Recent from talks
Nothing was collected or created yet.
Factor IX
View on Wikipedia
| F9 | |||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 | |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| Identifiers | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Aliases | F9, F9 p22, FIX, HEMB, P19, PTC, THPH8, coagulation factor IX, Blood coagulation factor IX, Christmas Factor | ||||||||||||||||||||||||||||||||||||||||||||||||||
| External IDs | OMIM: 300746; MGI: 88384; HomoloGene: 106; GeneCards: F9; OMA:F9 - orthologs | ||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| Wikidata | |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
Factor IX (EC 3.4.21.22), also known as Christmas factor, is one of the serine proteases involved in coagulation; it belongs to peptidase family S1. Deficiency of this protein causes haemophilia B.
It was discovered in 1952 after a young boy named Stephen Christmas was found to be lacking this exact factor, leading to haemophilia.[5] Coagulation factor IX is on the World Health Organization's List of Essential Medicines.[6]
Physiology
[edit]
Factor IX is produced as a zymogen, an inactive precursor. It is processed to remove the signal peptide, glycosylated and then cleaved by factor XIa (of the contact pathway) or factor VIIa (of the tissue factor pathway) to produce a two-chain form, where the chains are linked by a disulfide bridge.[7][8] When activated into factor IXa, in the presence of Ca2+, membrane phospholipids, and a Factor VIII cofactor, it hydrolyses one arginine-isoleucine bond in factor X to form factor Xa.
Factor IX is inhibited by antithrombin.[7]
Factor IX expression increases with age in humans and mice. In mouse models, mutations within the promoter region of factor IX have an age-dependent phenotype.[9]
Domain architecture
[edit]Factors VII, IX, and X all play key roles in blood coagulation and also share a common domain architecture.[10] The factor IX protein is composed of four protein domains: the Gla domain, two tandem copies of the EGF domain and a C-terminal trypsin-like peptidase domain which carries out the catalytic cleavage.

The N-terminal EGF domain has been shown to at least in part be responsible for binding tissue factor.[10] Wilkinson et al. conclude that residues 88 to 109 of the second EGF domain mediate binding to platelets and assembly of the factor X activating complex.[11]
The structures of all four domains have been solved. A structure of the two EGF domains and the trypsin-like domain was determined for the pig protein.[12] The structure of the Gla domain, which is responsible for Ca(II)-dependent phospholipid binding, was also determined by NMR.[13]
Several structures of 'super active' mutants have been solved,[14] which reveal the nature of factor IX activation by other proteins in the clotting cascade.
Genetics
[edit]
Because the gene for factor IX is located on the X chromosome (Xq27.1-q27.2), loss-of-function mutations thereof are X-linked recessive: males experience the disease phenotype much more frequently than females. At least 534 disease-causing mutations in this gene have been discovered.[15] The F9 gene was first cloned in 1982 by Kotoku Kurachi and Earl Davie.[16]
Polly, a transgenic cloned Poll Dorset sheep carrying the gene for factor IX, was produced by Dr Ian Wilmut at the Roslin Institute in 1997.[17]
Role in disease
[edit]
| Clinical data | |
|---|---|
| Trade names | Benefix |
| License data | |
| ATC code |
|
| Legal status | |
| Legal status |
|
| Clinical data | |
|---|---|
| Trade names | Rixubis |
| Routes of administration | Intravenous |
| ATC code |
|
| Legal status | |
| Legal status |
|
| Clinical data | |
|---|---|
| Trade names | Idelvion |
| License data | |
| ATC code |
|
| Legal status | |
| Legal status | |
| Clinical data | |
|---|---|
| Trade names | Alprolix |
| License data | |
| ATC code |
|
| Legal status | |
| Legal status | |
| Clinical data | |
|---|---|
| Trade names | Refixia |
| ATC code |
|
| Legal status | |
| Legal status | |
Deficiency of factor IX causes Christmas disease (hemophilia B).[5] Over 3000 variants of factor IX have been described, affecting 73% of the 461 residues;[22] some cause no symptoms, but many lead to a significant bleeding disorder. The original Christmas disease mutation was identified by sequencing of Christmas' DNA, revealing a mutation which changed a cysteine to a serine.[23] Recombinant factor IX is used to treat Christmas disease. Formulations include:
- nonacog alfa (brand name Benefix)[24]
- nonacog gamma (brand name Rixubis)[18]
- albutrepenonacog alfa (brand name Idelvion)[25]
- eftrenonacog alfa (brand name Alprolix)[26]
- nonacog beta pegol (brand name Refixia)[27]
- coagulation factor IX [recombinant] (Benefix)[28]
- coagulation factor IX [recombinant] (Idelvion)[29]
- coagulation factor IX (recombinant), Fc fusion protein (Alprolix)[30]
- coagulation factor IX [recombinant] (Ixinity)[31][32]
- coagulation factor IX [recombinant] (Rebinyn)[33]
- coagulation factor IX [recombinant] (Rixubis)[34]
- coagulation factor IX (human) (Alphanine SD)[35]
Some rare mutations of factor IX result in elevated clotting activity, and can result in clotting diseases, such as deep vein thrombosis. This gain of function mutation renders the protein hyperfunctional and is associated with familial early-onset thrombophilia.[36]
Factor IX deficiency is treated by injection of purified factor IX produced through cloning in various animal or animal cell vectors. Tranexamic acid may be of value in patients undergoing surgery who have inherited factor IX deficiency in order to reduce the perioperative risk of bleeding.[37]
A list of all the mutations in Factor IX is compiled and maintained by EAHAD.[38]
Coagulation factor IX is on the World Health Organization's List of Essential Medicines.[6]
References
[edit]- ^ a b c GRCh38: Ensembl release 89: ENSG00000101981 – Ensembl, May 2017
- ^ a b c GRCm38: Ensembl release 89: ENSMUSG00000031138 – Ensembl, May 2017
- ^ "Human PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- ^ "Mouse PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- ^ a b Biggs R, Douglas AS, Macfarlane RG, Dacie JV, Pitney WR (December 1952). "Christmas disease: a condition previously mistaken for haemophilia". British Medical Journal. 2 (4799): 1378–82. doi:10.1136/bmj.2.4799.1378. PMC 2022306. PMID 12997790.
- ^ a b World Health Organization (2025). The selection and use of essential medicines, 2025: WHO Model List of Essential Medicines, 24th list. Geneva: World Health Organization. doi:10.2471/B09474. hdl:10665/382243.
- ^ a b Di Scipio RG, Kurachi K, Davie EW (June 1978). "Activation of human factor IX (Christmas factor)". The Journal of Clinical Investigation. 61 (6): 1528–38. doi:10.1172/JCI109073. PMC 372679. PMID 659613.
- ^ Taran LD (July 1997). "Factor IX of the blood coagulation system: a review". Biochemistry. Biokhimiia. 62 (7): 685–93. PMID 9331959.
- ^ Boland EJ, Liu YC, Walter CA, Herbert DC, Weaker FJ, Odom MW, et al. (September 1995). "Age-specific regulation of clotting factor IX gene expression in normal and transgenic mice". Blood. 86 (6): 2198–205. doi:10.1182/blood.V86.6.2198.bloodjournal8662198. PMID 7662969.
- ^ a b Zhong D, Bajaj MS, Schmidt AE, Bajaj SP (February 2002). "The N-terminal epidermal growth factor-like domain in factor IX and factor X represents an important recognition motif for binding to tissue factor". The Journal of Biological Chemistry. 277 (5): 3622–31. doi:10.1074/jbc.M111202200. PMID 11723140.
- ^ Wilkinson FH, Ahmad SS, Walsh PN (February 2002). "The factor IXa second epidermal growth factor (EGF2) domain mediates platelet binding and assembly of the factor X activating complex". The Journal of Biological Chemistry. 277 (8): 5734–41. doi:10.1074/jbc.M107753200. PMID 11714704.
- ^ Brandstetter H, Bauer M, Huber R, Lollar P, Bode W (October 1995). "X-ray structure of clotting factor IXa: active site and module structure related to Xase activity and hemophilia B". Proceedings of the National Academy of Sciences of the United States of America. 92 (21): 9796–800. Bibcode:1995PNAS...92.9796B. doi:10.1073/pnas.92.21.9796. PMC 40889. PMID 7568220.
- ^ Freedman SJ, Furie BC, Furie B, Baleja JD (September 1995). "Structure of the calcium ion-bound gamma-carboxyglutamic acid-rich domain of factor IX". Biochemistry. 34 (38): 12126–37. doi:10.1021/bi00038a005. PMID 7547952.
- ^ Zögg T, Brandstetter H (December 2009). "Structural basis of the cofactor- and substrate-assisted activation of human coagulation factor IXa". Structure. 17 (12): 1669–78. doi:10.1016/j.str.2009.10.011. PMID 20004170.
- ^ Šimčíková D, Heneberg P (December 2019). "Refinement of evolutionary medicine predictions based on clinical evidence for the manifestations of Mendelian diseases". Scientific Reports. 9 (1) 18577. Bibcode:2019NatSR...918577S. doi:10.1038/s41598-019-54976-4. PMC 6901466. PMID 31819097.
- ^ Kurachi K, Davie EW (November 1982). "Isolation and characterization of a cDNA coding for human factor IX". Proceedings of the National Academy of Sciences of the United States of America. 79 (21): 6461–4. Bibcode:1982PNAS...79.6461K. doi:10.1073/pnas.79.21.6461. PMC 347146. PMID 6959130.
- ^ Nicholl D. (2002). An Introduction to Genetic Engineering Second Edition. Cambridge University Press. p. 257.
- ^ a b "Rixubis EPAR". European Medicines Agency (EMA). 19 December 2014. Retrieved 1 June 2024.
- ^ "Health Canada New Drug Authorizations: 2016 Highlights". Health Canada. 14 March 2017. Retrieved 7 April 2024.
- ^ "Alprolix EPAR". European Medicines Agency (EMA). 8 June 2007. Retrieved 7 June 2024.
- ^ "Refixia (Novo Nordisk Pharmaceuticals Pty Ltd)". Therapeutic Goods Administration (TGA). 13 September 2024. Retrieved 15 September 2024.
- ^ Goodeve AC (2015). "Hemophilia B: Molecular pathogenesis and mutation analysis". Journal of Thrombosis and Haemostasis. 13 (7): 1184–1195. doi:10.1111/jth.12958. PMC 4496316. PMID 25851415.
- ^ Taylor SA, Duffin J, Cameron C, Teitel J, Garvey B, Lillicrap DP (January 1992). "Characterization of the original Christmas disease mutation (cysteine 206----serine): from clinical recognition to molecular pathogenesis". Thrombosis and Haemostasis. 67 (1): 63–5. doi:10.1055/s-0038-1648381. PMID 1615485. S2CID 25251813.
- ^ "Benefix EPAR". European Medicines Agency (EMA). 17 September 2018. Archived from the original on 17 June 2020. Retrieved 17 June 2020.
- ^ "Idelvion EPAR". European Medicines Agency (EMA). 17 September 2018. Archived from the original on 17 June 2020. Retrieved 17 June 2020.
- ^ "Alprolix EPAR". European Medicines Agency (EMA). 17 September 2018. Archived from the original on 11 August 2020. Retrieved 17 June 2020.
- ^ "Refixia EPAR". European Medicines Agency (EMA). 17 September 2018. Archived from the original on 18 June 2020. Retrieved 17 June 2020.
- ^ "Benefix (coagulation factor ix- recombinant kit". DailyMed. 1 March 2023. Archived from the original on 29 January 2023. Retrieved 23 March 2024.
- ^ "Idelvion- coagulation factor ix recombinant human kit". DailyMed. 30 June 2023. Archived from the original on 27 January 2023. Retrieved 23 March 2024.
- ^ "Alprolix (coagulation factor ix- recombinant, fc fusion protein kit". DailyMed. 25 May 2023. Archived from the original on 7 February 2023. Retrieved 23 March 2024.
- ^ "Ixinity (coagulation factor ix- recombinant kit". DailyMed. 23 February 2021. Archived from the original on 28 September 2023. Retrieved 23 March 2024.
- ^ "Ixinity (coagulation factor ix- recombinant kit". DailyMed. 9 January 2024. Archived from the original on 3 December 2022. Retrieved 23 March 2024.
- ^ "Rebinyn ((coagulation factor ix- recombinant, glycopegylated kit". DailyMed. 11 August 2022. Archived from the original on 29 November 2022. Retrieved 23 March 2024.
- ^ "Rixubis (coagulation factor ix- recombinant kit". DailyMed. 22 March 2023. Archived from the original on 2 July 2022. Retrieved 23 March 2024.
- ^ "Alphanine SD (coagulation factor ix- human kit". DailyMed. 18 January 2024. Archived from the original on 18 February 2024. Retrieved 23 March 2024.
- ^ Simioni P, Tormene D, Tognin G, Gavasso S, Bulato C, Iacobelli NP, et al. (October 2009). "X-linked thrombophilia with a mutant factor IX (factor IX Padua)". The New England Journal of Medicine. 361 (17): 1671–5. doi:10.1056/NEJMoa0904377. hdl:11577/2438365. PMID 19846852.
- ^ Rossi M, Jayaram R, Sayeed R (September 2011). "Do patients with haemophilia undergoing cardiac surgery have good surgical outcomes?". Interactive Cardiovascular and Thoracic Surgery. 13 (3): 320–31. doi:10.1510/icvts.2011.272401. PMID 21712351.
- ^ "Home: EAHAD Factor 9 Gene Variant Database". Archived from the original on 28 October 2020. Retrieved 23 October 2020.
Further reading
[edit]- Davie EW, Fujikawa K (1975). "Basic mechanisms in blood coagulation". Annual Review of Biochemistry. 44: 799–829. doi:10.1146/annurev.bi.44.070175.004055. PMID 237463.
- Sommer SS (July 1992). "Assessing the underlying pattern of human germline mutations: lessons from the factor IX gene". FASEB Journal. 6 (10): 2767–74. doi:10.1096/fasebj.6.10.1634040. PMID 1634040. S2CID 15211597.
- Lenting PJ, van Mourik JA, Mertens K (December 1998). "The life cycle of coagulation factor VIII in view of its structure and function". Blood. 92 (11): 3983–96. doi:10.1182/blood.V92.11.3983. PMID 9834200.
- Lowe GD (December 2001). "Factor IX and thrombosis" (PDF). British Journal of Haematology. 115 (3): 507–13. doi:10.1046/j.1365-2141.2001.03186.x. PMID 11736930. S2CID 44650866. Archived (PDF) from the original on 19 June 2021. Retrieved 11 December 2019.
- O'Connell NM (June 2003). "Factor XI deficiency--from molecular genetics to clinical management". Blood Coagulation & Fibrinolysis. 14 (Suppl 1): S59-64. doi:10.1097/00001721-200306001-00014. PMID 14567539.
- Du X (May 2007). "Signaling and regulation of the platelet glycoprotein Ib-IX-V complex". Current Opinion in Hematology. 14 (3): 262–9. doi:10.1097/MOH.0b013e3280dce51a. PMID 17414217. S2CID 39904506.
External links
[edit]- Overview of all the structural information available in the PDB for UniProt: P00740 (Coagulation factor IX) at the PDBe-KB.
- GeneReviews/NCBI/NIH/UW entry on Hemophilia B
- The MEROPS online database for peptidases and their inhibitors: S01.214 Archived 2005-05-05 at the Wayback Machine
Factor IX
View on GrokipediaStructure and Biochemistry
Domain Architecture
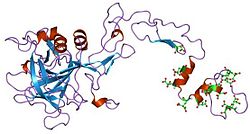
Factor IX is a single-chain zymogen glycoprotein comprising 415 amino acids and exhibiting a molecular weight of approximately 55 kDa.[6] This structure is synthesized in the liver as an inactive precursor, featuring a modular domain organization essential for its role in hemostasis. The protein includes an N-terminal gamma-carboxyglutamic acid (Gla) domain (residues 1–40), which facilitates binding to calcium ions and phospholipid membranes; two tandem epidermal growth factor-like (EGF) domains—EGF1 (residues 46–84) and EGF2 (residues 85–127)—that mediate specific protein-protein interactions; and a C-terminal serine protease domain (residues 181–415), which adopts a trypsin-like fold responsible for catalytic activity following zymogen activation.[7][8] The Gla domain contains 12 gamma-carboxylated glutamic acid residues critical for metal ion coordination, while the EGF domains each bind one calcium ion, contributing to structural rigidity.[9] Post-translational modifications are integral to Factor IX's functionality and stability. These include gamma-carboxylation of the 12 Gla residues in a vitamin K-dependent manner, N- and O-linked glycosylation at four sites (primarily Asn157, Asn167, and Asn262 for N-glycosylation, with additional O-glycosylation in the activation peptide), tyrosine sulfation at position 155, and multiple disulfide bonds—totaling 11 intra- and inter-domain linkages—that maintain the protein's compact conformation.[10][11][8] The sulfation at Tyr155, located in the EGF1 domain, enhances binding affinity to cofactors, while the disulfide bridges, such as the interchain link between Cys132 and Cys289 in the activated form, prevent unfolding under physiological conditions. Glycosylation contributes to approximately 17% of the protein's mass and influences secretion and clearance. Structural studies have elucidated the atomic details of these domains through complementary techniques. Nuclear magnetic resonance (NMR) spectroscopy has revealed the dynamic, largely unstructured nature of the Gla domain in solution without calcium, transitioning to a compact helix-loop-helix fold upon metal binding.[13] X-ray crystallography has provided high-resolution insights, including the EGF1 domain at 1.5 Å resolution, showcasing its beta-sheet core stabilized by a calcium-binding loop with six oxygen ligands from main-chain carbonyls and side chains.[14] The EGF2 and protease domains have been crystallized as part of activated Factor IXa at resolutions up to 1.37 Å, highlighting the 110° interdomain angle between EGF modules and the active-site geometry featuring the catalytic triad His221-Asp269-Ser365.[15] These structures underscore the evolutionary conservation of Factor IX across mammals, sharing approximately 80% sequence identity with mouse and 85-86% with dog orthologs, preserving key residues in the Gla and protease domains for functional equivalence.[16][17]Activation Mechanism
Factor IX, a zymogen serine protease, is primarily activated through limited proteolysis in two distinct pathways of the coagulation cascade. In the intrinsic pathway, Factor XIa initiates activation by cleaving the peptide bond after Arg^{145} (Arg^{145}-Ala^{146}), generating the intermediate Factor IXα, followed by cleavage after Arg^{180} (Arg^{180}-Val^{181}) to yield the fully active Factor IXaβ and release a 35-residue activation peptide (Ala^{146}-Arg^{180}).[18][19] This sequential process is calcium-dependent, with the γ-carboxyglutamic acid (Gla) domain of Factor IX binding to a specific exosite on the A3 domain of Factor XIa, enhancing catalytic efficiency for the second cleavage by approximately sevenfold compared to the first.[18] The reaction occurs in solution without requiring phospholipid surfaces, though platelet activation may facilitate it in vivo.[20] In the extrinsic pathway, the complex of Factor VIIa and tissue factor similarly activates Factor IX by cleaving the same Arg^{145}-Ala^{146} and Arg^{180}-Val^{181} bonds, providing an alternative route for rapid initiation of coagulation upon vascular injury.[21][22] This mechanism bypasses the contact activation steps and is particularly important for early thrombin burst generation.[23] Activation induces structural rearrangements, separating the light chain (Gla and epidermal growth factor-like domains) from the heavy chain (protease domain) via disulfide linkage while exposing the catalytic triad (His^{221}, Asp^{269}, Ser^{365}) in the active site of the heavy chain.[24][25] These allosteric changes transform the inactive zymogen into a functional enzyme, with the light chain facilitating membrane binding. The activated Factor IXa then assembles into the intrinsic tenase complex with Factor VIIIa on phospholipid surfaces (e.g., activated platelets), stabilized by calcium ions, which dramatically boosts its proteolytic activity toward Factor X.[26] In this complex, kinetic parameters for Factor X activation include a of approximately 0.2 μM and up to 20 s^{-1}, reflecting a million-fold enhancement over Factor IXa alone.[27] Minor activation pathways include limited proteolysis by plasmin, which can cleave Factor IX at similar sites under fibrinolytic conditions, though this often leads to subsequent inactivation.[28] Additionally, autoactivation of Factor IX occurs at high concentrations in vitro, potentially contributing to amplification in concentrated plasma environments.[29] For recombinant Factor IX used in hemophilia B therapeutics, production systems must ensure efficient γ-carboxylation and sulfation/phosphorylation at key residues (e.g., Tyr^{155}, Ser^{158}) to yield activation-competent forms with near-native activity, as deficiencies reduce recovery by 1.5–2-fold compared to plasma-derived products.[24] The EGF1 domain aids activation by mediating exosite interactions during cleavage.[30]Genetics
F9 Gene
The F9 gene, located on the long arm of the human X chromosome at cytogenetic band q27.1, spans approximately 34 kb of genomic DNA and consists of eight exons interrupted by seven introns. In the GRCh38.p14 human genome assembly, it occupies coordinates X:139,530,739-139,563,459 on the forward strand, encoding a primary transcript that produces a 461-amino-acid pre-pro-protein, including a 28-residue signal peptide and an 18-residue propeptide, which is processed to yield the mature 415-amino-acid Factor IX protein.[1][31][32] The promoter region of F9 is regulated by hepatocyte nuclear factor 4 alpha (HNF4α), a key liver-enriched transcription factor that binds to specific motifs to drive expression, alongside other factors such as C/EBP that contribute to tissue-specific control. Expression of F9 is predominantly restricted to hepatocytes in the liver, where it achieves high transcript levels (RPKM 181.1), with minimal detection in other tissues, reflecting its role in hepatic synthesis of coagulation factors. The primary mRNA transcript measures about 2.8 kb, undergoes standard splicing to remove introns, and produces a main isoform, though alternative splicing yields at least two variants encoding slightly different proteins, with such events being rare in normal physiology.[33][1][34] Evolutionarily, the F9 gene exhibits high conservation across vertebrates, with amino acid sequence homology reaching 83% between human and bovine Factor IX, and intron-exon boundaries largely preserved in mammalian orthologs, underscoring its ancient origin in the coagulation system. The gene was first cloned in 1982 through screening of a human liver cDNA library, yielding partial sequences that revealed the coding region, followed by publication of the full genomic sequence in 1984, which confirmed the eight-exon structure and enabled early studies of its expression.[31][35][32] Biosynthesis of Factor IX begins with transcription of F9 in hepatocytes, followed by translation of the pre-pro-mRNA into the pre-pro-protein on endoplasmic reticulum ribosomes, where the signal peptide is cleaved co-translationally to produce pro-Factor IX. Post-translational modifications occur primarily in the endoplasmic reticulum, including γ-carboxylation of 12 glutamic acid residues to γ-carboxyglutamic acid (Gla) by the vitamin K-dependent γ-glutamyl carboxylase enzyme, which requires reduced vitamin K as a cofactor; the protein then traffics to the Golgi apparatus for additional processing, such as O-glycosylation and sulfation, before secretion into plasma.[2][36]Mutations and Inheritance
Factor IX deficiency, known as hemophilia B, follows an X-linked recessive inheritance pattern, where the F9 gene is located on the X chromosome. Males, being hemizygous for the X chromosome, express the mutant allele and typically manifest the disease if they inherit the defective gene from their carrier mother. Females, who are heterozygous carriers, generally have sufficient functional Factor IX from their normal X chromosome and remain asymptomatic, though they transmit the mutation to 50% of their sons (who will be affected) and 50% of their daughters (who will be carriers).[31][37] The spectrum of genetic mutations in the F9 gene is diverse, with 1,692 unique pathogenic variants documented in comprehensive databases as of 2023. These include predominantly point mutations, accounting for approximately 73% of cases, with missense variants comprising the majority (around 58-66%) that alter amino acid sequences without abolishing protein production entirely. Nonsense mutations, which introduce premature stop codons, represent about 5-10%, while small deletions and insertions together make up roughly 15-20%, often leading to frameshifts and truncated proteins. Splice-site mutations, affecting about 8%, disrupt intron-exon boundaries and result in aberrant mRNA processing. Large gross deletions, occurring in 5-6% of cases, remove entire exons or the whole gene and are associated with severe phenotypes. The updated interactive F9 variant database, maintained by the Scientific and Standardization Committee (SSC) on Factor IX of the International Society on Thrombosis and Haemostasis (ISTH), serves as a key repository for these entries, facilitating genotype-phenotype correlations.[38][39][40] Mutations in F9 can be classified based on their impact on circulating Factor IX protein levels and activity, distinguishing between cross-reacting material positive (CRM+) variants, where antigen levels are normal but functional activity is reduced due to qualitative defects, and CRM- variants, characterized by both low antigen and low activity from quantitative deficiencies like null alleles. CRM+ mutations often involve missense changes in critical domains, such as the activation peptide or catalytic site; for instance, the Arg145His substitution impairs proteolytic cleavage by Factor XIa or VIIa, preventing proper zymogen activation to the active enzyme. These distinctions are crucial for understanding disease severity and potential therapeutic responses.[38][41] Approximately one-third of hemophilia B cases arise from de novo mutations in the F9 gene, with this rate higher in sporadic (non-familial) presentations compared to inherited ones, reflecting the elevated mutation rate in male germ cells. This phenomenon contributes significantly to the disease's incidence, independent of family history.[37][42] In female carriers, detection of the mutation is complicated by X-chromosome inactivation, or Lyonization, a random process early in embryonic development that silences one X chromosome per cell, leading to mosaic expression and variable Factor IX levels (typically 20-80% of normal, but occasionally <20% in skewed cases, potentially causing mild symptoms). Carrier status is confirmed through genetic testing, including linkage analysis for familial cases or direct sequencing of the F9 gene for de novo or unknown variants; prenatal diagnosis employs similar methods, often via chorionic villus sampling or amniocentesis to assess fetal genotype.[43][44] Historical efforts to model F9 mutations and explore therapeutic production included the 1997 generation of transgenic sheep expressing human Factor IX via nuclear transfer from transfected fetal fibroblasts, akin to the cloning of Dolly, which demonstrated the feasibility of large-scale recombinant protein production in milk for hemophilia B treatment.[45]Physiological Role
Coagulation Cascade Involvement
Factor IX serves as a key serine protease zymogen in the blood coagulation cascade, primarily within the intrinsic pathway, where it is activated by factor XIa following contact activation involving factors XII and XI. This activation occurs through proteolytic cleavages at Arg145-Ala146 and Arg180-Val181, converting factor IX to its active form, factor IXa.[2] In the extrinsic pathway, factor IX can also be activated by the tissue factor/factor VIIa complex at sites of vascular injury, providing an alternative initiation route that bridges the two pathways.[2] These activation mechanisms position factor IXa as a central amplifier in hemostasis, ensuring rapid progression toward clot formation upon endothelial disruption.[46] Once activated, factor IXa assembles with its cofactor, activated factor VIIIa, and calcium ions on negatively charged phospholipid membranes—typically exposed on activated platelets—to form the intrinsic tenase complex. This complex dramatically enhances the activation of factor X to factor Xa, with the cofactor factor VIIIa accelerating factor IXa's catalytic efficiency by approximately 20,000-fold.[2] The tenase complex amplifies factor X activation by over 1,000,000-fold compared to factor IXa alone, representing a critical rate-limiting step in the propagation phase of coagulation.[2] Downstream, factor Xa then forms the prothrombinase complex with factor Va to convert prothrombin into thrombin, which cleaves fibrinogen to fibrin and activates additional cofactors, culminating in stable clot formation.[2] In human plasma, factor IX circulates at a concentration of approximately 5 μg/mL (about 90 nM), with a half-life of 18–24 hours, ensuring availability for rapid response to injury.[2] Hemostasis can be maintained with factor IX activity levels as low as 1–5% of normal (corresponding to moderate hemophilia B), though bleeding risks increase with trauma or surgery at these reduced levels.[47] In vivo studies using factor IX knockout mice demonstrate severe bleeding phenotypes, such as prolonged tail bleeding leading to death without intervention, underscoring factor IX's essential role.[48] Infusion of human factor IX restores hemostasis in these models, confirming its functional conservation across species.[48]Regulation and Inhibitors
Factor IX activity is primarily regulated by several natural inhibitors to maintain hemostatic balance and prevent excessive thrombosis. The main plasma inhibitor of activated Factor IX (FIXa) is antithrombin III (ATIII), which forms a 1:1 covalent complex with FIXa through a suicide substrate mechanism involving the reactive center loop of ATIII and the active site of FIXa.[49] This inhibition proceeds slowly in the absence of cofactors, with a second-order rate constant of approximately 3.1 × 10³ M⁻¹ min⁻¹ (equivalent to ~5 × 10¹ M⁻¹ s⁻¹), limiting FIXa's contribution to the coagulation cascade under basal conditions.[49] Heparin and related glycosaminoglycans, such as fondaparinux or unfractionated heparin, dramatically accelerate this process by inducing a conformational change in ATIII that enhances its reactivity toward FIXa by 60- to 80-fold, primarily through bridging interactions and exosite engagement on FIXa.[49][2] The Protein C pathway provides indirect regulation of FIXa by targeting the intrinsic tenase complex, in which FIXa associates with activated Factor VIII (FVIIIa) on phospholipid surfaces to activate Factor X. Activated Protein C (APC), generated when thrombin binds thrombomodulin on endothelial cells, proteolytically inactivates FVIIIa in a reaction accelerated by its cofactor Protein S, thereby dismantling the tenase complex and curtailing FIXa-mediated amplification of coagulation.[2] This mechanism is particularly important on endothelial surfaces, where Protein S enhances APC's specificity for FVIIIa cleavage at Arg336 and Arg562 sites, reducing tenase activity by over 90% under physiological conditions.[2] Another specific inhibitor of FIXa is the Protein Z-dependent protease inhibitor (ZPI), a serpin that targets FIXa particularly when it is bound to phospholipid membranes in the tenase complex. ZPI, in complex with its cofactor Protein Z (a vitamin K-dependent protein), inhibits FIXa through a similar covalent mechanism to ATIII, but with enhanced efficiency on procoagulant surfaces due to Protein Z anchoring ZPI near FIXa.[50] This inhibition is calcium- and phospholipid-dependent, downregulating FIXa activity in the factor Xase complex by forming a stable acyl-enzyme intermediate that prevents further substrate turnover.[50] ZPI's role complements ATIII by providing localized control at sites of clot formation.[2] Factor IX levels exhibit age-related changes, with plasma activity in newborns and infants typically at 20-50% of adult values due to immature hepatic synthesis.[51] These levels progressively increase 2- to 3-fold, reaching adult ranges (50-150%) by around 6 months to 1 year of age and stabilizing by early adulthood (around age 20), reflecting developmental maturation of the coagulation system.[51][52] Pathophysiological dysregulation of Factor IX regulation contributes to thrombophilia, where elevated FIX activity (>120%) is associated with increased risk of venous thrombosis. For instance, plasma FIX levels exceeding 130-150 IU/dL correlate with a twofold higher incidence of deep vein thrombosis, independent of other factors like FVIII, due to enhanced tenase complex formation and thrombin generation.[53] Such elevations may arise from genetic variants or acquired conditions disrupting inhibitory controls like ATIII or ZPI.[2]Clinical Aspects
Hemophilia B
Hemophilia B, also known as factor IX deficiency or Christmas disease, is a rare X-linked recessive bleeding disorder characterized by insufficient levels of functional factor IX, leading to impaired blood clotting and a predisposition to prolonged bleeding episodes.[47] It primarily affects males, with females serving as carriers, and manifests through a range of clinical severities depending on the residual factor IX activity in the blood.[54] The condition arises from mutations in the F9 gene, as detailed in the genetics section, and has significant implications for affected individuals' quality of life due to recurrent hemorrhages.[55] Epidemiologically, hemophilia B affects approximately 1 in 25,000 to 30,000 male births worldwide, representing about 15-20% of all hemophilia cases.[37] The global prevalence is estimated at approximately 3.8 per 100,000 males, with around 45,600 diagnosed individuals as of 2024.[56] The total number of people with hemophilia A and B combined is estimated to exceed 1 million globally, though significant underdiagnosis persists in low- and middle-income countries.[56] Prevalence is notably higher in regions with elevated rates of consanguineous marriages, such as parts of the Middle East, North Africa, and South Asia, where inbreeding increases the likelihood of homozygous mutations in offspring.[57] The disorder was first identified as distinct from hemophilia A in 1952, when researchers described it in a young patient named Stephen Christmas, using early coagulation assays that revealed differences in clotting factor responses during the 1950s.[58] Severity is classified based on plasma factor IX activity levels: severe cases exhibit less than 1% activity and are prone to spontaneous bleeding; moderate cases have 1-5% activity with bleeds typically triggered by injury; and mild cases show 6-40% activity, where bleeding occurs mainly after mild trauma or surgery.[37] Common symptoms include hemarthroses, or joint bleeds, most frequently affecting the knees, ankles, and elbows, which can lead to chronic pain and mobility issues if recurrent.[54] Muscle hematomas, deep tissue bleeding causing swelling and compartment syndrome, are also prevalent, alongside a 3-5% risk of intracranial hemorrhage in neonates, often presenting as unexplained neurological symptoms shortly after birth.[59] In chronic, inadequately managed cases, repeated hemorrhages may form pseudotumors—encapsulated cystic masses from organized blood clots that erode bone and soft tissue, mimicking neoplasms.[60] Pathophysiologically, the deficiency reduces intrinsic tenase complex activity, where factor IXa, in complex with factor VIIIa, calcium, and phospholipids, normally activates factor X; this impairment hampers thrombin generation, prolonging the activated partial thromboplastin time (aPTT) and destabilizing fibrin clot formation.[37] Complications include the development of alloantibodies (inhibitors) against factor IX in 1-3% of patients, particularly those with severe disease and certain genetic mutations, which neutralize replacement factors and exacerbate bleeding risk.[61] Repeated joint bleeds also contribute to hemophilic arthropathy, a progressive degenerative joint disease involving synovitis, cartilage destruction, and bony overgrowth, often resulting in lifelong disability without preventive measures.[62]Diagnosis and Treatment
Diagnosis of Factor IX (FIX) deficiency, also known as hemophilia B, begins with laboratory assays to measure FIX activity levels in plasma, which classify the condition as severe (<1% activity), moderate (1-5%), or mild (5-40%). The one-stage clotting assay, based on activated partial thromboplastin time (aPTT), is the most widely used method globally, involving dilution of patient plasma with FIX-deficient plasma and comparison of clotting times to a calibrator curve.[63] The chromogenic assay serves as an alternative or confirmatory test, particularly when the one-stage assay yields normal results despite clinical suspicion, by quantifying FIX through enzymatic generation of factor Xa and measurement of optical density changes.[63] These functional assays guide initial diagnosis, often prompted by symptoms such as prolonged bleeding after injury or surgery. Genetic testing via next-generation sequencing of the F9 gene identifies causative mutations, including single nucleotide variants, insertions, deletions, and copy number variants, for definitive diagnosis and family counseling.[64] Carrier testing for females uses the same DNA analysis to detect heterozygous mutations, enabling risk assessment for offspring.[64] The primary treatment for hemophilia B is replacement therapy with FIX concentrates to restore hemostasis during bleeding episodes or prophylactically. Plasma-derived FIX products, such as prothrombin complex concentrates, provide effective replacement but carry a small risk of viral transmission despite purification processes.[65] Recombinant FIX concentrates, preferred for their lower immunogenicity and viral safety, include standard half-life options like Benefix, approved by the FDA in 1997 for on-demand and prophylactic use.[65] Extended half-life recombinant products, such as Alprolix (Fc fusion protein) and Idelvion (albumin fusion protein), extend FIX circulation, allowing dosing intervals of 7-14 days and reducing infusion frequency compared to standard products.[65] Prophylaxis with regular FIX infusions is standard for severe hemophilia B to prevent spontaneous bleeds and joint damage, typically administered weekly at doses maintaining trough FIX activity above 1%.[65] This approach achieves 80-90% reduction in annualized bleeding rates compared to on-demand therapy, with extended half-life products showing mean rates as low as 1.29 versus 3.12 for standard formulations.[65] Gene therapy using adeno-associated virus (AAV) vectors represents a transformative one-time treatment, with etranacogene dezaparvovec (Hemgenix) approved by the FDA in 2022 and the European Commission in 2023 for adults with hemophilia B on FIX prophylaxis.[66] As of 2025, four-year follow-up data from the phase 3 HOPE-B trial demonstrate sustained mean FIX activity of 37% in treated patients, with 94% remaining off prophylaxis and annualized bleeding rates reduced by approximately 90% (from 4.16 to 0.40).[66] Challenges include transient elevations in liver enzymes in about 17% of patients, managed with corticosteroids, and potential preexisting immunity to AAV vectors limiting eligibility or necessitating redosing strategies.[66] Emerging therapies aim to further simplify management and address limitations of current options. B-cell-mediated cell therapy, such as BE-101 using CRISPR/Cas9-engineered B cells to produce FIX, entered phase 1/2 trials in 2025 with the first patient dosed, showing potential for durable FIX expression without preconditioning chemotherapy.[67] Non-factor therapies like fitusiran, an siRNA that reduces antithrombin III levels to enhance thrombin generation, received FDA approval in March 2025 for once-monthly subcutaneous prophylaxis in hemophilia B patients with or without inhibitors.[68] Ongoing monitoring of therapy involves pharmacokinetic studies to assess FIX half-life, which is 18-24 hours for standard concentrates, guiding individualized dosing.[69] Inhibitor development, occurring in 1-3% of patients, is screened using the Bethesda assay, which quantifies neutralizing antibodies by measuring residual FIX activity after incubation with patient plasma; titers ≥0.6 Bethesda units on two occasions confirm positivity.[69]References
- https://www.sciencedirect.com/topics/[neuroscience](/page/Neuroscience)/factor-ix