Recent from talks
Nothing was collected or created yet.
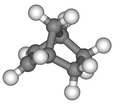
Norbornene
View on Wikipedia
| |||
 | |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
Bicyclo[2.2.1]hept-2-ene | |||
| Other names
Norbornylene
Norcamphene | |||
| Identifiers | |||
3D model (JSmol)
|
|||
| ChEBI | |||
| ChemSpider | |||
| ECHA InfoCard | 100.007.152 | ||
| EC Number |
| ||
PubChem CID
|
|||
| UNII | |||
CompTox Dashboard (EPA)
|
|||
| |||
| |||
| Properties | |||
| C7H10 | |||
| Molar mass | 94.157 g·mol−1 | ||
| Appearance | White solid | ||
| Melting point | 42 to 46 °C (108 to 115 °F; 315 to 319 K) | ||
| Boiling point | 96 °C (205 °F; 369 K) | ||
| Hazards | |||
| NFPA 704 (fire diamond) | |||
| Flash point | −15 °C (5 °F; 258 K) | ||
| Related compounds | |||
Related compounds
|
Nadic anhydride | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||
Norbornene or norbornylene or norcamphene is a highly strained bridged cyclic hydrocarbon. It is a white solid with a pungent sour odor. The molecule consists of a cyclohexene ring with a methylene bridge between carbons 1 and 4. The molecule carries a double bond which induces significant ring strain and significant reactivity.
The name comes from norbornane, from bornane.
Production
[edit]Norbornene is made by a Diels–Alder reaction of cyclopentadiene and ethylene.[dubious – discuss] Many substituted norbornenes can be prepared similarly.[2][3] Related bicyclic compounds are norbornadiene, which has the same carbon skeleton but with two double bonds, and norbornane which is prepared by hydrogenation of norbornene.
Reactions
[edit]Norbornene undergoes an acid-catalyzed hydration reaction to form norborneol. This reaction was of great interest in the elucidation of the non-classical carbocation controversy.
Norbornene is used in the Catellani reaction and in norbornene-mediated meta-C−H activation.[4]
Certain substituted norbornenes undergo unusual substitution reactions owing to the generation of the 2-norbornyl cation.
Being a strained ene, norbornenes react readily with thiols in the thiol-ene reaction to form thioethers. This makes norbornene-functionalized monomers ideal for polymerization with thiol-based monomers to form thiol-ene networks.[5]
Polynorbornenes
[edit]Norbornenes are important monomers in ring-opening metathesis polymerizations (ROMP). Typically these conversions are effected with ill-defined catalysts. Polynorbornenes exhibit high glass transition temperatures and high optical clarity.[6]

ROMP reaction giving polynorbornene. Like most commercial alkene metathesis processes, this reaction does not employ a well-defined molecular catalyst.
In addition to ROMP, norbornene monomers also undergo vinyl-addition polymerization, and is a popular monomer for use in cyclic olefin copolymers.
Polynorbornene is used mainly in the rubber industry for antivibration (rail, building, industry), antiimpact (personal protective equipment, shoe parts, bumpers) and grip improvement (toy tires, racing tires, transmission systems, transports systems for copiers, feeders, etc.)
Ethylidene norbornene is a related monomer derived from cyclopentadiene and butadiene.
See also
[edit]References
[edit]- ^ Norbornene MSDS
- ^ Binger, Paul; Wedemann, Petra; Brinker, Udo H. "Cyclopropene: A New Simple Synthesis and its Diels-Alder Reaction with Cyclopentadiene". Organic Syntheses; Collected Volumes, vol. 10, p. 231.
- ^ Oda, Masaji; Kawase, Takeshi; Okada, Tomoaki; Enomoto, Tetsuya. "2-Cyclohexene-1,4-dione". Organic Syntheses; Collected Volumes, vol. 9, p. 186.
- ^ Thansandote, Praew; Chong, Eugene; Feldmann, Kai-Oliver; Lautens, Mark (21 May 2010). "Palladium-Catalyzed Domino C−C/C−N Coupling Using a Norbornene Template: Synthesis of Substituted Benzomorpholines, Phenoxazines, and Dihydrodibenzoxazepines". The Journal of Organic Chemistry. 75 (10): 3495–3498. doi:10.1021/jo100408p. PMID 20423091.
- ^ Hoyle, Charles E.; Bowman, Christopher N. (2010). "Thiol–Ene Click Chemistry". Angewandte Chemie International Edition. 49 (9): 1540–1573. doi:10.1002/anie.200903924.
- ^ Delaude, Lionel; Noels, Alfred F. (2005). "Metathesis". Kirk-Othmer Encyclopedia of Chemical Technology. Weinheim: Wiley-VCH. doi:10.1002/0471238961.metanoel.a01. ISBN 978-0471238966.
Norbornene
View on GrokipediaStructure and Properties
Molecular Formula and Nomenclature
Norbornene has the molecular formula .[1] Its systematic IUPAC name is bicyclo[2.2.1]hept-2-ene.[1] Common names for the compound include norbornene, norbornylene, and norcamphene.[6] The molecular structure of norbornene is that of a bridged bicyclic alkene, specifically a seven-carbon framework with bridgehead carbons at positions 1 and 4 connected by two two-carbon bridges and one methylene bridge, featuring a double bond between carbons 2 and 3 in one of the two-carbon bridges.[7] This arrangement results in a rigid, strained system that can be visualized as a cyclohexene ring with a methylene bridge spanning positions 1 and 4.[8] The name "norbornene" derives from norbornane, its saturated hydrocarbon analog (bicyclo[2.2.1]heptane), which was historically obtained through the reduction of norcamphor, a derivative of the natural terpenoid camphor.[9] The prefix "nor-" in norbornane signifies the removal of one or more methyl groups from bornane (1,7,7-trimethylbicyclo[2.2.1]heptane), the parent hydrocarbon skeleton of camphor, reflecting early synthetic degradations of this bicyclic framework in organic chemistry.[10]Stereoisomers
The parent norbornene is achiral and has no stereoisomers. Norbornene derivatives featuring substituents at the 2- or 3-positions, arising from Diels-Alder cycloadditions of cyclopentadiene with alkenes, exhibit stereoisomerism classified as endo and exo based on the orientation of the substituent relative to the bicyclic framework. In the endo isomer, the substituent is directed toward the methylene bridge at position 7, positioning it on the same side as the bridgehead hydrogens at carbons 1 and 4. Conversely, the exo isomer has the substituent oriented away from the methylene bridge, toward the open face of the molecule.[11][12] These configurations can be visualized in 3D representations where the rigid bicyclo[2.2.1]heptene skeleton constrains the substituent: the endo form places it in closer proximity to the C7 methylene group and the σ-bonds of the bridges, while the exo form extends it outward, minimizing interactions with the endo face. The bridgehead carbons (1 and 4) serve as reference points, with the double bond between carbons 2 and 3 defining the plane across which the stereochemistry is assessed.[13] The Diels-Alder synthesis of such norbornene derivatives typically yields a mixture favoring the endo isomer, with common ratios around 70:30 to 80:20 endo:exo, depending on the dienophile and conditions; for instance, the reaction with acrylic acid derivatives often produces approximately 75:25 endo:exo.[14][15] The exo isomer is slightly more stable than the endo isomer, primarily due to reduced steric hindrance between the substituent and the methylene bridge, which alleviates strain in the endo configuration.[12][16] Pure endo and exo isomers can be isolated from mixtures via fractional distillation, exploiting subtle differences in boiling points, or through column chromatography, which separates based on polarity variations.[17][18] In terms of reactivity, the endo isomer often displays enhanced rates in certain cycloaddition reactions, such as epoxidations, attributable to favorable frontier orbital alignments that facilitate interaction with the approaching reagent.[19]Physical Properties
Norbornene appears as a white solid with a pungent odor.[8][20] Its melting point ranges from 44 to 46 °C, while the boiling point is 96 °C at 760 mmHg, and the flash point is -8 °C.[7][20] The density of the liquid form is 0.845 g/mL at 50 °C.[2] Norbornene is sparingly soluble in water (134 mg/L at 20 °C) but soluble in organic solvents such as ethanol and ether.[2] Infrared spectroscopy reveals a characteristic C=C stretch absorption at approximately 1600 cm⁻¹, indicative of the strained alkene functionality.[21] The ¹H NMR spectrum shows signals for the olefinic protons between 5.9 and 6.2 ppm.[22] Norbornene exhibits a vapor pressure of about 39 mmHg at 25 °C.[23] It demonstrates thermal stability under normal conditions, remaining intact up to around 200 °C without significant decomposition.[2]Chemical Properties
Norbornene possesses significant ring strain energy of approximately 27 kcal/mol arising from its bridged bicyclic [2.2.1] system, which distorts bond angles and introduces torsional strain, rendering it far more reactive than typical cycloalkenes like cyclohexene with negligible strain (~1 kcal/mol).[24] This elevated strain primarily stems from the constrained geometry of the five-membered ring fused within the structure and the methylene bridge, promoting facile ring-opening processes.[25] The olefinic double bond in norbornene is electron-rich due to the surrounding strained framework, which enhances its nucleophilicity and susceptibility to electrophilic addition reactions, often proceeding with high regioselectivity favoring exo attack.[26] Additionally, the bicyclic architecture enforces Bredt's rule, prohibiting stable double bonds at the bridgehead positions because such configurations would require a trans double bond in a small ring, leading to excessive geometric strain.[27] Norbornene demonstrates good chemical stability under ambient conditions, remaining unreactive in air without initiators, though it is prone to polymerization upon heating or exposure to catalysts, driven by strain relief.[2] It undergoes slow oxidation in the presence of oxygen to form epoxides, reflecting the moderate reactivity of its strained alkene. Regarding acid-base properties, norbornene is non-acidic overall, with its bridgehead hydrogens particularly resistant to deprotonation; base-catalyzed exchange occurs sluggishly due to the inability of the resultant carbanion to achieve planarity without introducing prohibitive strain.[28]Synthesis
Industrial Production
Norbornene is industrially produced on a large scale primarily through the Diels-Alder cycloaddition reaction between cyclopentadiene and ethylene. Cyclopentadiene, the diene component, is generated in situ by the thermal cracking of dicyclopentadiene at temperatures of 170–200 °C.[29] The reaction proceeds as follows: This process occurs under elevated conditions of 150–200 °C and pressures of 50–100 bar to ensure efficient conversion, with excess ethylene often used to suppress side reactions.[30] The cycloaddition typically achieves yields of 95–98%.[31] The crude product is purified by fractional distillation to isolate high-purity norbornene suitable for downstream applications.[31] Norbornene is produced on a significant industrial scale, with the majority of manufacturing facilities located in Asia to supply polymer precursor demands.[32] An alternative route involves the catalytic hydrogenation of norbornadiene, but this method is less prevalent owing to higher costs associated with the starting material.[33]Laboratory Methods
In laboratory settings, norbornene is commonly synthesized via the Diels-Alder cycloaddition of cyclopentadiene with ethylene, where cyclopentadiene is generated in situ by heating dicyclopentadiene to 170–200°C to induce retro-Diels-Alder cracking. The monomeric diene is then reacted with ethylene in a sealed steel autoclave under high pressure (initial 800–900 psi at 25°C) and elevated temperature (190–200°C for 7 hours), with careful control of heating to mitigate the exothermic nature of the reaction. Yields typically range from 57–71% after distillation of the crude product (b.p. 94–97°C/740 mm), providing the bicyclic alkene in sufficient purity for research purposes.[34] Safety considerations are paramount due to the use of high-pressure ethylene, necessitating a pressure-rated vessel, pressure relief mechanisms, and gradual heating (e.g., ~50°C per hour) to prevent runaway reactions or vessel rupture. This method is adaptable for small-scale (1–2 mol) preparations but requires specialized equipment not typically found in undergraduate labs.[34] For substituted norbornene analogs, the Diels-Alder approach employs alternative dienophiles to introduce functional groups, enabling the synthesis of variants tailored for specific studies in polymerization or materials science. Reaction of freshly distilled cyclopentadiene with maleic anhydride in ethyl acetate or a mixed solvent (e.g., ethyl acetate/hexanes) at ambient temperature (20–25°C) for 1–2 hours affords the endo-5-norbornene-2,3-dicarboxylic anhydride in 85–95% yield after recrystallization, with the endo selectivity driven by secondary orbital interactions. Similarly, cyclopentadiene with acrylic acid under mild heating (40–60°C) in diethyl ether yields 5-norbornene-2-carboxylic acid (bicyclo[2.2.1]hept-5-ene-2-carboxylic acid) in 80–90% isolated yield, often as a mixture of endo and exo isomers that can be used directly or purified further. These procedures are straightforward, solvent-based, and avoid high pressures, making them ideal for bench-scale synthesis of functionalized monomers.[35][12] Alternative synthetic routes to norbornene include thermal pyrolysis of norbornyl acetate precursors, which proceeds via elimination to form the alkene. For instance, gas-phase pyrolysis of exo-2-norbornyl acetate at 480°C in a quartz tube yields norbornene alongside minor isomers, with product distribution influenced by stereochemistry and temperature; this method is useful for isotopic labeling studies but requires vacuum pyrolysis apparatus and achieves moderate yields (40–60%) due to side reactions like skeletal rearrangement. Olefin metathesis of cycloheptene derivatives offers another pathway for substituted norbornenes, such as ring-closing metathesis of diene-functionalized cycloheptenes using Grubbs' catalysts to construct the bicyclic framework, though it is less prevalent for the unsubstituted parent compound and typically employed for analogs with pendant groups, yielding 70–85% under reflux in dichloromethane with second-generation ruthenium catalysts.[36] The Diels-Alder reaction often produces mixtures of endo and exo isomers, particularly with unsymmetrical dienophiles, necessitating separation for stereospecific applications. Preparative gas chromatography on silver nitrate-impregnated columns effectively resolves endo- and exo-norbornene derivatives (e.g., retention times differing by 1–2 min at 100–120°C), allowing isolation of pure isomers in gram quantities with >98% purity. Selective crystallization from solvents like ethanol or petroleum ether can also purify the endo isomer of norbornene dicarboxylic anhydride (m.p. 145–150°C), exploiting its lower solubility compared to the exo form, while lab-scale yields for purified fractions reach 70–80% overall. These techniques ensure access to stereochemically defined materials for subsequent reactions.[37]Reactions
Polymerization Reactions
Norbornene can be polymerized through ring-opening metathesis polymerization (ROMP), a chain-growth process initiated by transition metal carbene complexes that exploit the molecule's ring strain to open the cyclic olefin, forming a linear polymer while preserving the number of double bonds as a polyalkenamer.[38] Schrock-type catalysts, based on molybdenum or tungsten, were among the first highly active systems for this reaction, proceeding via a [2+2] cycloaddition between the metal carbene and the norbornene double bond, followed by cycloreversion to propagate the chain.[38] Ruthenium-based Grubbs' catalysts, particularly second- and third-generation variants, offer greater functional group tolerance and enable living polymerization, yielding polymers with mixed cis and trans double bonds in the backbone (typically favoring trans under standard conditions).[38] ROMP of norbornene occurs readily at room temperature in organic solvents such as dichloromethane or toluene, with catalyst loadings as low as 0.1 mol% producing high molecular weight polynorbornenes exceeding 1,000,000 g/mol and narrow polydispersities when using Grubbs' third-generation catalysts.[38] The endo and exo stereoisomers of norbornene exhibit differing reactivities and influence polymer tacticity; the endo isomer, with its inward-oriented double bond, often leads to more syndiotactic sequences due to steric interactions during propagation, while exo favors isotactic tendencies in controlled systems.[39] These stereochemical effects arise from the monomer's bicyclic rigidity, which directs approach angles in the catalytic cycle. In contrast, vinyl-addition polymerization of norbornene employs late transition metal catalysts such as nickel, palladium, or iron complexes with chelating (pyridyl)imine or pincer ligands, activated by methylaluminoxane (MMAO), to insert the monomer across the metal-carbon bond via coordination-insertion mechanism, resulting in a saturated backbone of linked bicyclic units.[41] This process retains the norbornane structure without incorporating double bonds in the main chain, though minor ROMP side reactions can introduce unsaturation suitable for subsequent crosslinking in copolymer applications.[41] Optimal conditions include non-polar solvents like chlorobenzene at 25°C with Al/M ratios of 1000–1500, yielding polymers with molecular weights up to 1,180,000 g/mol and polydispersities of 1.7–2.5, where nickel catalysts show the highest activities (up to 81,900 g PNBE mol⁻¹ h⁻¹).[41] Ethylidene norbornene (ENB), a diene derivative of norbornene, is commonly used as a comonomer in the terpolymerization with ethylene and propylene to produce EPDM rubbers, where its exocyclic double bond provides pendant unsaturation sites for efficient sulfur vulcanization and crosslinking.[42] This incorporation enhances the rubber's processability and cure rate without significantly altering the saturated backbone.[42] Recent advancements in 2025 have focused on stereoselective vinyl-addition polymerization using CNN and PCN pincer Pd(II) complexes, enabling the production of ultra-high molecular weight endo-polynorbornenes with activities reaching 9.6 × 10⁶ g PNB mol⁻¹ Pd h⁻¹ at 20–40°C and near-quantitative conversions (up to 99.5%) in the presence of Et₂AlCl or MAO cocatalysts.[43] These systems demonstrate improved control over tacticity for endo-enriched monomers, yielding thermally stable polymers (decomposition >400°C) with vinyl-type microstructures.[43]Addition and Substitution Reactions
Norbornene undergoes acid-catalyzed hydration to form exo-norborneol as the major product through a Markovnikov addition mechanism involving protonation of the double bond, followed by water addition to the resulting carbocation intermediate. This stereoselectivity arises from the preference for exo attack due to the bicyclic structure, which minimizes steric hindrance and aligns with the stability of the bridged carbocation species. The overall reaction is represented as: This process highlights the reactivity of norbornene's strained double bond toward electrophilic addition, consistent with its general chemical properties.[44] In radical-mediated processes, norbornene participates in thiol-ene click reactions, where thiols add across the double bond to form thioether linkages via a step-growth mechanism initiated by photo or thermal radicals.[45] The reaction proceeds through thiyl radical addition to the alkene, followed by hydrogen abstraction, yielding high-efficiency coupling suitable for crosslinking in material synthesis.[46] This orthogonal chemistry exploits norbornene's electron-rich alkene for selective functionalization, though in certain conditions such as photoinitiated hydrogel formation, norbornene homopolymerization can occur as a competing side reaction.[47] Palladium-catalyzed substitution reactions of norbornene enable advanced arene functionalizations, notably through the Catellani reaction, where norbornene acts as a transient mediator in the ortho-functionalization of aryl halides. In this process, oxidative addition of the aryl halide to Pd(0) forms a palladacycle with norbornene via migratory insertion, directing subsequent ortho C-H activation and coupling with nucleophiles or electrophiles, ultimately regenerating norbornene catalytically. This cooperative catalysis allows selective installation of diverse groups at the ortho position, demonstrating norbornene's role in facilitating remote C-H bond activation. Recent extensions (as of 2025) include triple cross-electrophile couplings using Pd/norbornene catalysis for multi-component arene functionalization.[48] The solvolysis of norbornyl derivatives derived from norbornene leads to the formation of the 2-norbornyl cation, a landmark example of a non-classical carbocation characterized by σ-delocalization and Wagner-Meerwein rearrangements. Upon ionization, the cation exhibits partial bridging between C1-C2 and C6, resulting in equivalent bridgehead and exo/endo positions, as evidenced by NMR and kinetic studies showing accelerated rates due to anchimeric assistance.[44] This rearrangement underscores the unique electronic structure imposed by norbornene's bicyclic framework. Norbornene-mediated meta-C-H activation extends substitution capabilities to distal positions on arenes using directing groups, leveraging Pd/norbornene cooperative catalysis to relay palladation from ortho to meta sites.[49] In this strategy, an ortho-directed palladacycle inserts norbornene, enabling a second C-H activation at the meta position for arylation or alkylation, with norbornene extruded after functionalization. This method achieves high regioselectivity for simple directing groups like amines or amides, advancing synthetic efficiency in arene diversification.Applications
Polymer Materials
Polynorbornene materials are primarily produced through two polymerization routes: ring-opening metathesis polymerization (ROMP) and vinyl addition polymerization. ROMP-derived polynorbornenes, such as Norsorex, are elastomeric with a glass transition temperature (Tg) of approximately 35–45 °C and exhibit optical clarity, making them suitable for applications requiring flexibility and transparency. In contrast, addition polymers like Avatrel feature a rigid polycyclic backbone, yielding high Tg values exceeding 200 °C and low dielectric constants (around 2.3–2.6), which position them as effective low-k dielectrics in microelectronics.[50][51] These materials demonstrate robust mechanical properties, including tensile strengths typically in the range of 24–60 MPa and low gas permeability coefficients (e.g., 2.5 Barrer for oxygen), which contribute to their durability and barrier performance.[52][53] Polynorbornene's inherent vibration-damping characteristics, stemming from its viscoelastic behavior, enable its use in anti-vibration rubber compounds for rail fasteners and automotive components.[54] Processing of polynorbornene-based materials often involves solution casting to form thin films for dielectric applications or extrusion for bulk elastomeric parts, allowing compatibility with techniques like injection molding.[55] For elastomer formulations, crosslinking with organic peroxides enhances network formation, improving elasticity and thermal resistance without compromising processability.[56] C copolymers of norbornene derivatives, such as ethylene-propylene-diene monomer (EPDM) incorporating ethylidene norbornene (ENB) as the diene, are widely used in automotive seals due to their weather resistance and flexibility.[57] Recent advances in 2025 have introduced stereoisomer-specific polydichloronorbornene (PDCNB), where exo- and endo-isomers exhibit Tg values above 300 °C and wide bandgaps (4.3 eV), enabling high-temperature capacitive energy storage with enhanced dielectric performance.[58] Polynorbornene materials offer thermal stability up to 350 °C, attributed to their robust bicyclic structure, but they are susceptible to UV-induced degradation due to the presence of unsaturated bonds, leading to chain scission and reduced mechanical integrity.[59][60]Other Industrial and Medicinal Uses
Norbornene derivatives serve as valuable scaffolds in pharmaceutical synthesis, particularly for anticancer drug development through click chemistry approaches. For instance, norbornene-based structures enable bioorthogonal labeling via inverse electron-demand Diels-Alder reactions with tetrazines, facilitating targeted drug conjugation and delivery systems. Specific examples include biperiden, which reduces pancreatic cancer tumor size by 83% in mouse models by inhibiting MALT1 protease activity, and endo-IWR-1, which suppresses Wnt/β-catenin signaling in colon and osteosarcoma cells, enhancing efficacy when combined with doxorubicin (IC₅₀ values improved by up to 10-fold).[61] Additionally, norcantharidin derivatives promote apoptosis and autophagy in hepatocellular carcinoma, prostate, and colon cancers by modulating key signaling pathways.[62] In agriculture, norbornene acts as a precursor for pesticide ingredients and other agrochemical compounds, contributing to the synthesis of active agents for crop protection.[63] Norbornene derivatives find applications in electronics and energy sectors, including as components in microelectronics adhesives and solar cell encapsulants, where their cyclic structure provides thermal stability and bonding efficiency. Exo-polynorbornene variants are utilized in gas separation membranes, offering tunable permeability—for example, CO₂ permeability ranging from 28 to 104 Barrer with selectivities around 13–19 for CO₂/CH₄—enabling efficient separation in industrial processes.[64] Ferrocene-norbornene hybrid polymeric materials exhibit redox stability, >95% transmittance in the 550–1000 nm range, and 28% optical contrast at 420 nm, suitable for electrochromic devices in energy applications.[65] Beyond these, norbornene is employed in specialty fragrances, leveraging its bridged cyclic motif for scent profile enhancement in perfumes and cosmetics.[63] Derivatives such as pentacyclo[8.2.1.1⁴,⁷.0²,⁹.0³,⁸]tetradecane (PCTD), synthesized via photoinduced [2+2] cycloaddition of norbornene, serve as high-energy-density additives for rocket fuels, achieving a density of 1.024 g/mL and volumetric net heat of combustion of 42.98 MJ/L, outperforming conventional JP-10 in aerospace propulsion.[66] Safety considerations for handling norbornene include its flammability, with a flash point of -8 °C, necessitating storage away from ignition sources and use of explosion-proof equipment. It causes serious eye irritation and requires protective gloves, eyewear, and handling under a fume hood to minimize inhalation and skin exposure risks.[67] Thiol-ene derivatives of norbornene enable ultrafast photo-crosslinking (<1 s gelation) in air for opaque silicone elastomers and nanocomposites, suitable for high-throughput industrial coatings, soft robotics components, and additive manufacturing in automotive parts.[68]References
- https://www.[researchgate](/page/ResearchGate).net/publication/324592946_EndoExo_Reactivity_Ratios_in_Living_Vinyl_Addition_Polymerization_of_Substituted_Norbornenes