Recent from talks
Nothing was collected or created yet.
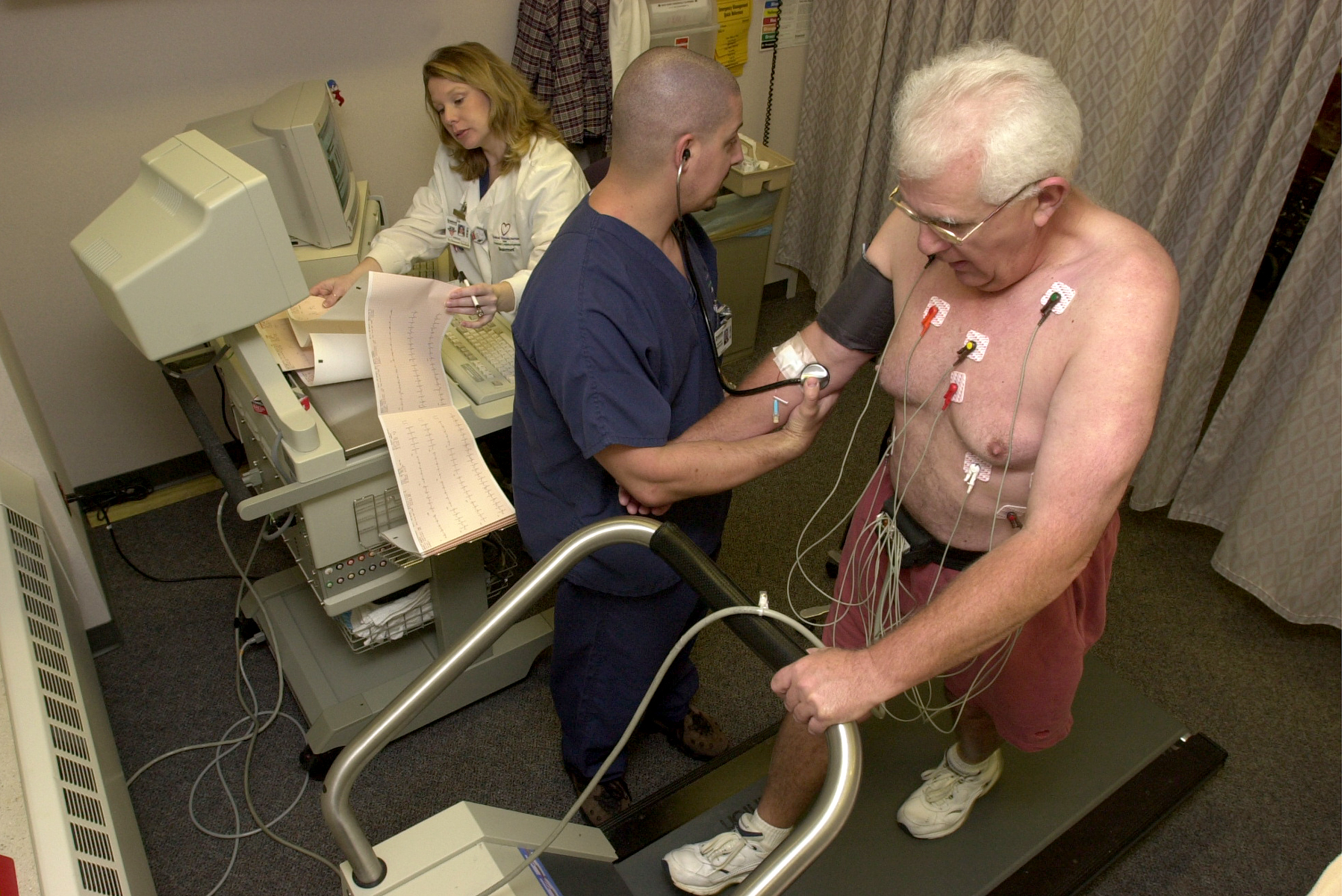
Cardiac stress test
View on WikipediaIt has been suggested that Cardiopulmonary exercise test be merged into this article. (Discuss) Proposed since September 2025. |
| Cardiac stress test | |
|---|---|
 A male patient walks on a stress test treadmill to have his heart's function checked. | |
| Other names | Cardiopulmonary exercise test |
| ICD-9-CM | 89.4 |
| MeSH | D025401 |
| MedlinePlus | 003878 |
A cardiac stress test is a cardiological examination that evaluates the cardiovascular system's response to external stress within a controlled clinical setting. This stress response can be induced through physical exercise (usually a treadmill) or intravenous pharmacological stimulation of heart rate.[1]
As the heart works progressively harder (stressed) it is monitored using an electrocardiogram (ECG) monitor. This measures the heart's electrical rhythms and broader electrophysiology. Pulse rate, blood pressure and symptoms such as chest discomfort or fatigue are simultaneously monitored by attending clinical staff. Clinical staff will question the patient throughout the procedure asking questions that relate to pain and perceived discomfort. Abnormalities in blood pressure, heart rate, ECG or worsening physical symptoms could be indicative of coronary artery disease.[2]
Stress testing does not accurately diagnose all cases of coronary artery disease, and can often indicate that it exists in people who do not have the condition. The test can also detect heart abnormalities such as arrhythmias, and conditions affecting electrical conduction within the heart such as various types of fascicular blocks.[3]
A "normal" stress test does not offer any substantial reassurance that a future unstable coronary plaque will not rupture and block an artery, inducing a heart attack. As with all medical diagnostic procedures, data is only from a moment in time. A primary reason stress testing is not perceived as a robust method of CAD detection — is that stress testing generally only detects arteries that are severely narrowed (~70% or more).[4][5][6]
Stress testing and echocardiography
[edit]A stress test may be accompanied by echocardiography.[7] The echocardiography is performed both before and after the exercise so that structural differences can be compared.
A resting echocardiogram is obtained prior to stress. The ultrasound images obtained are similar to the ones obtained during a full surface echocardiogram, commonly referred to as transthoracic echocardiogram. The patient is subjected to stress in the form of exercise or chemically (often dobutamine). After the target heart rate is achieved, 'stress' echocardiogram images are obtained. The two echocardiogram images are then compared to assess for any abnormalities in wall motion of the heart. This is used to detect obstructive coronary artery disease.[8]
Cardiopulmonary exercise stress testing
[edit]
While also measuring breathing gases (e.g., oxygen saturation, maximal oxygen consumption), the test is often referred to as a cardiopulmonary exercise test. Common indications for a cardiopulmonary exercise test include evaluation of shortness of breath, workup before heart transplantation, and prognosis and risk assessment of heart failure patients.
The test is also common in sport science for measuring athletes' maximal oxygen consumption, V̇O2 max.[9] In 2016, the American Heart Association published an official scientific statement advocating that cardiorespiratory fitness, quantifiable as V̇O2 max and measured during a cardiopulmonary exercise test, be categorized as a clinical vital sign and should be routinely assessed as part of clinical practice.[10]
The CPX test can be done on a treadmill or cycle ergometer. In untrained subjects, V̇O2 max is 10% to 20% lower when using a cycle ergometer compared with a treadmill.[11]
Stress testing using injected nuclear markers
[edit]A nuclear stress test uses a gamma camera to image radioisotopes injected into the bloodstream. The best known example is myocardial perfusion imaging. Typically, a radiotracer (Tc-99 sestamibi, Myoview or thallous chloride 201) may be injected during the test. After a suitable waiting period to ensure proper distribution of the radiotracer, scans are acquired with a gamma camera to capture images of the blood flow. Scans acquired before and after exercise are examined to assess the state of the coronary arteries of the patient. By showing the relative amounts of radioisotope within the heart muscle, the nuclear stress tests more accurately identify regional areas of reduced blood flow.[12]
Stress and potential cardiac damage from exercise during the test is a problem in patients with ECG abnormalities at rest or in patients with severe motor disability. Pharmacological stimulation from vasodilators such as dipyridamole or adenosine, or positive chronotropic agents such as dobutamine can be used. Testing personnel can include a cardiac radiologist, a nuclear medicine physician, a nuclear medicine technologist, a cardiology technologist, a cardiologist, and/or a nurse. The typical dose of radiation received during this procedure can range from 9.4 to 40.7 millisieverts.[13]
Recommended utility of this procedure
[edit]
The American Heart Association recommends ECG treadmill testing as the first choice for patients with medium risk of coronary heart disease according to risk factors of smoking, family history of coronary artery stenosis, hypertension, diabetes and high cholesterol. In 2013, in its "Exercise Standards for Testing and Training", the AHA indicated that high frequency QRS analysis during ECG treadmill test have useful test performance for detection of coronary heart disease.[14]
- Perfusion stress test (with 99mTc labelled sestamibi[15]) is appropriate for select patients, especially those with an abnormal resting electrocardiogram.
- Intracoronary ultrasound or angiogram can provide more information but is invasive and carries the risk of complications associated with cardiac catheterization procedures.[16]
Diagnostic value
[edit]The common approach for stress testing recommended by the American College of Cardiology[17][18] and the American Heart Association[19] involves several methods to assess cardiac health. These methods provide information for diagnosing and managing heart-related conditions. Two primary stress tests utilized are a treadmill test using ECG/electrophysiology metrics and nuclear testing, each have unique sensitivity and specificity values.
The treadmill test, employing the modified Bruce protocol,[20] demonstrates a sensitivity range of around 73-90% and a specificity range of around 50-74%. Sensitivity refers to the percentage of individuals with the condition correctly identified by the test, while specificity denotes the percentage of individuals without the condition correctly identified as not having it.[21] The nuclear stress test exhibits a sensitivity of 81% and a specificity ranging from 85 to 95%.[22]
To arrive at the patient's post test likelihood of disease, the interpretation of the stress test result necessitates the integration of the patient's pretest likelihood with the test's sensitivity and specificity. This method, initially introduced by Diamond and Forrester in the 1970s, provides an estimate of the patient's post-test likelihood of disease.[23][24] Stress tests have limitations in assessing the significance and nature of cardiac problems, they should be seen in context - as an initial assessment that can lead to a number of other diagnostic approaches in the broader management of cardiac diseases.[25]
According to data from the US Centers for Disease Control and Prevention (CDC) common first systems of coronary artery disease is a heart attack. According to the American Heart Association, a significant percentage of individuals, approximately 65% of men and 47% of women, present with a heart attack or sudden cardiac arrest as their first symptom of cardiovascular disease. Consequently, stress tests performed shortly before these events may not be highly relevant for predicting infarction in the majority of individuals tested.[26][27]
Contraindications and termination conditions
[edit]Stress cardiac imaging is not recommended for asymptomatic, low-risk patients as part of their routine care.[28] Some estimates show that such screening accounts for 45% of cardiac stress imaging, and evidence does not show that this results in better outcomes for patients.[28] Unless high-risk markers are present, such as diabetes in patients aged over 40, peripheral arterial disease, or a risk of coronary heart disease greater than 2 percent yearly, most health societies do not recommend the test as a routine procedure.[28][29][30][31]
Absolute contraindications to cardiac stress test include:
- Acute myocardial infarction within 48 hours
- Unstable angina not yet stabilized with medical therapy
- Uncontrolled cardiac arrhythmia, which may have significant hemodynamic responses (e.g. ventricular tachycardia)
- Severe symptomatic aortic stenosis, aortic dissection, pulmonary embolism, and pericarditis
- Multivessel coronary artery diseases that have a high risk of producing an acute myocardial infarction
- Decompensated or inadequately controlled congestive heart failure[32]
- Uncontrolled hypertension (blood pressure > 200/110 mmHg)[32]
- Severe pulmonary hypertension[32]
- Acute aortic dissection[32]
- Acutely ill for any reason[32]
Indications for termination: A cardiac stress test should be terminated before completion under the following circumstances:[33][34]
Absolute indications for termination include:
- Systolic blood pressure decreases by more than 10 mmHg with increase in work rate, or drops below baseline in the same position, with other evidence of ischemia.
- Increase in nervous system symptoms: Dizziness, ataxia or near syncope
- Moderate to severe anginal pain (above 3 on standard 4-point scale[34])
- Signs of poor perfusion,[33] e.g. cyanosis or pallor[34]
- Request of the test subject
- Technical difficulties (e.g. difficulties in measuring blood pressure or EGC[34])
- ST Segment elevation of more than 1 mm in aVR, V1 or non-Q wave leads
- Sustained ventricular tachycardia
Relative indications for termination include:
- Systolic blood pressure decreases by more than 10 mmHg with increase in work rate, or drops below baseline in the same position, without other evidence of ischemia.
- ST or QRS segment changes,[34] e.g. more than 2 mm[33] horizontal or downsloping[34] ST segment depression in non-Q wave leads, or marked axis shift
- Arrhythmias other than sustained ventricular tachycardia e.g. Premature ventricular contractions, both multifocal or triplet; heart block; supraventricular tachycardia or bradyarrhythmias[34]
- Intraventricular conduction delay or bundle branch block or that cannot be distinguished from ventricular tachycardia
- Increasing chest pain
- Fatigue, shortness of breath, wheezing, claudication or leg cramps
- Hypertensive response (systolic blood pressure > 250 mmHg or diastolic blood pressure > 115 mmHg)
Adverse effects
[edit]Side effects from cardiac stress testing may include[citation needed]
- Palpitations, chest pain, myocardial infarction, shortness of breath, headache, nausea or fatigue.
- Adenosine and dipyridamole can cause mild hypotension.
- As the radioactive tracers used for this test are chemically carcinogenic, frequent use of these tests carries a small risk of cancer.[35]
Use of pharmacological agents to stress the heart
[edit]Pharmacologic stress testing relies on coronary steal. Vasodilators are used to dilate coronary vessels, which causes increased blood velocity and flow rate in normal vessels and less of a response in stenotic vessels. This difference in response leads to a steal of flow and perfusion defects appear in cardiac nuclear scans or as ST-segment changes.[36]
The choice of pharmacologic stress agents used in the test depends on factors such as potential drug interactions with other treatments and concomitant diseases.
Pharmacologic agents such as adenosine, regadenoson (Lexiscan), or dipyridamole is generally used when a patient cannot achieve adequate work level with treadmill exercise, or has poorly controlled hypertension or left bundle branch block. However, an exercise stress test may provide more information about exercise tolerance than a pharmacologic stress test.[37]
Commonly used agents include:
- Vasodilators acting as adenosine receptor agonists, such as adenosine itself, and dipyridamole (Persantine),[38] which acts indirectly at the receptor.
- Regadenoson (Lexiscan), which acts specifically at the adenosine A2A receptor, thus affecting the heart more than the lung.
- Dobutamine – The effects of beta-agonists such as dobutamine can be reversed by administering beta-blockers such as propranolol.
Regadenoson or dobutamine is often used in patients with severe reactive airway disease (asthma or COPD) as adenosine and dipyridamole can cause acute exacerbation of these conditions. If the patient's asthma is treated with an inhaler then it should be used as a pre-treatment prior to the injection of the pharmacologic stress agent. In addition, if the patient is actively wheezing then the physician should determine the benefits versus the risk to the patient of performing a stress test especially outside of a hospital setting. Caffeine is usually held 24 hours prior to an adenosine stress test, as it is a competitive antagonist of the A2A adenosine receptor and can attenuate the vasodilatory effects adenosine.[citation needed]
Aminophylline may be used to attenuate severe and/or persistent adverse reactions to adenosine and regadenoson.[39]
History
[edit]Cardiac stress testing, used since the 1960s, has a history rooted in the diagnostic and prognostic assessment of patients with suspected coronary artery disease. It has evolved to evaluate inducible myocardial ischemia as an indicator of adverse outcomes. The factors influencing mortality risk have changed over time due to decreasing angina symptoms, increasing prevalence of conditions like diabetes and obesity, and the rise in pharmacologic testing for patients unable to exercise during stress tests.[40]
See also
[edit]References
[edit]- ^ "Stress Tests: MedlinePlus Medical Test". medlineplus.gov. Retrieved 2023-11-09.
- ^ "Exercise ECG". British Heart Foundation. Retrieved 2023-11-09.
- ^ Ladapo JA, Blecker S, O'Donnell M, Jumkhawala SA, Douglas PS (2016-08-18). "Appropriate Use of Cardiac Stress Testing with Imaging: A Systematic Review and Meta-Analysis". PLOS ONE. 11 (8) e0161153. Bibcode:2016PLoSO..1161153L. doi:10.1371/journal.pone.0161153. ISSN 1932-6203. PMC 4990235. PMID 27536775.
- ^ Vilcant V, Zeltser R (2023), "Treadmill Stress Testing", StatPearls, Treasure Island (FL): StatPearls Publishing, PMID 29763078, retrieved 2023-11-09
- ^ Schoenhagen P, Ziada KM, Kapadia SR, Crowe TD, Nissen SE, Tuzcu EM (2000-02-15). "Extent and Direction of Arterial Remodeling in Stable Versus Unstable Coronary Syndromes: An Intravascular Ultrasound Study". Circulation. 101 (6): 598–603. doi:10.1161/01.CIR.101.6.598. ISSN 0009-7322. PMID 10673250.
- ^ Steeds RP, Wheeler R, Bhattacharyya S, Reiken J, Nihoyannopoulos P, Senior R, Monaghan MJ, Sharma V (2019-03-28). "Stress echocardiography in coronary artery disease: a practical guideline from the British Society of Echocardiography". Echo Research and Practice. 6 (2): G17 – G33. doi:10.1530/ERP-18-0068. ISSN 2055-0464. PMC 6477657. PMID 30921767.
- ^ Rimmerman C (2009-05-05). The Cleveland Clinic Guide to Heart Attacks. Kaplan Publishing. pp. 113–. ISBN 978-1-4277-9968-5. Retrieved 25 September 2011.[permanent dead link]
- ^ "Stress echocardiography: MedlinePlus Medical Encyclopedia". medlineplus.gov. Retrieved 2023-11-09.
- ^ Wasserman K, Hansen JE, Sue DY, Stringer WW, Whipp BJ (2004). Principles of Exercise Testing and Interpretation: Including Pathophysiology and Clinical Applications (4th ed.). Philadelphia: Lippincott Williams and Wilkins.
- ^ Ross R, Blair SN, Arena R, Church TS, Després JP, Franklin BA, Haskell WL, Kaminsky LA, Levine BD, Lavie CJ, Myers J, Niebauer J, Sallis R, Sawada SS, Sui X (2016-12-13). "Importance of Assessing Cardiorespiratory Fitness in Clinical Practice: A Case for Fitness as a Clinical Vital Sign: A Scientific Statement From the American Heart Association". Circulation. 134 (24): e653 – e699. doi:10.1161/CIR.0000000000000461. ISSN 0009-7322. PMID 27881567. S2CID 3372949.
- ^ Kaminsky LA, Imboden MT, Arena R, Myers J (2017). "Reference Standards for Cardiorespiratory Fitness Measured With Cardiopulmonary Exercise Testing Using Cycle Ergometry: Data From the Fitness Registry and the Importance of Exercise National Database (FRIEND) Registry". Mayo Clinic Proceedings. 92 (2): 228–233. doi:10.1016/j.mayocp.2016.10.003. PMID 27938891. S2CID 3465353.
- ^ Gupta A, Samarany S (2023), "Dipyridamole Nuclear Stress Test", StatPearls, Treasure Island (FL): StatPearls Publishing, PMID 31335041, retrieved 2023-11-10
- ^ Mettler FA J, Huda W, Yoshizumi TT, Mahesh M (July 2008). "Effective doses in radiology and diagnostic nuclear medicine: a catalog". Radiology. 248 (1): 254–63. doi:10.1148/radiol.2481071451. PMID 18566177. S2CID 7018130. Archived from the original on 2014-08-04.
- ^ Gerald F., Philip A., Kligfield P., et al., Exercise Standards for Testing and Training A Scientific Statement From the American Heart Association. Circulation. 2013; 128: 873-934
- ^ Rizk TH, Nagalli S (2023), "Technetium 99m Sestamibi", StatPearls, Treasure Island (FL): StatPearls Publishing, PMID 31985941, retrieved 2023-11-10
- ^ "Cardiac Catheterization". www.hopkinsmedicine.org. 2021-08-08. Retrieved 2023-11-10.
- ^ Gibbons RJ, Balady GJ, Beasley JW, Faafp, Bricker JT, Duvernoy WF, Froelicher VF, Mark DB, Marwick TH, McCallister BD, Thompson PD, Facsm, Winters WL, Yanowitz FG (July 1997). "ACC/AHA Guidelines for Exercise Testing: Executive Summary: A Report of the American College of Cardiology/ American Heart Association Task Force on Practice Guidelines (Committee on Exercise Testing)". Circulation. 96 (1): 345–354. doi:10.1161/01.CIR.96.1.345. ISSN 0009-7322. PMID 9236456.
- ^ "Why You May Not Need a Stress Test". Cleveland Clinic. 2020-10-27. Retrieved 2023-11-08.
- ^ "Exercise Stress Test". www.heart.org. Retrieved 2023-11-08.
- ^ "Stress Test: Purpose, Procedure, Risks and Results". Cleveland Clinic. Retrieved 2023-11-08.
- ^ Vilcant V, Zeltser R (2023), "Treadmill Stress Testing", StatPearls, Treasure Island (FL): StatPearls Publishing, PMID 29763078, retrieved 2023-11-08
- ^ Morgenstern J (2019-03-13). "Stress Tests Part 3: Stress test accuracy". First10EM. Retrieved 2023-11-08.
- ^ Darrow MD (1999-01-15). "Ordering and Understanding the Exercise Stress Test". American Family Physician. 59 (2): 401–410. PMID 9930131.
- ^ Versteylen MO, Joosen IA, Shaw LJ, Narula J, Hofstra L (2011). "Comparison of Framingham, PROCAM, SCORE, and Diamond Forrester to predict coronary atherosclerosis and cardiovascular events". Journal of Nuclear Cardiology. 18 (5): 904–911. doi:10.1007/s12350-011-9425-5. ISSN 1071-3581. PMC 3175044. PMID 21769703.
- ^ Bilal M, Haseeb A, Arshad MH, Jaliawala AA, Farooqui I, Minhas A, Hussaini A, Khan AA, Ahmad S, Saleem Z, Awan O, Sabahat NU, Ayaz A, Rizwan H (2018). "Frequency and Determinants of Inappropriate Use of Treadmill Stress Test for Coronary Artery Disease". Cureus. 10 (1) e2101. doi:10.7759/cureus.2101. ISSN 2168-8184. PMC 5898845. PMID 29662724.
- ^ CDC (2021-07-19). "Coronary Artery Disease | cdc.gov". Centers for Disease Control and Prevention. Retrieved 2023-11-10.
- ^ "Exercise Stress Test". www.heart.org. Retrieved 2023-11-10.
- ^ a b c American College of Cardiology, "Five Things Physicians and Patients Should Question" (PDF), Choosing Wisely: an initiative of the ABIM Foundation, American College of Cardiology, archived from the original (PDF) on 2012-06-24, retrieved August 17, 2012
- ^ Taylor AJ, Cerqueira M, Hodgson JM, Mark D, Min J, O'Gara P, Rubin GD, American College of Cardiology Foundation Appropriate Use Criteria Task Force, Society of Cardiovascular Computed Tomography, American College Of R, American Heart A, American Society of Echocardiography, American Society of Nuclear Cardiology, North American Society for Cardiovascular Imaging, Society for Cardiovascular Angiography Interventions, Society for Cardiovascular Magnetic Resonance, Kramer CM, Berman, Brown, Chaudhry FA, Cury RC, Desai MY, Einstein AJ, Gomes AS, Harrington R, Hoffmann U, Khare R, Lesser, McGann, Rosenberg A (2010). "ACCF/SCCT/ACR/AHA/ASE/ASNC/NASCI/SCAI/SCMR 2010 Appropriate Use Criteria for Cardiac Computed Tomography". Journal of the American College of Cardiology. 56 (22): 1864–1894. doi:10.1016/j.jacc.2010.07.005. PMID 21087721.
- ^ Douglas PS, Garcia MJ, Haines DE, Lai WW, Manning WJ, Patel AR, Picard MH, Polk DM, Ragosta M, Ward RP, Douglas RB, Weiner RB, Society for Cardiovascular Angiography Interventions, Society of Critical Care Medicine, American Society of Echocardiography, American Society of Nuclear Cardiology, Heart Failure Society of America, Society for Cardiovascular Magnetic Resonance, Society of Cardiovascular Computed Tomography, American Heart Association, Heart Rhythm Society (2011). "ACCF/ASE/AHA/ASNC/HFSA/HRS/SCAI/SCCM/SCCT/SCMR 2011 Appropriate Use Criteria for Echocardiography". Journal of the American College of Cardiology. 57 (9): 1126–1166. doi:10.1016/j.jacc.2010.11.002. PMID 21349406.
- ^ Hendel RC, Abbott BG, Bateman TM, Blankstein R, Calnon DA, Leppo JA, Maddahi J, Schumaecker MM, Shaw LJ, Ward RP, Wolinsky DG, American Society of Nuclear Cardiology (2010). "The role of radionuclide myocardial perfusion imaging for asymptomatic individuals". Journal of Nuclear Cardiology. 18 (1): 3–15. doi:10.1007/s12350-010-9320-5. PMID 21181519. S2CID 27605594.
- ^ a b c d e Henzlova M, Cerqueira, Hansen, Taillefer, Yao (January 2009). "Stress Protocols and Tracers". Journal of Nuclear Cardiology. 16 (2): 331. doi:10.1007/s12350-009-9062-4.
- ^ a b c Weisman IM, Zeballos RJ, eds. (2002). Clinical exercise testing. Basel: Karger. p. 111. ISBN 978-3-8055-7298-9. Retrieved 26 November 2014.
- ^ a b c d e f g American College of Sports Medicine (2013). ACSM's Guidelines for Exercise Testing and Prescription. Lippincott Williams & Wilkins. p. 131. ISBN 978-1-4698-2666-0. Retrieved 26 November 2014.
- ^ Gopal S, Murphy C (2023), "Nuclear Medicine Stress Test", StatPearls, Treasure Island (FL): StatPearls Publishing, PMID 32491614, retrieved 2023-11-10
- ^ Akinpelu D (17 October 2021). "Pharmacologic Stress Testing: Background, Indications, Contraindications". Medscape Reference. Retrieved 26 March 2022.
- ^ Weissman NJ, Adelmann GA (2004). Cardiac imaging secrets. Elsevier Health Sciences. pp. 126–. ISBN 978-1-56053-515-7. Retrieved 25 September 2011.
- ^ Nicholls SJ, Worthley S (January 2011). Cardiovascular Imaging for Clinical Practice. Jones & Bartlett Learning. pp. 198–. ISBN 978-0-7637-5622-2. Retrieved 25 September 2011.
- ^ Abidov A, Dilsizian V, Doukky R, Duvall WL, Dyke C, Elliott MD, Hage FG, Henzlova MJ, Johnson NP, Schwartz RG, Thomas GS, Einstein AJ (2018-12-20). "Aminophylline shortage and current recommendations for reversal of vasodilator stress: an ASNC information statement endorsed by SCMR". Journal of Cardiovascular Magnetic Resonance. 20 (1): 87. doi:10.1186/s12968-018-0510-7. ISSN 1097-6647. PMC 6300896. PMID 30567577.
- ^ Rozanski A, Sakul S, Narula J, Uretsky S, Lavie CJ, Berman D (2023). "Assessment of lifestyle-related risk factors enhances the effectiveness of cardiac stress testing". Prog Cardiovasc Dis. 77: 95–106. doi:10.1016/j.pcad.2023.03.004. PMID 36931544. S2CID 257592720.
External links
[edit]- Preparing for the exercise stress test Archived 2021-02-28 at the Wayback Machine
- "A Simple Exercise Tolerance Test for Circulatory Efficiency with Standard Tables for Normal Individuals," American Journal of the Medical Sciences
- "Optimal Medical Therapy with or without PCI for Stable Coronary Disease," New England Journal of Medicine
- Stress test information from the American Heart Association
- Nuclear stress test information at NIH MedLine
Cardiac stress test
View on GrokipediaOverview and Purpose
Definition and Principles
A cardiac stress test is a non-invasive or minimally invasive diagnostic procedure designed to evaluate the heart's function and blood flow by simulating physical or physiological stress, thereby increasing myocardial oxygen demand to uncover abnormalities such as ischemia, arrhythmias, or other dysfunctions that may not be apparent during rest.[7][8] This approach allows clinicians to assess how the cardiovascular system responds under conditions of elevated workload, revealing potential limitations in coronary perfusion or electrical stability.[2] The underlying physiological principles of the cardiac stress test revolve around the heart's response to increased demand, which elevates heart rate, blood pressure, and myocardial oxygen consumption through sympathetic nervous system activation and enhanced preload. In healthy individuals, cardiac output rises via the Frank-Starling mechanism, where greater venous return stretches myocardial fibers to augment stroke volume, combined with chronotropic effects on heart rate.[9] However, in coronary artery disease (CAD), this heightened demand often exceeds the available oxygen supply due to stenotic vessels, resulting in supply-demand mismatch that manifests as myocardial ischemia; this can be detected through electrocardiographic (ECG) changes like ST-segment depression, regional wall motion abnormalities, or perfusion defects.[9][2] These principles, rooted in the early 20th-century observations of the Frank-Starling law and the pathophysiology of CAD, underscore the test's ability to provoke and identify latent cardiac vulnerabilities.[9] Key components of a cardiac stress test include an initial baseline assessment of resting vital signs, ECG, and symptoms; induction of stress, commonly via exercise such as treadmill walking to replicate natural exertion; continuous monitoring of ECG, heart rate, blood pressure, and oxygenation throughout the procedure; and a recovery phase to observe return to baseline and any delayed abnormalities.[7][8] This structured framework ensures safe provocation of stress while capturing dynamic responses, with exercise serving as the preferred method when feasible due to its physiological fidelity.[9]Clinical Indications and Utility
Cardiac stress testing is primarily indicated for the evaluation of patients presenting with chest pain suggestive of stable angina, particularly those with an intermediate pretest probability of obstructive coronary artery disease (CAD), where it aids in confirming or excluding ischemia as the cause. According to the 2021 AHA/ACC Chest Pain Guideline, stress testing is recommended (Class 1, Level of Evidence B-NR) for intermediate- to high-risk patients with stable chest pain and no known CAD to diagnose myocardial ischemia and guide management.[10] This approach integrates Bayesian principles, using pretest probability models such as the Diamond-Forrester or CAD Consortium scores—based on age, sex, and chest pain characteristics—to select appropriate candidates, as low-probability patients may defer testing while high-probability cases warrant direct angiography.[11] Additional key indications include risk stratification following acute myocardial infarction (MI) and preoperative assessment for noncardiac surgery in elevated-risk patients. In stable patients post-non-ST-elevation acute coronary syndrome (NSTEACS), predischarge stress testing is useful for identifying residual ischemia and stratifying long-term risk, provided symptoms have resolved and the patient is clinically stable, typically at least 12-24 hours for unstable angina and 2-5 days after for NSTEMI; the 2025 ACC/AHA/ACEP/NAEMSP/SCAI Guideline for the Management of Patients With Acute Coronary Syndromes recommends noninvasive stress testing prior to hospital discharge in select low- to intermediate-risk patients without prior invasive evaluation.[12][13] For perioperative evaluation, the 2024 AHA/ACC Guideline recommends stress testing (Class 1) in high-risk patients (e.g., Revised Cardiac Risk Index ≥1 with poor functional capacity <4 metabolic equivalents) undergoing intermediate- or high-risk surgery to detect significant ischemia that may alter management.[14] The test holds utility in specific populations, such as asymptomatic individuals with multiple CAD risk factors like diabetes or hypertension, where it may support risk assessment in select cases per appropriate use criteria, though routine screening is not endorsed without symptoms or functional changes.[15] It also facilitates monitoring the efficacy of revascularization or medical therapy in patients with chronic coronary disease (CCD) who experience persistent symptoms despite guideline-directed medical therapy (GDMT), as outlined in the 2023 AHA/ACC CCD Guideline (Class 1, Level of Evidence B-NR).[16] Guideline frameworks from the ACC/AHA emphasize evidence-based selection, with Class 1 recommendations for intermediate-risk patients to optimize diagnostic yield.[17] Prognostically, a normal stress test result indicates a low annual event rate of less than 1% for cardiac death or MI, enabling reassurance and conservative management, while abnormal findings prompt further interventions such as coronary angiography.[18] This utility spans exercise, pharmacological, or imaging-enhanced modalities, depending on patient factors.[10]Types of Cardiac Stress Tests
Exercise-Based Tests
Exercise-based cardiac stress tests, also known as exercise electrocardiography (ECG) tests, involve physical exertion to evaluate cardiac function under stress, primarily using treadmill or bicycle protocols. These tests are suitable for patients capable of ambulating or pedaling, allowing direct assessment of the heart's response to increased demand. The standard approach uses incremental workloads to gradually elevate heart rate and myocardial oxygen consumption, monitoring for ischemic changes via ECG.[2] The most common protocol is the Bruce protocol, introduced in 1963 and widely adopted as the gold standard for treadmill testing in adults. It consists of three-minute stages with progressive increases in speed and incline: stage 1 begins at 1.7 mph and 10% grade (approximately 5 metabolic equivalents, or METs), stage 2 at 2.5 mph and 12% grade, and subsequent stages further escalate until the patient reaches exhaustion or an endpoint. For patients with lower functional capacity, such as those recovering from myocardial infarction, the Naughton protocol employs a constant speed of 2.0 mph with gradual incline increases over two-minute stages (e.g., 0% to 3.5% in early stages), while the Balke protocol maintains a fixed speed of 3.3 mph and increments the grade every one to two minutes. These protocols aim to achieve at least 85% of the maximum predicted heart rate, calculated as 220 minus the patient's age, though tests may be symptom-limited if fatigue, angina, or significant ECG changes occur first.[9][2][19] Equipment typically includes a motorized treadmill for walking or running, or an upright/recumbent cycle ergometer for pedaling, selected based on patient mobility and preference. Continuous 12-lead ECG monitoring tracks ST-segment changes, arrhythmias, and heart rate, while automated blood pressure cuffs measure systolic and diastolic pressures at rest, during each stage, and in recovery. Safety features, such as emergency stop buttons and resuscitation equipment, are standard in the testing environment.[2][20] Key physiological responses evaluated include heart rate progression, which normally increases by about 10 beats per minute per MET achieved; exercise capacity, quantified in METs (e.g., >10 METs indicates good prognosis); and heart rate recovery, defined as a drop of at least 12 beats per minute in the first minute post-exercise, reflecting vagal reactivation. Endpoints are either target heart rate attainment for diagnostic adequacy or symptom-limited cessation to avoid undue risk, providing insights into chronotropic competence and overall cardiovascular reserve.[9][21][22] Advantages of exercise-based tests include direct evaluation of functional capacity through METs achieved, reproduction of patient-specific exertional symptoms like dyspnea or chest pain, and cost-effectiveness without exposure to radiation or pharmacological agents. These tests offer prognostic value, with higher exercise tolerance correlating to lower mortality risk, and can integrate briefly with imaging modalities for enhanced detection if needed.[23][2][21]Pharmacological Stress Tests
Pharmacological stress tests are employed to simulate the cardiovascular effects of exercise in patients who are unable to perform physical activity due to conditions such as orthopedic limitations, physical deconditioning, or chronic obstructive pulmonary disease (COPD). These tests induce stress through medications that either increase myocardial oxygen demand or enhance coronary blood flow, allowing assessment of cardiac function under simulated exertion. Approximately 52% of cardiac stress tests utilize pharmacological agents, reflecting their widespread application in clinical practice.[24] The primary pharmacological agents fall into two categories: vasodilators, which promote coronary hyperemia by dilating coronary arteries, and inotropic agents, which elevate heart rate and contractility to mimic exercise-induced demand. Common vasodilators include adenosine, administered as an intravenous infusion at 140 mcg/kg/min for 4 to 6 minutes to achieve maximal coronary vasodilation.[25] Another vasodilator is dipyridamole, given intravenously at 0.56 mg/kg over 4 minutes, which inhibits adenosine uptake and thereby induces similar hyperemic effects.[26] Regadenoson, a selective adenosine A2A receptor agonist, is also frequently used at a fixed dose of 0.4 mg as a rapid intravenous bolus followed by a saline flush, offering ease of administration and comparable coronary flow augmentation.[27] In contrast, dobutamine serves as an inotropic and beta-adrenergic stimulant, starting at 5 mcg/kg/min and titrated upward in 3-minute stages to a maximum of 40 mcg/kg/min, thereby increasing myocardial oxygen consumption akin to physical exertion.[28] Hemodynamically, vasodilators like adenosine produce a 3- to 5-fold increase in coronary blood flow but may cause transient atrioventricular block and carry a risk of bronchospasm due to non-selective adenosine receptor activation.[25] Dipyridamole similarly elevates coronary perfusion 3.8- to 7-fold, with effects peaking around 6.5 minutes post-infusion.[26] Dobutamine, by contrast, raises heart rate and contractility without direct vasodilation, targeting 85% of the age-predicted maximum heart rate to replicate exercise physiology.[27] Protocols for pharmacological stress tests involve continuous electrocardiographic monitoring and vital sign assessment throughout the procedure to detect ischemic changes or hemodynamic instability. The infusion is typically paired with radiotracer administration for imaging, though the core stress induction remains drug-driven. Reversal agents, such as aminophylline (50-250 mg intravenously) for vasodilator-induced effects or beta-blockers like metoprolol for dobutamine, are available to terminate symptoms if needed, administered at least 1 minute after tracer injection to avoid interference.[25]Imaging-Enhanced Tests
Imaging-enhanced cardiac stress tests integrate functional imaging modalities with physiological stress to detect coronary artery disease (CAD) by visualizing myocardial ischemia or perfusion defects, offering greater diagnostic accuracy than electrocardiography alone. These tests typically combine exercise or pharmacological stress induction with either echocardiography or nuclear imaging to assess regional wall motion abnormalities or blood flow distribution, respectively. Such enhancements allow for the identification of inducible ischemia in patients unable to achieve adequate exercise or those with baseline ECG abnormalities.[29] Stress echocardiography employs ultrasound imaging to evaluate left ventricular wall motion and thickening before and immediately after stress, detecting ischemia-induced abnormalities such as hypokinesis or akinesis. Protocols include treadmill exercise echocardiography, where images are acquired at baseline, peak exercise, and recovery, or pharmacological variants using dobutamine infusion (up to 40 mcg/kg/min) with atropine if needed to simulate stress in non-exercising patients. This modality demonstrates a sensitivity of 80-85% and specificity of 80-88% for detecting significant CAD, comparable to nuclear methods but with advantages in portability and absence of ionizing radiation.[30][31][29] Nuclear myocardial perfusion imaging (MPI) uses single-photon emission computed tomography (SPECT) or positron emission tomography (PET) with radiotracers to map myocardial blood flow at rest and during stress, identifying reversible perfusion defects indicative of ischemia. Common SPECT tracers include technetium-99m sestamibi or tetrofosmin, administered in a rest-stress or stress-rest sequence, while PET employs rubidium-82 or ammonia-13 for higher resolution; SPECT MPI achieves sensitivity of 85-90% and specificity of 70-75% for CAD, with PET offering superior accuracy (sensitivity up to 90%, specificity 80-85%) particularly in obese patients or multivessel disease. The effective radiation dose for a typical SPECT rest-stress protocol is approximately 10-15 mSv, depending on tracer and dosing. Gated imaging during acquisition enables simultaneous assessment of left ventricular ejection fraction and volumes, enhancing prognostic value.[32][33][34][35] Compared to nuclear MPI, stress echocardiography is preferred for its lower cost, lack of radiation exposure, and bedside applicability, making it ideal for initial evaluation in low-to-intermediate risk patients; however, nuclear imaging excels in detecting three-vessel or left main CAD due to its quantitative perfusion assessment and attenuation correction capabilities. Both modalities follow similar stress induction methods, such as exercise or vasodilators like adenosine, but imaging-specific protocols optimize detection of subtle defects. Acquisition typically sequences rest imaging first followed by stress to minimize artifacts, though stress-first approaches reduce radiation in low-risk cases.[36][15]Specialized Variants
Cardiopulmonary exercise testing (CPET) represents a specialized variant of cardiac stress testing that integrates respiratory gas exchange analysis with incremental exercise to evaluate integrated cardiopulmonary function. During CPET, key parameters such as peak oxygen uptake (VO₂ max), anaerobic threshold, and ventilatory efficiency (measured as the VE/VCO₂ slope) are quantified using breath-by-breath analysis to assess aerobic capacity and gas exchange dynamics. Protocols typically involve a ramped treadmill or cycle ergometer protocol progressing to symptom-limited exhaustion, allowing for the detection of exercise-limiting factors beyond standard electrocardiographic changes.[37][38][39] CPET is particularly indicated for differentiating cardiac from pulmonary contributions to exercise intolerance, such as in unexplained dyspnea, where patterns like elevated VE/VCO₂ slopes (>34) suggest cardiac limitation due to impaired perfusion or ventilation-perfusion mismatch. In heart failure patients, it provides prognostic insights; for instance, a peak VO₂ <14 mL/kg/min is associated with increased mortality risk and serves as a criterion for advanced therapies like transplantation, especially in those on beta-blocker therapy. These metrics help stratify disease severity and guide therapeutic decisions, outperforming resting assessments in predictive accuracy.[40][41][42] The procedure requires specialized equipment, including a metabolic cart equipped with rapid-response gas analyzers for continuous measurement of oxygen consumption (VO₂) and carbon dioxide production (VCO₂), connected via a face mask or mouthpiece to capture expired air. Ventilatory volumes are monitored through flow sensors, enabling derivation of parameters like the anaerobic threshold via the V-slope method. In heart failure evaluation, the Weber classification system utilizes peak VO₂ thresholds to categorize patients into classes A-D (e.g., class C: 10-14 mL/kg/min; class D: <10 mL/kg/min), correlating with functional capacity and survival prognosis.[37][43][44] Beyond CPET, pharmacological myocardial perfusion imaging (MPI) with positron emission tomography (PET) serves as a specialized option for high-risk patients unable to exercise, employing vasodilators like adenosine to induce stress while quantifying absolute myocardial blood flow and detecting multivessel disease with higher sensitivity than SPECT. Emerging stress cardiac magnetic resonance (CMR) imaging, often using dobutamine for inotropic stress, excels in viability assessment by combining perfusion, wall motion, and late gadolinium enhancement to identify hibernating myocardium without ionizing radiation, aiding revascularization decisions in ischemic cardiomyopathy.[45][46][47]Procedure and Protocols
Patient Preparation
Patient preparation for a cardiac stress test is essential to ensure both safety and diagnostic accuracy, involving adjustments to medications and diet, comprehensive baseline evaluations, and logistical arrangements at the testing facility. Guidelines recommend reviewing the patient's medical history to identify any absolute or relative contraindications, such as unstable angina or severe aortic stenosis, prior to proceeding.[48] Specific instructions on medications aim to optimize heart rate response and minimize interference with stress induction. For exercise-based tests, beta-blockers and calcium channel blockers are typically withheld for 24 to 48 hours beforehand to allow achievement of adequate heart rate increases, though this is not always routine and depends on the clinical context; timing and dosage should be documented if continued.[48][23] In pharmacological stress tests using dobutamine, beta-blockers should be withheld for at least 24 hours, while for vasodilator agents like adenosine or regadenoson, xanthine derivatives (e.g., theophylline) are discontinued 12 to 24 hours prior to avoid blunting the response.[25] Other antianginal medications, such as nitrates or digoxin, may also require withholding, particularly if ST-segment changes could be obscured, with digoxin effects persisting up to two weeks after discontinuation.[48] Patients are advised to bring a list of all current medications for review. Dietary and lifestyle preparations focus on avoiding substances that could alter physiological responses. Caffeine-containing products, including coffee, tea, chocolate, and certain medications, must be avoided for 12 to 24 hours prior, as they can block adenosine receptors in pharmacological tests or provoke arrhythmias in exercise protocols.[48][25] Fasting is not strictly required, but patients should abstain from food for at least three hours before the test to enhance exercise tolerance; a light meal earlier in the day is permissible, and routine medications can be taken with small sips of water.[48] Smoking and alcohol should be avoided for at least 24 hours to prevent impacts on heart rate and blood pressure. Baseline assessments begin with a thorough review of the patient's history, including symptoms, cardiovascular risk factors, allergies, and recent illnesses, followed by a physical examination to evaluate vital signs, cardiac auscultation, and overall fitness.[48] A resting 12-lead electrocardiogram (ECG) is performed supine and standing to identify baseline abnormalities, such as ST-segment depression greater than 1 mm or left bundle branch block, which may affect test interpretation; the test is postponed if resting systolic blood pressure exceeds 200 mm Hg or diastolic exceeds 110 mm Hg.[48][2] Informed consent is obtained after explaining the procedure, potential benefits for diagnosing coronary artery disease, and risks such as arrhythmias or chest pain, ensuring the patient understands termination criteria.[48] Laboratory tests are selectively ordered based on the test modality. For tests involving contrast agents or pharmacological drugs, electrolytes, renal function (e.g., creatinine), and complete blood count may be checked to assess eligibility and mitigate risks like nephrotoxicity.[49] Women of childbearing potential undergo a pregnancy test prior to nuclear imaging or radiation-involving procedures to avoid fetal exposure.[50] The testing facility must be equipped for immediate intervention, with intravenous access established (e.g., a 20- to 24-gauge cannula) for potential medication administration or hydration.[51] Emergency equipment, including a defibrillator, crash cart with resuscitation drugs, and oxygen, must be readily available, and the procedure is supervised by qualified personnel trained in advanced cardiac life support.[52]Test Execution and Monitoring
The execution of a cardiac stress test begins with a baseline recording phase lasting 2 to 3 minutes, during which the patient is positioned supine and then standing to obtain initial electrocardiographic (ECG) tracings and vital signs, establishing a reference for subsequent changes.[2] For exercise-based tests, patients walk on a treadmill or pedal a stationary bike while connected to monitoring equipment, with intensity gradually increasing via faster speed or incline; the procedure tracks heart rate, blood pressure, ECG, and symptoms, and takes 30–60 minutes with close monitoring.[1] This is followed by the stress induction phase, where exercise or pharmacological agents progressively increase myocardial demand; for exercise-based tests, this typically involves a treadmill or cycle ergometer using standardized protocols such as the Bruce protocol, with stages escalating in workload every 2 to 3 minutes until an endpoint is reached, aiming for a total duration of 6 to 12 minutes.[9] The recovery phase then ensues, involving a cool-down period of 5 to 10 minutes of monitoring while the patient rests or walks slowly, allowing observation of heart rate deceleration and resolution of any abnormalities.[2] Continuous monitoring occurs throughout the test to detect physiological responses in real time. A 12-lead ECG is recorded continuously via hardwired or telemetry systems, with ST-segment analysis performed at 60 to 80 milliseconds after the J-point to identify ischemic changes, and tracings printed at least every minute or stage.[9] Blood pressure is measured every 1 to 2 minutes using an automated cuff, tracking systolic increases (typically 30 to 50 mm Hg) and any drops exceeding 10 mm Hg.[2] Symptoms such as chest pain, dyspnea, or fatigue are assessed subjectively using the Borg scale (ratings 6 to 20) at regular intervals, while heart rate is monitored to reach a target of 85% of the age-predicted maximum, calculated as (220 minus age) multiplied by 0.85.[9][53] The test is overseen by a qualified physician or advanced practice clinician with Advanced Cardiac Life Support certification, who interprets ECG changes and symptoms in real time, while a trained technician manages equipment setup, electrode placement, and data acquisition.[9] For submaximal tests in elderly or deconditioned patients, protocols adjust workloads to avoid excessive strain, prioritizing safety over peak achievement.[2] Endpoints for terminating the stress phase include attainment of the target heart rate, patient fatigue or volitional exhaustion, evidence of ischemia such as horizontal or downsloping ST-segment depression greater than 1 mm in two contiguous leads, significant hypotension (systolic drop >10 mm Hg despite increased workload), or development of arrhythmias like frequent ventricular ectopy.[9][2] In pharmacological variants, endpoints align similarly but are triggered by peak drug infusion or comparable physiological thresholds.[54]Post-Test Evaluation
Following the completion of a cardiac stress test, patients undergo a supervised recovery phase to ensure hemodynamic stability and monitor for potential delayed complications. In exercise-based tests, individuals typically engage in a cool-down period of slow walking to prevent venous pooling and abrupt drops in blood pressure, with continuous monitoring of heart rate (HR), blood pressure (BP), and electrocardiogram (ECG) for 6 to 8 minutes or until these parameters return to near-baseline levels. Vital signs are checked every 5 minutes, and observation continues for arrhythmias, ischemic changes, or symptoms such as chest pain or dyspnea, which may occur post-exercise due to vagal reactivation or residual effects. For pharmacological stress tests, recovery involves continuous ECG monitoring for at least 4 minutes and BP assessments every minute for 3 to 5 minutes or until stable, with readiness to administer reversal agents like aminophylline for vasodilator-induced complications such as severe hypotension (systolic BP <80 mm Hg) or bronchospasm. Supervised rest persists until HR falls below 100 beats per minute, prioritizing patient safety during this transition. Documentation during post-test evaluation captures essential metrics to support preliminary analysis and future comparisons. Key elements recorded include peak HR achieved, metabolic equivalents (METs) attained, exercise duration or pharmacological infusion details, symptoms experienced, ECG tracings (including ST-segment changes), and the reason for test termination (e.g., fatigue, angina, or target HR reached). A preliminary report is generated promptly, summarizing these findings and baseline comparisons, which is shared with the patient and referring physician to guide immediate management decisions. In imaging-enhanced tests, such as stress echocardiography or myocardial perfusion imaging, post-stress images are documented alongside rest images to note any wall motion abnormalities or perfusion defects. Patients receive clear discharge instructions to facilitate safe recovery and ongoing vigilance. They are advised to resume normal activities gradually unless contraindicated by test results, such as avoiding vigorous exertion if ischemia was detected, and to report any new or persistent symptoms like chest pain, shortness of breath, or palpitations immediately to their healthcare provider. Medications withheld prior to the test, such as beta-blockers, should be resumed as prescribed, and hydration is encouraged to aid clearance of pharmacological agents if used. For those with implantable devices like pacemakers, activity levels are tailored based on documented HR responses to prevent inappropriate device activation. Quality control measures ensure the reliability and usability of test data for clinical and longitudinal purposes. If imaging modalities were employed, technicians verify the quality of acquired images—checking for artifacts, adequate resolution, and proper protocol adherence (e.g., gated imaging post-stress in SPECT studies)—to confirm diagnostic validity. All raw data, including ECG strips, vital sign logs, and imaging files, are archived securely in electronic health records for serial testing comparisons, adhering to standards that minimize radiation exposure (e.g., <9 mSv effective dose in optimized protocols) while maintaining traceability. This process supports reproducibility and integration with broader patient care pathways.Interpretation and Diagnostic Value
Analyzing Results
Interpreting the results of a cardiac stress test involves a systematic evaluation of electrocardiographic (ECG) changes, imaging findings if applicable, and patient symptoms to identify evidence of myocardial ischemia or other abnormalities. The primary focus is on detecting inducible ischemia, which manifests as reversible perfusion defects or wall motion abnormalities under stress compared to baseline. Analysis typically occurs post-test, with clinicians correlating multiple modalities for diagnostic accuracy. In ECG-based stress testing, key indicators of ischemia include ST-segment depression, measured 80 ms after the J-point. A horizontal or downsloping ST depression of ≥1 mm is considered a standard marker for ischemia, as it reflects subendocardial oxygen supply-demand mismatch during stress. Upsloping ST depression is less specific and often not diagnostic on its own, potentially arising from non-ischemic causes like rate-related changes. Additional ECG findings, such as T-wave inversions or stress-induced arrhythmias (e.g., ventricular ectopy), may support ischemia but require correlation with other data for confirmation. For imaging-enhanced tests, echocardiography assesses regional wall motion using a standardized 16-segment model of the left ventricle, scoring each segment from 1 (normal) to 4 (dyskinetic). Inducible ischemia is indicated by new or worsening wall motion abnormalities in ≥2 contiguous segments during stress, reflecting reduced perfusion to affected myocardial territories. In nuclear perfusion imaging, the summed stress score (SSS) quantifies defect extent and severity across 17 segments, with scores of 0-4 typically normal, 4-8 mildly abnormal, and >8 suggesting high-risk ischemia based on perfusion defect size. Symptom integration enhances result interpretation, particularly when angina coincides with ECG or imaging changes, as this combination strengthens the likelihood of true ischemia. The Duke Treadmill Score provides a composite metric for exercise ECG tests, calculated as exercise duration in minutes minus 5 times the maximum ST-segment deviation in mm minus 4 times the angina index (0 for no angina, 1 for non-limiting, 2 for exercise-limiting). Higher scores indicate lower risk, aiding in risk stratification alongside clinical context. False-positive results, which can mimic ischemia, must be scrutinized to avoid misdiagnosis. Left ventricular hypertrophy (LVH) often causes baseline repolarization abnormalities that exaggerate ST changes during stress. Digoxin therapy induces secondary ST depression, reducing test specificity in treated patients. Motion artifacts in imaging modalities, such as patient movement during nuclear scans, can create apparent perfusion defects unrelated to coronary disease.Sensitivity, Specificity, and Prognostic Insights
The diagnostic accuracy of cardiac stress tests varies by modality and is typically evaluated against coronary artery disease (CAD) as confirmed by invasive angiography. For exercise electrocardiography (ECG), a meta-analysis of over 24,000 patients across 147 studies reported a sensitivity of 68% and specificity of 77% in detecting CAD. Stress echocardiography demonstrates improved performance, with meta-analyses indicating sensitivities ranging from 80% to 88% and specificities from 77% to 86% for identifying significant CAD, particularly when assessing wall motion abnormalities under stress. Nuclear myocardial perfusion imaging (MPI), such as single-photon emission computed tomography (SPECT), achieves sensitivities of 85% to 90%, though specificities are generally lower at around 70% to 80%, due to potential artifacts from soft tissue attenuation. Beyond diagnosis, cardiac stress tests provide substantial prognostic value for future cardiac events, including myocardial infarction and death. A normal stress test result is associated with a low annual event rate of 0.5% to 1%, reflecting excellent negative predictive value for adverse outcomes over 1 to 5 years of follow-up in patients with suspected CAD. In contrast, high-risk features—such as early-onset ischemia, extensive perfusion defects, or low exercise capacity—correlate with elevated annual risks of 5% to 10%, enabling effective risk stratification to guide therapy intensity. Evidence from landmark trials underscores these metrics in clinical management. The COURAGE trial, involving over 2,200 patients with stable CAD, demonstrated that stress testing effectively identifies candidates for optimal medical therapy versus revascularization, with no significant difference in hard outcomes between percutaneous coronary intervention plus medical therapy and medical therapy alone, highlighting the test's role in safe deferral of invasive procedures. Recent updates in the 2023 AHA/ACC guideline for chronic coronary disease emphasize stress testing for risk stratification, recommending its use to categorize patients into low-, intermediate-, or high-risk groups based on ischemia extent and functional capacity, thereby informing personalized treatment plans. Despite these strengths, accuracy can be reduced in certain populations, necessitating cautious interpretation. In women, exercise ECG sensitivity and specificity are lower (often 50% to 60% for both) due to higher rates of false positives from baseline ST-segment changes and lower pretest CAD probability. Obese patients face challenges with image quality in echocardiography and attenuation artifacts in nuclear MPI, potentially decreasing specificity by 10% to 20% and increasing nondiagnostic rates up to 30%. In such cases, complementary tests like coronary computed tomography angiography (CCTA) are often recommended to enhance diagnostic confidence.Limitations and Complementary Tests
Cardiac stress testing, while valuable for detecting ischemia, has inherent limitations in accurately localizing coronary lesions, particularly in distinguishing between single-vessel and multivessel disease. In cases of multivessel coronary artery disease, the test may fail to pinpoint specific stenoses due to diffuse perfusion abnormalities that obscure territorial defects. This challenge is exacerbated in scenarios of balanced ischemia, where uniform reductions in myocardial blood flow across multiple territories lead to reduced sensitivity, often resulting in false-negative results on myocardial perfusion imaging.[55] Additionally, stress echocardiography exhibits significant operator dependency, as image acquisition and interpretation rely heavily on the technician's skill in obtaining clear views and assessing wall motion abnormalities, which can introduce variability and reduce reproducibility.[56] Patient-specific factors further compromise the reliability of cardiac stress tests. In individuals with obesity, acoustic windows are often obscured by excess adipose tissue, limiting the feasibility of echocardiography and potentially necessitating contrast agents or alternative modalities to achieve diagnostic-quality images.[57] Patients with left bundle branch block (LBBB) face a high risk of false-positive septal perfusion defects during nuclear stress testing, particularly with exercise or vasodilator protocols, which can mimic ischemia and lead to unnecessary downstream testing.[58] Moreover, stress testing is contraindicated in acute coronary syndrome, as it may provoke further myocardial injury in unstable patients.[8] To address these gaps, complementary tests are often employed to refine diagnosis and guide management. Coronary computed tomography angiography (CCTA) serves as a useful adjunct for low-risk patients with suspected coronary artery disease, providing detailed anatomic visualization of stenoses without the functional limitations of stress testing.[59] For individuals with high-risk stress test results indicating severe ischemia, invasive coronary angiography is recommended to confirm lesion severity and facilitate revascularization decisions.[60] In cases where other modalities are contraindicated—such as in patients unable to exercise, with poor echocardiographic windows, or contraindications to nuclear agents—stress cardiac magnetic resonance imaging (MRI) offers a robust alternative, enabling assessment of perfusion and viability with high spatial resolution.[61] Emerging technologies, including AI-assisted interpretation, are addressing interpretive variability in stress testing. Machine learning algorithms applied to myocardial perfusion imaging have demonstrated improved detection of perfusion defects, with 2024 studies showing enhanced sensitivity and reduced inter-observer discordance in identifying coronary artery disease.[62]Safety and Risks
Contraindications
Cardiac stress testing, whether exercise-based or pharmacological, carries risks that necessitate careful patient selection. Contraindications are categorized as absolute, where the procedure should not be performed due to high risk of adverse events, and relative, where the test may be considered only after weighing risks and benefits or opting for alternatives. These recommendations are based on class III indications (no benefit or potential harm) from the American College of Cardiology (ACC) and American Heart Association (AHA) guidelines.[9][2] Absolute contraindications include conditions posing immediate life-threatening risks during stress induction:- Acute myocardial infarction within 48 hours.[11]
- Unstable angina.[11]
- Uncontrolled arrhythmias causing hemodynamic compromise or symptoms.[11]
- Symptomatic severe aortic stenosis.[11]
- Decompensated heart failure.[11]
- Acute pulmonary embolism or aortic dissection.[11]
- Acute myocarditis or pericarditis.[11]
- Severe pulmonary hypertension (systolic pressure >60 mm Hg).[11]
- Severe hypertension (systolic blood pressure >200 mmHg or diastolic >110 mmHg).[11]
- Known left main coronary artery disease.[11]
- Moderate stenotic valvular heart disease.[11]
- Electrolyte imbalances.[11]
- Recent stroke.[63]