing heat vertically and latitudinally.[117] The greenhouse effect quantifies this process, elevating the surface temperature by about 33 K above the effective radiating temperature through back-radiation of approximately 333 W/m² to the surface.[117] Water vapor dominates absorption in the lower troposphere, responsible for over 50% of the effect, followed by clouds and CO₂. Empirical data from radiative transfer models and flux measurements confirm that without these absorbers, surface temperatures would resemble those on airless bodies like the Moon, averaging -18°C rather than 15°C.[117] Perturbations to this balance, such as increased greenhouse gas concentrations, alter emission profiles; for instance, CO₂'s absorption bands in the 15 μm region trap outgoing radiation, requiring higher emission altitudes with cooler temperatures to restore equilibrium, per the Schwarzschild equation governing radiative transfer.[116] Satellite-derived OLR spectra validate these mechanisms, showing reduced flux in gaseous absorption windows.[114]Refractive Effects
The refractive index of air, which governs light bending in the atmosphere, varies with density and composition; for dry air at 15°C and 1013.25 hPa, it measures approximately 1.000276 for sodium D-line light (589 nm).[118] This index increases nearly linearly with pressure (roughly proportional to density) and decreases with rising temperature, with a typical sensitivity of about -9 × 10^{-6} per Kelvin near standard conditions, while humidity introduces minor corrections via water vapor's lower index.[119] [120] Vertical gradients in temperature and pressure create a refractive index profile that decreases with altitude, causing light rays from space to curve concave to Earth, elevating apparent celestial positions.[121] Astronomical refraction displaces stars and the Sun upward, with the effect maximal near the horizon at about 35 arcminutes, rendering the Sun visible for roughly two additional minutes at sunrise and sunset compared to geometric positions.[122] This refraction, computed via models like those integrating the index gradient along ray paths, follows Snell's law adapted for continuous media, yielding corrections essential for precise navigation and surveying; for instance, at zenith the displacement drops to under 1 arcminute.[123] Turbulent eddies further modulate this, producing scintillation or "twinkling" in stars—intensity fluctuations from refractive index fluctuations on scales of centimeters to meters—more pronounced near horizons where path lengths through unstable layers lengthen.[124] Terrestrial refraction manifests in mirages from strong near-surface gradients, such as hot ground creating inferior mirages (e.g., illusory puddles via downward-bending rays) or cold air aloft yielding superior mirages that elevate or invert distant objects like ships or mountains.[125] These effects, quantified by ray-tracing through measured lapse rates, can extend visible ranges beyond geometric horizons by 10-20% under sub-refraction conditions, influencing optical signaling and aviation; conversely, super-refraction from temperature inversions compresses ranges, as documented in military propagation studies.[126] Dispersion across wavelengths slightly varies refraction, contributing to chromatic distortions in high-precision applications but negligible for most visual phenomena.[127]Atmospheric Dynamics
Global Circulation Patterns
The global atmospheric circulation patterns arise primarily from the uneven distribution of solar heating, with the equator receiving more intense insolation than the poles due to the Earth's spherical shape and axial tilt.[128] This thermal gradient drives air from high-pressure regions at the poles toward low-pressure areas near the equator, establishing a three-cell model in each hemisphere: the Hadley cell between 0° and 30° latitude, the Ferrel cell between 30° and 60° latitude, and the Polar cell between 60° and 90° latitude.[129] [128] In the Hadley cell, surface air flows equatorward as the trade winds, converges at the Intertropical Convergence Zone (ITCZ)—a band of low pressure near the equator where northeast trades from the Northern Hemisphere and southeast trades from the Southern Hemisphere meet—rises due to heating, cools aloft, and sinks around 30° latitude, forming subtropical high-pressure belts.[128] [130] The ITCZ position shifts seasonally, migrating northward in boreal summer by up to 10° latitude following the thermal equator defined by maximum solar heating.[131] The Ferrel cell, an indirect circulation, features poleward surface flow as prevailing westerlies between 30° and 60° latitude, driven by interactions with adjacent cells rather than direct solar forcing, with air rising near 60° latitude and sinking near 30°.[128] [129] The Polar cell involves cold, dense air sinking at the poles, flowing equatorward as polar easterlies, and rising around 60° latitude where it meets warmer mid-latitude air.[128] The Coriolis effect, resulting from Earth's rotation at approximately 1670 km/h at the equator decreasing poleward, deflects moving air masses to the right in the Northern Hemisphere and to the left in the Southern Hemisphere, preventing direct north-south flow and establishing the east-west components of prevailing winds.[132] [133] Thus, equatorward trades become northeast in the north and southeast in the south; mid-latitude westerlies blow from west to east; and polar easterlies flow from the east.[134] [135] These patterns transport heat poleward, moderating global temperatures, with the Hadley cell alone accounting for about half of the meridional heat flux in the tropics.[128] Jet streams, narrow bands of strong westerly winds aloft near the tropopause—such as the polar jet around 60° latitude and subtropical jet near 30°—mark boundaries between cells and influence weather systems by steering storms.[129]Weather Formation and Variability
Weather on Earth forms primarily through the uneven heating of the planet's surface by solar radiation, which creates temperature gradients that drive atmospheric motion.[128] This differential heating, stronger at the equator than the poles, generates pressure differences: warmer air expands and rises, forming low-pressure areas, while cooler air sinks, creating high-pressure zones.[136] Winds arise as air flows from high to low pressure, influenced by Earth's rotation via the Coriolis effect, which deflects flows and organizes them into circulation cells like the Hadley, Ferrel, and polar cells.[128] These processes redistribute heat and moisture, setting the stage for localized weather phenomena.[137] Convection plays a central role in initiating many weather events, particularly precipitation and storms. Warm, moist air near the surface rises due to buoyancy, expands and cools adiabatically in the lower troposphere, reaching saturation where water vapor condenses into cloud droplets.[138] As droplets collide and coalesce, they grow heavy enough to fall as rain, snow, or hail when updrafts can no longer support them.[139] In unstable atmospheres, intense convection fuels thunderstorms, with updrafts exceeding 20 m/s in severe cases, producing lightning, heavy rain, and sometimes tornadoes through shear-induced rotation.[140] Frontal lifting at boundaries between contrasting air masses—warm and cold—similarly promotes cloud formation and precipitation by forcing air upward along sloping surfaces.[141] Variability in weather arises from interactions among these mechanisms modulated by geographic and temporal factors. Diurnal cycles cause daily fluctuations, with afternoon convection peaks in tropical regions leading to thunderstorms on about 50% of days in some areas.[142] Seasonal changes, driven by Earth's 23.5° axial tilt, shift insolation patterns, enhancing mid-latitude storm tracks in winter when temperature contrasts are greatest.[143] Topography amplifies variability: mountains orogenically lift air, increasing orographic precipitation by up to 10 times on windward slopes, while oceans moderate temperatures via heat capacity, fostering persistent weather like monsoons from land-sea thermal contrasts.[136] Short-term oscillations, such as those from jet stream meanders, introduce unpredictability, with synoptic-scale cyclones forming every 3-7 days in mid-latitudes, transporting heat poleward.[141] Volcanic eruptions can temporarily enhance variability by injecting aerosols that alter radiation balance, cooling surfaces and shifting storm paths for months.[144]Long-Term Climate Drivers
Long-term climate drivers operate on timescales of thousands to millions of years, primarily influencing Earth's atmospheric temperature, circulation patterns, and composition through variations in solar insolation, greenhouse gas concentrations, and surface-albedo feedbacks. These mechanisms, distinct from short-term weather variability, have paced glacial-interglacial cycles and broader climatic shifts, with empirical evidence from ice cores, sediment records, and astronomical calculations confirming their causal roles.[145][146] Milankovitch cycles, arising from periodic changes in Earth's orbital parameters, represent a dominant driver on 20,000–100,000-year scales by modulating the geographic and seasonal distribution of incoming solar radiation. These include eccentricity variations (cycle ~100,000 years, altering orbital shape and Earth-Sun distance by up to 3%), obliquity shifts (41,000-year cycle, changing axial tilt from 22.1° to 24.5° and thus polar insolation), and precession (23,000-year cycle, wobbling the tilt axis relative to perihelion). Reduced summer insolation at high northern latitudes, for instance, has triggered ice sheet growth during glacial maxima, amplifying atmospheric cooling via increased albedo and CO2 drawdown into oceans and biomass; benthic foraminifera oxygen isotope records from deep-sea cores correlate precisely with these insolation minima, explaining ~50–70% of observed temperature variance over the past 800,000 years.[147][148][145] Plate tectonics exerts control over multimillion-year climate via modulation of atmospheric CO2 levels and ocean-atmosphere circulation. Subduction zones recycle carbon into the mantle, while mid-ocean ridge volcanism and arc degassing release ~5–7 × 10^11 kg CO2 annually on average, balancing silicate weathering that sequesters it; faster plate speeds historically elevated CO2, fostering hothouse climates like the Cretaceous (CO2 ~1,000 ppm, temperatures 5–10°C above modern), whereas slowed tectonics and enhanced weathering during supercontinent assembly lowered CO2, promoting cooler states. Continental drift also reshapes atmospheric dynamics by altering ocean gateway configurations—e.g., Panama Isthmus closure ~3 million years ago intensified Atlantic meridional overturning, drying subtropical atmospheres and contributing to Northern Hemisphere glaciation onset. Proxy data from stomatal indices and boron isotopes in foraminifera validate these CO2-climate links, with tectonics explaining ~60% of Phanerozoic CO2 variance.[145][149][150] Solar luminosity variations contribute modestly on intermediate timescales, with the Sun's total irradiance fluctuating ~0.1% over 11-year cycles (impacting global temperatures by ~0.1°C) and rarer grand minima like the Maunder (1645–1715) correlating with ~0.3–0.5°C regional coolings via reduced radiative forcing. Over billions of years, solar output has risen ~30% since the Archean faint young Sun paradox, necessitating higher early atmospheric greenhouse gases (CH4, CO2) to avert global freeze, as evidenced by faint early irradiance models and geological warmth proxies like banded iron formations. However, these long-term trends are overshadowed by internal planetary feedbacks for Holocene-era climate.[151][152] Episodic large-scale volcanism, often tied to tectonics via flood basalts, drives transient warmings by injecting massive CO2 pulses—e.g., Siberian Traps eruptions ~252 million years ago released ~10^4–10^5 Gt CO2, elevating levels to ~2,000–4,000 ppm and triggering Permian-Triassic hothouse conditions with ~6–10°C warming and ocean anoxia. Sulfate aerosol veils from explosive events cause decadal coolings (e.g., Pinatubo 1991: ~0.5°C global drop), but long-term net effects favor greenhouse forcing when CO2 dominates, as reconstructed from mercury and iridium spikes in sediments. These drivers interact; for instance, tectonic uplift enhances weathering, countering volcanic CO2, while Milankovitch-modulated insolation amplifies glacial responses to baseline forcings.[153][154][155]Evolutionary History
Earliest Atmosphere
The proto-atmosphere of Earth, formed during the planet's accretion approximately 4.54 billion years ago, primarily consisted of hydrogen and helium captured from the solar nebula, mirroring the composition of gas giants.[156] This primary atmosphere was rapidly lost to space through hydrodynamic escape driven by the young Sun's intense solar wind and Earth's insufficient gravitational retention for light gases, particularly following the Moon-forming giant impact around 4.5 billion years ago, which further heated the planet and enhanced escape rates.[157] Models indicate that terrestrial planets like Earth retained only trace amounts of these primordial volatiles, with helium loss continuing at rates of about 50 g/s even today via polar wind mechanisms.[158] Following the dissipation of the proto-atmosphere, the earliest secondary atmosphere emerged in the Hadean eon (4.6–4.0 billion years ago) through volcanic outgassing from a differentiating mantle and degassing during magma ocean solidification.[157] This process released volatiles trapped during accretion, including water vapor, carbon dioxide, and nitrogen, with estimates suggesting an initial post-impact atmosphere of up to 560 bar H2O and 100 bar CO2 from a global steam envelope.[159] Transient reducing conditions post-giant impact may have produced methane- or carbon monoxide-rich layers via impact degassing, though these were short-lived due to oxidation and precipitation.[157] Dominant models favor a CO2- and N2-rich composition over earlier reducing scenarios (e.g., high CH4 or NH3 proposed by Urey in 1952), as geochemical evidence from Hadean zircons and sulfur isotope ratios in metasediments (>3.8 Ga) indicates neutral to mildly oxidizing conditions incompatible with sustained reductants.[160] CO2 pressures likely exceeded several bars initially, declining over the Hadean through subduction and carbonate formation, while nitrogen delivery occurred via inner solar system dust and planetesimals.[161] No free oxygen existed, and the atmosphere's opacity from steam and greenhouse gases delayed ocean formation until late Hadean impacts subsided.[162] These inferences rely on thermodynamic modeling and proxy records, as direct Hadean atmospheric samples are absent, with critiques noting potential overreliance on Venus analogies despite differing evolutionary paths.[163]Secondary Atmosphere Development
The secondary atmosphere of Earth developed after the dissipation of the primary atmosphere, which consisted mainly of hydrogen and helium captured from the solar nebula during planetary formation around 4.5 billion years ago. This primary atmosphere was lost due to high surface temperatures exceeding thousands of degrees Celsius during accretion, combined with insufficient gravitational retention for light gases and intense solar wind stripping in the early solar system.[157] [164] Volcanic outgassing from the planet's interior became the dominant mechanism for secondary atmosphere formation, commencing during the Hadean eon as the crust differentiated and mantle convection drove widespread magmatism. This process released volatiles trapped in the silicate mantle, with peak activity linked to the solidification of a global magma ocean post the moon-forming impact approximately 4.5 billion years ago. Geochemical models indicate that outgassing rates were orders of magnitude higher than modern levels, potentially building atmospheric pressure to several bars within the first 100-200 million years. Evidence includes isotopic signatures in ancient zircons from Western Australia, dated to 4.4 billion years ago, which record interaction with liquid water and imply an overlying steam-rich atmosphere.[157] [165] [166] The initial composition mirrored modern volcanic emissions but scaled for early flux: predominantly water vapor (70-90% by volume), carbon dioxide (10-15%), nitrogen (trace to a few percent), sulfur dioxide, and minor hydrogen and methane depending on mantle redox state. These proportions derive from thermodynamic modeling of mantle degassing under high-pressure conditions, where oxidized magmas favored CO2 and SO2 over reduced species like H2. Unlike the reducing primary atmosphere, the secondary was relatively oxidized, setting the stage for eventual ocean formation as temperatures dropped below 200°C around 4.4-4.0 billion years ago, condensing H2O and sequestering much of it into surface oceans while leaving a CO2-N2 dominated envelope.[164] [166] [157] Subsequent modifications involved partial loss via hydrodynamic escape and impact erosion, particularly during the Late Heavy Bombardment around 4.1-3.8 billion years ago, but outgassing replenished the inventory, stabilizing a dense, greenhouse-forced atmosphere conducive to prebiotic chemistry. Mantle nitrogen isotopes and carbon cycling proxies support that N2 accumulation occurred gradually through devolatilization, reaching near-modern levels by the Archean. This development contrasts with capture hypotheses, as noble gas ratios (e.g., low 36Ar/38Ar) align better with internal degassing than solar nebula inheritance.[167] [157]oxygenation and Biosphere Coupling
The oxygenation of Earth's atmosphere arose from the biological innovation of oxygenic photosynthesis, developed by cyanobacteria around 2.7 billion years ago.[168] This process utilizes sunlight, water, and carbon dioxide to generate organic compounds and molecular oxygen (O₂), fundamentally linking biospheric productivity to atmospheric composition: the reaction 6CO₂ + 6H₂O → C₆H₁₂O₆ + 6O₂ represents the primary source of free oxygen.[169] Despite early onset, significant atmospheric accumulation was delayed due to efficient sinks, including oxidation of reduced crustal materials like ferrous iron (Fe²⁺) in seawater, which precipitated as banded iron formations (BIFs) spanning 3.8 to 1.8 billion years ago.[170] The Great Oxidation Event (GOE), dated to approximately 2.4 billion years ago, initiated widespread oxygenation when photosynthetic O₂ production exceeded these geological sinks, evidenced by the cessation of BIF deposition, appearance of red beds (oxidized sediments), and shifts in sulfur and chromium isotopes.[171] [172] This threshold event exemplifies biosphere-atmosphere coupling: cyanobacterial proliferation, possibly facilitated by ecological niche expansions or reduced nitrogenase inhibition, overwhelmed sinks, raising O₂ from trace levels (<0.001% present atmospheric level, PAL) to 0.1–1% PAL.[170] [173] Post-GOE, feedback loops stabilized and modulated O₂ levels through biogeochemical cycles. Photosynthetic burial of organic carbon in sediments acts as a long-term O₂ source by sequestering reduced carbon away from oxidation, while aerobic respiration and organic matter decay serve as sinks; net O₂ thus tracks the fraction of primary production escaping respiration, estimated at 0.1–1% globally.[174] Tectonic uplift exposes reduced minerals (e.g., sulfides, Fe²⁺) for weathering, consuming O₂ and closing the cycle, with Phanerozoic models indicating O₂ fluctuations between 15–30% driven by such imbalances.[175] [176] This coupling extended evolutionary impacts: rising O₂ enabled aerobic metabolisms, fostering eukaryotic diversification around 1.8–0.8 billion years ago, yet initially stressed anaerobes, contributing to mass extinctions.[168] Later pulses, like the Neoproterozoic Oxygenation Event (~800–540 million years ago), correlated with biospheric expansions such as algal blooms, further intertwining biological innovation with atmospheric redox state.[177] Empirical proxies, including carbon-sulfur isotope records and uranium-thorium ratios, confirm O₂'s rise from near-zero in the Archean to modern 21% levels, underscoring the biosphere's causal role in transforming Earth's oxidative capacity.[178] [179]Holocene Stability and Recent Perturbations
The Holocene epoch, commencing approximately 11,700 years before present following the Younger Dryas cold reversal, featured a period of relative climatic stability characterized by interglacial conditions with subdued variability compared to the Pleistocene's glacial-interglacial oscillations. Proxy reconstructions from ice cores, lake sediments, and pollen records indicate global mean temperatures peaked during the Holocene Thermal Maximum around 6,400 years ago, with mid-Holocene conditions at least as warm as the late 20th century in many regions, followed by a gradual cooling trend of about 0.4–0.7°C over the subsequent millennia until the pre-industrial era. Atmospheric CO₂ concentrations remained confined within a narrow range of roughly 260–280 ppm throughout most of the epoch, exhibiting only minor millennial-scale fluctuations of 10–30 ppm as evidenced by high-resolution Antarctic ice core analyses such as those from Law Dome and WAIS Divide, with no excursions approaching modern levels. This stability facilitated the expansion of human agriculture and civilizations, underpinned by predictable seasonal cycles and reduced frequency of extreme events relative to earlier epochs.[180][181][182][183] Despite this overall equilibrium, the Holocene encompassed internal modes of variability, including centennial to millennial oscillations such as the 8.2 ka cooling event linked to freshwater outbursts disrupting Atlantic circulation and periodic cool intervals recurring roughly every 1,400 years in North American proxies. Temperature reconstructions reveal spatial heterogeneity, with Northern Hemisphere summer warmth declining post-optimum while winter variability persisted, influenced by orbital forcing (Milankovitch cycles) that reduced summer insolation by up to 50 W/m² at high latitudes since the early Holocene. Sea ice proxies from North Atlantic sediments and Greenland ice cores document fluctuations in extent, correlating with these temperature modes, yet without triggering widespread deglaciation or abrupt shifts beyond regional scales. Such dynamics underscore the atmosphere's resilience to internal forcings like volcanic eruptions and solar irradiance variations, which induced transient perturbations but failed to destabilize the epoch's baseline state.[184][185][186][187] In contrast, the past two centuries have witnessed accelerated perturbations, primarily manifested in the rapid accumulation of anthropogenic greenhouse gases. Ice core data confirm pre-industrial CO₂ at approximately 280 ppm, escalating to over 420 ppm by 2023 due to fossil fuel combustion and land-use changes, a rate exceeding any comparable Holocene flux by orders of magnitude. Instrumental records document a global surface temperature increase of about 1.1°C since 1880, with the post-1950 warming phase aligning temporally with emissions growth, though urban heat island effects and adjustments in datasets introduce uncertainties in precise attribution. Aerosol loading from industrial activities has modulated radiative forcing, exerting a net cooling influence that partially offsets GHG warming, while methane and nitrous oxide have risen concurrently, altering tropospheric chemistry. These changes coincide with reduced diurnal temperature ranges and shifts in precipitation patterns, deviating from Holocene norms.[188][183][189] Debates persist regarding the relative contributions of natural versus anthropogenic drivers to these recent shifts, with empirical evidence showing correlations between emissions and radiative imbalance but also influences from solar cycles (e.g., the Modern Grand Solar Minimum post-2000) and ocean-atmosphere oscillations like the Pacific Decadal Oscillation. Proxy-satellite comparisons highlight discrepancies, such as tropospheric warming rates slower than some models predict, suggesting potential overestimation of climate sensitivity to CO₂ doubling (estimated at 1.5–4.5°C in assessments, yet constrained lower by energy budget analyses). Natural forcings, including volcanic quiescence since the 1991 Pinatubo eruption, have amplified observed trends, while paleoclimate analogs indicate that Holocene-like variability could account for portions of the signal without invoking unprecedented forcing. Comprehensive attribution requires disentangling these factors, as institutional syntheses often emphasize anthropogenic dominance while underweighting empirical residuals in observational data.[190][181][191]Human Interactions
Natural vs. Anthropogenic Pollution Sources
Natural sources of atmospheric pollution include volcanic eruptions, dust storms, biogenic emissions from vegetation, wildfires, and sea spray, which collectively contribute substantial quantities of sulfur dioxide (SO2), particulate matter (PM), volatile organic compounds (VOCs), and aerosols. Volcanic activity releases approximately 20-25 teragrams (Tg) of SO2 annually from subaerial sources, with individual large eruptions like Mount Pinatubo in 1991 injecting up to 20 Tg in a single event, temporarily rivaling or exceeding annual human emissions. Dust storms, primarily from arid regions such as the Sahara and Gobi deserts, mobilize 1,000-3,000 million tonnes of PM annually, accounting for the majority of global coarse PM loading and influencing air quality across continents. Biogenic VOCs from terrestrial plants, such as isoprene and monoterpenes, emit around 1,000 Tg per year, comprising roughly 86% of total global VOC emissions and playing a key role in tropospheric ozone formation. Wildfires, often ignited by lightning in remote areas, release significant black carbon and organic aerosols; for instance, natural biomass burning contributes to seasonal PM spikes that can dominate regional aerosol budgets in fire-prone ecosystems.[192][193][194] Anthropogenic sources, driven by fossil fuel combustion, industrial processes, transportation, and solvent use, predominate for certain pollutants like nitrogen oxides (NOx) and fine PM2.5, though their global totals are smaller than natural contributions for PM and VOCs overall. Human activities emitted about 50-100 Tg of SO2 annually in the late 20th century from coal burning and smelting, but scrubber technologies and fuel switching reduced this to under 50 Tg by 2020, making volcanic outputs comparably significant in eruptive periods. Global anthropogenic NOx emissions reached approximately 40 Tg per year around 2010, mainly from vehicles and power plants, far exceeding natural sources like lightning (5-8 Tg) and soil microbes (10 Tg). Anthropogenic VOCs account for only 14% of global totals, estimated at 150 Tg annually, primarily from petroleum products and evaporation, yet they are concentrated in urban areas and contribute disproportionately to local ozone and secondary organic aerosol formation. Fine PM2.5 from human sources, including combustion and industry, totals around 100 Tg globally per year, representing a smaller fraction of total PM mass but with higher health impacts due to deeper lung penetration compared to natural coarse dust.[195][194][196]| Pollutant | Natural Annual Emission (Tg) | Anthropogenic Annual Emission (Tg) | Dominant Source Globally |
|---|---|---|---|
| SO2 | 20-25 (volcanic) | <50 (post-2000 reductions) | Variable; anthropogenic baseline, natural episodic |
| NOx | 15-20 (lightning, soils) | ~40 | Anthropogenic |
| PM (total) | 2,000-3,000 (dust dominant) | ~100 (fine PM focus) | Natural |
| VOCs | ~1,000 (biogenic) | ~150 | Natural (biogenic) |


