Recent from talks
Nothing was collected or created yet.
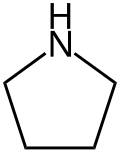
Pyrrolidine
View on Wikipedia
| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
Pyrrolidine[1] | |||
| Other names
Azolidine
Azacyclopentane Tetrahydropyrrole Prolamine Azolane | |||
| Identifiers | |||
3D model (JSmol)
|
|||
| 102395 | |||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
| ECHA InfoCard | 100.004.227 | ||
| EC Number |
| ||
| 1704 | |||
PubChem CID
|
|||
| RTECS number |
| ||
| UNII | |||
| UN number | 1922 | ||
CompTox Dashboard (EPA)
|
|||
| |||
| |||
| Properties | |||
| C4H9N | |||
| Molar mass | 71.123 g·mol−1 | ||
| Appearance | Clear colorless liquid | ||
| Density | 0.866 g/cm3 | ||
| Melting point | −63 °C (−81 °F; 210 K) | ||
| Boiling point | 87 °C (189 °F; 360 K) | ||
| Miscible | |||
| Acidity (pKa) | 11.27 (pKa of conjugate acid in water),[2] 19.56 (pKa of conjugate acid in acetonitrile)[3] | ||
| −54.8·10−6 cm3/mol | |||
Refractive index (nD)
|
1.4402 at 28°C | ||
| Hazards | |||
| Occupational safety and health (OHS/OSH): | |||
Main hazards
|
highly flammable, harmful, corrosive, possible mutagen | ||
| GHS labelling: | |||
  
| |||
| Danger | |||
| H225, H302, H314, H332 | |||
| P210, P233, P240, P241, P242, P243, P260, P261, P264, P270, P271, P280, P301+P312, P301+P330+P331, P303+P361+P353, P304+P312, P304+P340, P305+P351+P338, P310, P312, P321, P330, P363, P370+P378, P403+P235, P405, P501 | |||
| NFPA 704 (fire diamond) | |||
| Flash point | 3 °C (37 °F; 276 K) | ||
| 345 °C (653 °F; 618 K) | |||
| Safety data sheet (SDS) | MSDS | ||
| Related compounds | |||
Related nitrogen heterocyclic compounds
|
Pyrrole (aromatic with two double bonds) Pyrroline (one double bond) Pyrrolizidine (two pentagonal rings) | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||
Pyrrolidine, also known as tetrahydropyrrole, is an organic compound with the molecular formula (CH2)4NH. It is a cyclic secondary amine, also classified as a saturated heterocycle. It is a colourless liquid that is miscible with water and most organic solvents. It has a characteristic odor that has been described as "ammoniacal, fishy, shellfish-like".[4] In addition to pyrrolidine itself, many substituted pyrrolidines are known.
Production and synthesis
[edit]Industrial production
[edit]Pyrrolidine is prepared industrially by the reaction of 1,4-butanediol and ammonia at a temperature of 165–200 °C and a pressure of 17–21 MPa in the presence of a cobalt- and nickel oxide catalyst, which is supported on alumina.[5]
The reaction is carried out in the liquid phase in a continuous tube- or tube bundle reactor, which is operated in the cycle gas method. The catalyst is arranged as a fixed-bed and the conversion is carried out in the downflow mode. The product is obtained after multistage purification and separation by extractive and azeotropic distillation.[5]
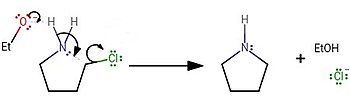
Laboratory synthesis
[edit]In the laboratory, pyrrolidine was usually synthesised by treating 4-chlorobutan-1-amine with a strong base:
Furthermore, 5-membered N-heterocyclic ring of the pyrrolidine derivatives can be synthesized via cascade reactions.[6]
Occurrence
[edit]Many modifications of pyrrolidine are found in natural and synthetic drugs and drug candidates.[6] The pyrrolidine ring structure is present in numerous natural alkaloids i.a. nicotine and hygrine. It is found in many drugs such as procyclidine and bepridil. It also forms the basis for the racetam compounds (e.g. piracetam, aniracetam). The amino acids proline and hydroxyproline are, in a structural sense, derivatives of pyrrolidine.
Reactions
[edit]Pyrrolidine is a base. Its basicity is typical of other dialkyl amines.[7] Relative to many secondary amines, pyrrolidine is distinctive because of its compactness, a consequence of its cyclic structure.
Pyrrolidine is used as a building block in the synthesis of more complex organic compounds. It is used to activate ketones and aldehydes toward nucleophilic addition by formation of enamines (e.g. used in the Stork enamine alkylation):[8]
References
[edit]- ^ International Union of Pure and Applied Chemistry (2014). Nomenclature of Organic Chemistry: IUPAC Recommendations and Preferred Names 2013. The Royal Society of Chemistry. p. 142. doi:10.1039/9781849733069. ISBN 978-0-85404-182-4.
- ^ Hall, H. K. (1957). "Correlation of the Base Strengths of Amines". Journal of the American Chemical Society. 79 (20): 5441–5444. Bibcode:1957JAChS..79.5441H. doi:10.1021/ja01577a030.
- ^ Kaljurand, I.; Kütt, A.; Sooväli, L.; Rodima, T.; Mäemets, V.; Leito, I.; Koppel, I. A. (2005). "Extension of the Self-Consistent Spectrophotometric Basicity Scale in Acetonitrile to a Full Span of 28 pKa Units: Unification of Different Basicity Scales". The Journal of Organic Chemistry. 70 (3): 1019–1028. doi:10.1021/jo048252w. PMID 15675863.
- ^ Pyrrolidine Archived 2017-11-21 at the Wayback Machine, The Good Scents Company
- ^ a b Bou Chedid, Roland; Melder, Johann-Peter; Dostalek, Roman; Pastre, Jörg; Tan, Aik Meam. "Process for the preparation of pyrrolidine". Google Patents. BASF SE. Archived from the original on 5 July 2019. Retrieved 5 July 2019.
- ^ a b Łowicki, Daniel; Przybylski, Piotr (2022). "Tandem construction of biological relevant aliphatic 5-membered N-heterocycles". European Journal of Medicinal Chemistry. 235 114303. doi:10.1016/j.ejmech.2022.114303. PMID 35344904. S2CID 247580048.
- ^ H. K. Hall Jr. (1957). "Correlation of the Base Strengths of Amines". J. Am. Chem. Soc. 79 (20): 5441. Bibcode:1957JAChS..79.5441H. doi:10.1021/ja01577a030.
- ^ R. B. Woodward, I. J. Pachter, and M. L. Scheinbaum (1974). "2,2-(Trimethylenedithio)cyclohexanone". Organic Syntheses. 54: 39
{{cite journal}}: CS1 maint: multiple names: authors list (link); Collected Volumes, vol. 6, p. 1014.
External links
[edit] Media related to Pyrrolidine at Wikimedia Commons
Media related to Pyrrolidine at Wikimedia Commons
Pyrrolidine
View on GrokipediaStructure and properties
Molecular structure
Pyrrolidine is an organic compound with the molecular formula C₄H₉N, featuring a five-membered saturated heterocyclic ring composed of four methylene (CH₂) groups and one secondary amine (NH) group.[1] This cyclic secondary amine structure positions the nitrogen atom within the ring, connected to two carbon atoms and bearing a hydrogen atom.[6] The preferred IUPAC name is pyrrolidine; azolidine is a systematic alternative, and it is also commonly referred to as tetrahydropyrrole.[1][7] In terms of atomic hybridization and geometry, all carbon and nitrogen atoms in the pyrrolidine ring exhibit sp³ hybridization, resulting in bond angles approximating the ideal tetrahedral value of 109.5°.[8] The ring adopts a puckered envelope conformation to minimize angle strain, with the nitrogen atom often positioned out of the plane formed by the adjacent four carbon atoms, as determined by ab initio calculations and electron diffraction studies.[9] This flexible pseudorotational behavior allows the ring to interconvert between envelope forms, contributing to its structural dynamics.[9] Unlike its aromatic counterpart pyrrole (C₄H₅N), which features a conjugated π-system where the nitrogen lone pair participates in delocalization, pyrrolidine's full saturation renders it non-aromatic and enables the lone pair on nitrogen to remain available in an sp³ orbital for protonation and basic behavior.[10] Similarly, compared to pyrroline, the partially unsaturated analog with one C=C double bond, pyrrolidine lacks such unsaturation, emphasizing its aliphatic nature. The structural diagram of pyrrolidine can be depicted as a pentagon with the nitrogen atom at one vertex bonded to two adjacent CH₂ groups, and the remaining three CH₂ groups completing the saturated ring.[11]Physical properties
Pyrrolidine appears as a clear, colorless to pale yellow liquid at room temperature, characterized by a strong ammoniacal, fishy odor.[12] Its molecular formula is C₄H₉N, with a molar mass of 71.12 g/mol.[12] The compound exhibits a dipole moment of 1.57 D, arising from the asymmetry of its five-membered ring structure.[13] Under standard conditions, pyrrolidine has a density of 0.86 g/cm³ at 20 °C and a refractive index of 1.443 at 20 °C.[14] It melts at -63 °C and boils at 87 °C, with the low melting point attributable to the flexible ring structure that limits intermolecular forces.[7] The vapor pressure is 128 mmHg at 39 °C.[14] Pyrrolidine is miscible with water and most organic solvents such as ethanol, chloroform, and ether.[15] It has a flash point of 3 °C (closed cup) and an autoignition temperature of 345 °C.[16]| Property | Value | Conditions/Source |
|---|---|---|
| Density | 0.86 g/cm³ | 20 °C [Sigma-Aldrich] |
| Refractive index | 1.443 | n₂₀/D [Sigma-Aldrich] |
| Melting point | -63 °C | [ChemicalBook] |
| Boiling point | 87 °C | [Sigma-Aldrich] |
| Vapor pressure | 128 mmHg | 39 °C [Sigma-Aldrich] |
| Flash point | 3 °C | Closed cup [Sigma-Aldrich] |
| Autoignition temperature | 345 °C | [Fisher Scientific] |