Bob
Have a question related to this hub?
Alice
Got something to say related to this hub?
Share it here.
 | |
| Names | |
|---|---|
| Other names
TL-358
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
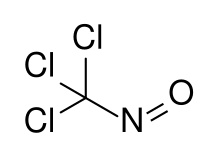
| CCl3NO | |
| Molar mass | 148.37 g·mol−1 |
| Appearance | Deep blue liquid |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Trichloronitrosomethane is a chlorinated nitrosoalkane. It is a deep blue liquid with powerful lachrymatory effects.[1]
Trichloronitrosomethane can be produced with following methods:[1][2]
Trichloronitrosomethane is an unstable substance. It slowly decomposes into nitrosyl chloride, nitrogen oxides, and chloropicrin over time.[1]
Trichloronitrosomethane can be reduced to phosgene oxime by hydrogen sulfide.[1]