Recent from talks
Nothing was collected or created yet.
Ventricle (heart)
View on Wikipedia| Ventricle | |
|---|---|
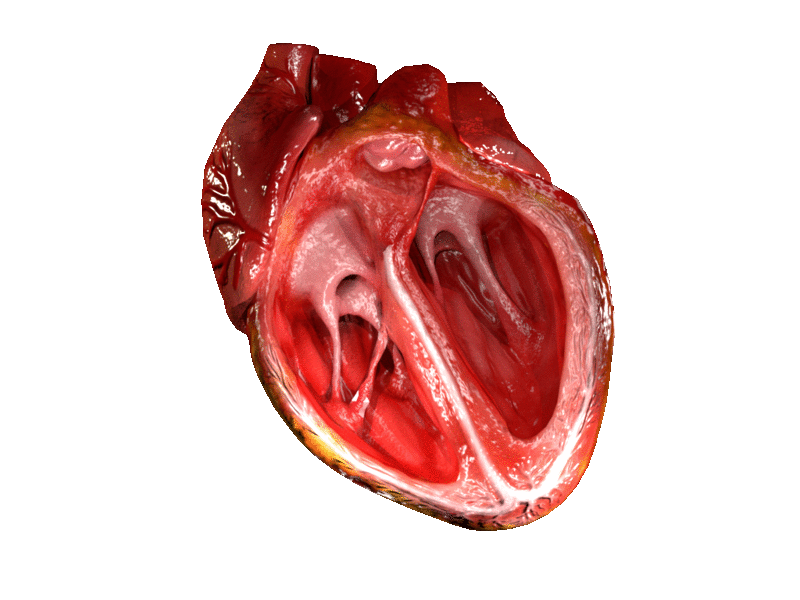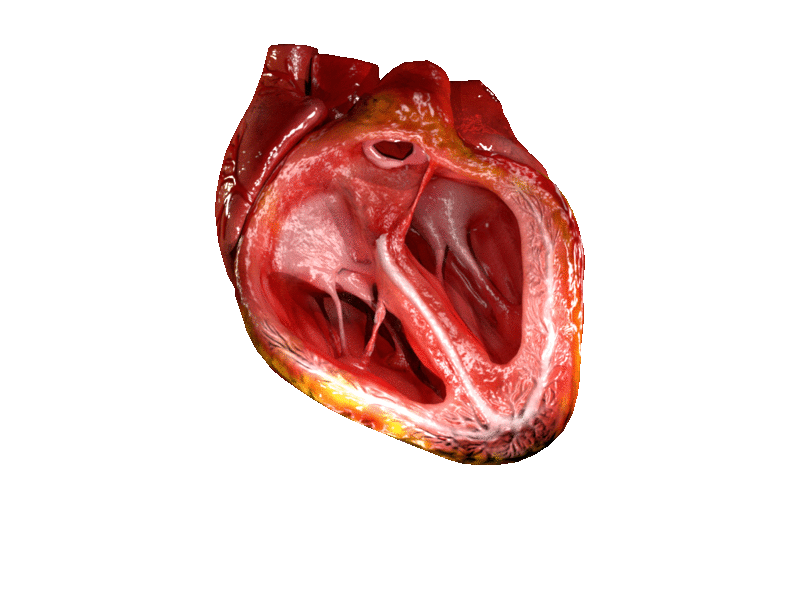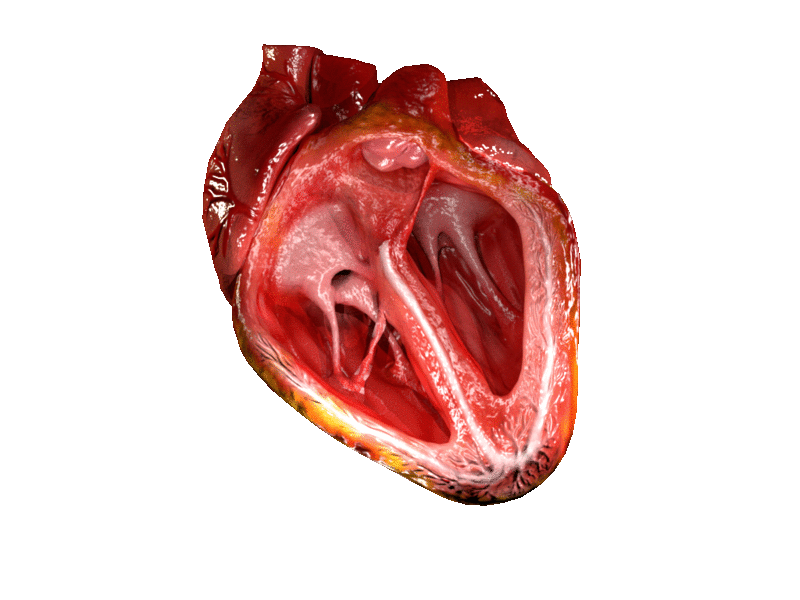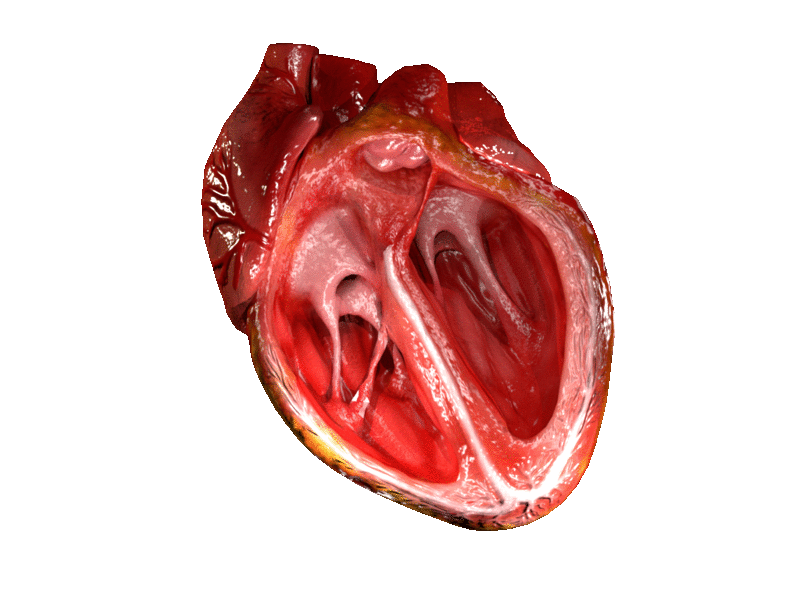
 Computer generated animation of cut section of the human heart showing both ventricles. | |
| Details | |
| Identifiers | |
| Latin | ventriculus cordis |
| MeSH | D006352 |
| TA98 | A12.1.00.012 |
| FMA | 7100 |
| Anatomical terminology | |
A ventricle is one of two large chambers located toward the bottom of the heart that collect and expel blood towards the peripheral beds within the body and lungs. The blood pumped by a ventricle is supplied by an atrium, an adjacent chamber in the upper heart that is smaller than a ventricle. Interventricular means between the ventricles (for example the interventricular septum), while intraventricular means within one ventricle (for example an intraventricular block).
In a four-chambered heart, such as that in humans, there are two ventricles that operate in a double circulatory system: the right ventricle pumps blood into the pulmonary circulation to the lungs, and the left ventricle pumps blood into the systemic circulation through the aorta.
Structure
[edit]
Ventricles have thicker walls than atria and generate higher blood pressures. The physiological load on the ventricles requiring pumping of blood throughout the body and lungs is much greater than the pressure generated by the atria to fill the ventricles. Further, the left ventricle has thicker walls than the right because it needs to pump blood to most of the body while the right ventricle fills only the lungs.[1][citation needed][2]
On the inner walls of the ventricles are irregular muscular columns called trabeculae carneae which cover all of the inner ventricular surfaces except that of the conus arteriosus, in the right ventricle. There are three types of these muscles. The third type, the papillary muscles, give origin at their apices to the chordae tendinae which attach to the cusps of the tricuspid valve and to the mitral valve.
The mass of the left ventricle, as estimated by magnetic resonance imaging, averages 143 g ± 38.4 g, with a range of 87–224 g.[3]
The right ventricle is equal in size to the left ventricle[citation needed] and contains roughly 85 millilitres (3 imp fl oz; 3 US fl oz) in the adult. Its upper front surface is circled and convex, and forms much of the sternocostal surface of the heart. Its under surface is flattened, forming part of the diaphragmatic surface of the heart that rests upon the diaphragm.
Its posterior wall is formed by the ventricular septum, which bulges into the right ventricle, so that a transverse section of the cavity presents a semilunar outline. Its upper and left angle forms a conical pouch, the conus arteriosus, from which the pulmonary artery arises. A tendinous band, called the tendon of the conus arteriosus, extends upward from the right atrioventricular fibrous ring and connects the posterior surface of the conus arteriosus to the aorta.[citation needed]
Shape
[edit]The left ventricle is longer and more conical in shape than the right, and on transverse section its concavity presents an oval or nearly circular outline. It forms a small part of the sternocostal surface and a considerable part of the diaphragmatic surface of the heart; it also forms the apex of the heart. The left ventricle is thicker and more muscular than the right ventricle because it pumps blood at a higher pressure.
The right ventricle is triangular in shape and extends from the tricuspid valve in the right atrium to near the apex of the heart. Its wall is thickest at the apex and thins towards its base at the atrium. When viewed via cross section however, the right ventricle seems to be crescent shaped.[4][5] The right ventricle is made of two components: the sinus and the conus. The Sinus is the inflow which flows away from the tricuspid valve.[6] Three bands made from muscle, separate the right ventricle: the parietal, the septal, and the moderator band.[6] The moderator band connects from the base of the anterior papillary muscle to the ventricular septum.[5][7]
Development
[edit]By young adulthood, the walls of the left ventricle have thickened from three to six times greater than that of the right ventricle. This reflects the typical five times greater pressure workload this chamber performs while accepting blood returning from the pulmonary veins at ~80mmHg pressure (equivalent to around 11 kPa) and pushing it forward to the typical ~120mmHg pressure (around 16.3 kPa) in the aorta during each heartbeat. (The pressures stated are resting values and stated as relative to surrounding atmospheric which is the typical "0" reference pressure used in medicine.)
Function
[edit]During systole, the ventricles contract, pumping blood through the body. During diastole, the ventricles relax and fill with blood again.
The left ventricle receives oxygenated blood from the left atrium via the mitral valve and pumps it through the aorta via the aortic valve, into the systemic circulation. The left ventricular muscle must relax and contract quickly and be able to increase or lower its pumping capacity under the control of the nervous system. In the diastolic phase, it has to relax very quickly after each contraction so as to quickly fill with the oxygenated blood flowing from the pulmonary veins. Likewise in the systolic phase, the left ventricle must contract rapidly and forcibly to pump this blood into the aorta, overcoming the much higher aortic pressure. The extra pressure exerted is also needed to stretch the aorta and other arteries to accommodate the increase in blood volume.
The right ventricle receives deoxygenated blood from the right atrium via the tricuspid valve and pumps it into the pulmonary artery via the pulmonary valve, into the pulmonary circulation.
Pumping volume
[edit]The typical healthy adult heart pumping volume is ~5 liters/min, resting. Maximum capacity pumping volume extends from ~25 liters/min for non-athletes to as high as ~45 liters/min for Olympic level athletes.
Volumes
[edit]In cardiology, the performance of the ventricles are measured with several volumetric parameters, including end-diastolic volume (EDV), end-systolic volume (ESV), stroke volume (SV) and ejection fraction (Ef).

| Ventricular volumes | ||
|---|---|---|
| Measure | Right ventricle | Left ventricle |
| End-diastolic volume | 144 mL (± 23 mL)[8] | 142 mL (± 21 mL)[9] |
| End-diastolic volume / body surface area (mL/m2) | 78 mL/m2 (± 11 mL/m2)[8] | 78 mL/m2 (± 8.8 mL/m2)[9] |
| End-systolic volume | 50 mL (± 14 mL)[8] | 47 mL (± 10 mL)[9] |
| End-systolic volume / body surface area (mL/m2) | 27 mL/m2 (± 7 mL/m2)[8] | 26 mL/m2 (± 5.1 mL/m2)[9] |
| Stroke volume | 94 mL (± 15 mL)[8] | 95 mL (± 14 mL)[9] |
| Stroke volume / body surface area (mL/m2) | 51 mL/m2 (± 7 mL/m2)[8] | 52 mL/m2 (± 6.2 mL/m2)[9] |
| Ejection fraction | 66% (± 6%)[8] | 67% (± 4.6%)[9] |
| Heart rate | 60–100 bpm[10] | 60–100 bpm[10] |
| Cardiac output | 4.0–8.0 L/minute[11] | 4.0–8.0 L/minute[11] |
Pressures
[edit]
Red = aortic pressure
Blue = left ventricular pressure
Yellow = left atrial pressure.
| Site | Normal pressure range (in mmHg)[12] | |
|---|---|---|
| Central venous pressure | 3–8 | |
| Right ventricular pressure | systolic | 15–30 |
| diastolic | 3–8 | |
| Pulmonary artery pressure | systolic | 15–30 |
| diastolic | 4–12 | |
| Pulmonary vein/ |
2–15 | |
| Left ventricular pressure | systolic | 100–140 |
| diastolic | 3–12 | |
Ventricular pressure is a measure of blood pressure within the ventricles of the heart.[13]
Left
[edit]During most of the cardiac cycle, ventricular pressure is less than the pressure in the aorta, but during systole, the ventricular pressure rapidly increases, and the two pressures become equal to each other (represented by the junction of the blue and red lines on the diagram on this page), the aortic valve opens, and blood is pumped to the body.
Elevated left ventricular end-diastolic pressure has been described as a risk factor in cardiac surgery.[14]
Noninvasive approximations have been described.[15]
An elevated pressure difference between the aortic pressure and the left ventricular pressure may be indicative of aortic stenosis.[16]
Right
[edit]Right ventricular pressure demonstrates a different pressure-volume loop than left ventricular pressure.[17]
Dimensions
[edit]The heart and its performance are also commonly measured in terms of dimensions, which in this case means one-dimensional distances, usually measured in millimeters. This is not as informative as volumes but may be much easier to estimate with (e.g., M-Mode echocardiography[18] or with sonomicrometry, which is mostly used for animal model research). Optimally, it is specified with which plane the distance is measured in, e.g. the dimension of the longitudinal plane.[19]
| Dimension | Abbreviation | Definition | Normally |
|---|---|---|---|
| End-diastolic dimension | EDD | The diameter across a ventricle at the end of diastole, if not else specified then usually referring to the transverse[20] (left-to-right) internal (luminal) distance, excluding thickness of walls, although it can also be measured as the external distance. | |
|
LVEDD or sometimes LVDD | The end-diastolic dimension of the left ventricle. | 48 mm,[21] Range 36 – 56 mm[22] |
|
RVEDD or sometimes RVDD | The end-diastolic dimension of the right ventricle. | Range 10 – 26 mm[22] |
| End-systolic dimension | ESD | ESD is similar to the end-diastolic dimension, but is measured at the end of systole (after the ventricles have pumped out blood) rather than at the end of diastole. | |
|
LVESD or sometimes LVSD | The end-systolic dimension of the left ventricle. | Range 20 – 40 mm[22] |
|
RVESD or sometimes RVSD | The end-systolic dimension of the right ventricle. | Range 10 – 26 mm[22] |
| Interventricular septal end diastolic dimension | IVSd | The thickness of the interventricular septum. | 8.3 mm,[21] Range 7 – 11 mm[22] |
| Left ventricular end diastolic posterior wall dimension | LVPWd | The thickness of the posterior left ventricular wall. | 8.3 mm,[21] Range 7 – 11 mm[22] |
| Mean left ventricular myocardial thickness | Mean LVMT | Average thickness of the left ventricle, with numbers given as 95% prediction interval for the short axis images at the mid-cavity level[23] | Women: 4 - 8 mm[23] Men: 5 - 9 mm[23] |
| Mean right ventricular myocardial thickness | Mean RVMT | Average thickness of the right ventricle, with numbers given as 95% prediction interval.[24] | 4 - 7 mm[24] |
| Left ventricular end systolic dimension | As above but measured during systole. This measurement is not commonly used clinically. | 16 mm[25] | |
| Left atrial dimension | LA | Range 24 – 40 mm[22] |
Fractional shortening (FS) is the fraction of any diastolic dimension that is lost in systole. When referring to endocardial luminal distances, it is EDD minus ESD divided by EDD (times 100 when measured in percentage).[26] Normal values may differ somewhat dependent on which anatomical plane is used to measure the distances. Normal range is 25–45%, Mild is 20–25%, Moderate is 15–20%, and Severe is <15%.[27] Cardiology Diagnostic Tests Midwall fractional shortening may also be used to measure diastolic/systolic changes for inter-ventricular septal dimensions[28] and posterior wall dimensions. However, both endocardial and midwall fractional shortening are dependent on myocardial wall thickness, and thereby dependent on long-axis function.[29] By comparison, a measure of short-axis function termed epicardial volume change (EVC) is independent of myocardial wall thickness and represents isolated short-axis function.[29]
Clinical significance
[edit]An arrhythmia is an irregular heartbeat that can occur in the ventricles or atria. Normally the heartbeat is initiated in the SA node of the atrium but initiation can also occur in the Purkinje fibres of the ventricles, giving rise to premature ventricular contractions, also called ventricular extra beats. When these beats become grouped the condition is known as ventricular tachycardia.[citation needed]
Another form of arrhythmia is that of the ventricular escape beat. This can happen as a compensatory mechanism when there is a problem in the conduction system from the SA node.[citation needed]
The most severe form of arrhythmia is ventricular fibrillation which is the most common cause of cardiac arrest and subsequent sudden death.
See also
[edit]References
[edit]- ^ Berman, Michelle N.; Tupper, Connor; Bhardwaj, Abhishek (2025), "Physiology, Left Ventricular Function", StatPearls, Treasure Island (FL): StatPearls Publishing, PMID 31082142, retrieved 2025-07-15
- ^ "How your Heart works?". HealthyWa.
- ^ Schlosser T, Pagonidis K, Herborn CU, Hunold P, Waltering KU, Lauenstein TC, Barkhausen J (March 2005). "Assessment of left ventricular parameters using 16-MDCT and new software for endocardial and epicardial border delineation". AJR. American Journal of Roentgenology. 184 (3): 765–73. doi:10.2214/ajr.184.3.01840765. PMID 15728595.
- ^ Leng J. Right ventricle. In: Weyman AE, ed. Principle andpractice of echocardiography. Philadelphia: Lippincott Williams& Wilkins, 1994:901–21
- ^ a b Haddad F, Couture P, Tousignant C, Denault AY. The right ventricle in cardiac surgery, a perioperative perspective: I. Anatomy, physiology, and assessment. Anesth Analg. 2009;108(2):407-21. doi:10.1213/ane.0b013e31818f8623.
- ^ a b Haddad F, Couture P, Tousignant C, Denault AY. The right ventricle in cardiac surgery, a perioperative perspective: I. Anatomy, physiology, and assessment. Anesth Analg. 2009;108(2):407-21. doi:10.1213/ane.0b013e31818f8623.
- ^ Farb A, Burke AP, Virmani R. Anatomy and pathology of theright ventricle (including acquired tricuspid and pulmonicvalve disease). Cardiol Clin 1992;10:1–21
- ^ a b c d e f g Maceira AM, Prasad SK, Khan M, Pennell DJ (December 2006). "Reference right ventricular systolic and diastolic function normalized to age, gender and body surface area from steady-state free precession cardiovascular magnetic resonance" (PDF). European Heart Journal. 27 (23): 2879–88. doi:10.1093/eurheartj/ehl336. PMID 17088316.
- ^ a b c d e f g Maceira A (2006). "Normalized Left Ventricular Systolic and Diastolic Function by Steady State Free Precession Cardiovascular Magnetic Resonance". Journal of Cardiovascular Magnetic Resonance. 8: 417–426. doi:10.1080/10976640600572889. (subscription required)
- ^ a b Normal ranges for heart rate are among the narrowest limits between bradycardia and tachycardia. See the Bradycardia and Tachycardia articles for more detailed limits.
- ^ a b "Normal Hemodynamic Parameters – Adult" (PDF). Edwards Lifesciences LLC. 2009.
- ^ Table 30-1 in: Goers TA, Klingensmith ME, Chen LE, Glasgow SC (2008). The Washington Manual of Surgery. Philadelphia: Wolters Kluwer Health/Lippincott Williams & Wilkins. ISBN 978-0-7817-7447-5.
- ^ Ventricular+pressure at the U.S. National Library of Medicine Medical Subject Headings (MeSH)
- ^ Salem R, Denault AY, Couture P, Bélisle S, Fortier A, Guertin MC, Carrier M, Martineau R (September 2006). "Left ventricular end-diastolic pressure is a predictor of mortality in cardiac surgery independently of left ventricular ejection fraction". British Journal of Anaesthesia. 97 (3): 292–7. doi:10.1093/bja/ael140. PMID 16835254.
- ^ Brenner JI, Baker KR, Berman MA (October 1980). "Prediction of left ventricular pressure in infants with aortic stenosis". British Heart Journal. 44 (4): 406–10. doi:10.1136/hrt.44.4.406. PMC 482419. PMID 7426202.
- ^ "Aortic Stenosis: Overview – eMedicine Emergency Medicine". Retrieved 2009-02-28.
- ^ Redington AN, Gray HH, Hodson ME, Rigby ML, Oldershaw PJ (January 1988). "Characterisation of the normal right ventricular pressure-volume relation by biplane angiography and simultaneous micromanometer pressure measurements". British Heart Journal. 59 (1): 23–30. doi:10.1136/hrt.59.1.23. PMC 1277068. PMID 3342146.
- ^ van Dam I, van Zwieten G, Vogel JA, Meijler FL (1980). "Left ventricular (diastolic) dimensions and relaxation in patients with atrial fibrillation". European Heart Journal. Suppl A: 149–56. doi:10.1093/eurheartj/1.suppl_1.149. PMID 7274225.
- ^ Kurita A (2008). "Longitudinal fractional shortening and its relation to diastolic cardiac function". Journal of Medical Ultrasonics. 35 (3): 113–118. doi:10.1007/s10396-008-0176-0. PMID 27278833. S2CID 22506795.
- ^ Grimsgaard S, Bønaa KH, Hansen JB, Myhre ES (July 1998). "Effects of highly purified eicosapentaenoic acid and docosahexaenoic acid on hemodynamics in humans". The American Journal of Clinical Nutrition. 68 (1): 52–9. doi:10.1093/ajcn/68.1.52. PMID 9665096.
- ^ a b c Basavarajaiah S, Wilson M, Naghavi R, Whyte G, Turner M, Sharma S (November 2007). "Physiological upper limits of left ventricular dimensions in highly trained junior tennis players". British Journal of Sports Medicine. 41 (11): 784–8. doi:10.1136/bjsm.2006.033993. PMC 2465269. PMID 17957014.
- ^ a b c d e f g Page 41 in: O'Connor, Simon (2009). Examination Medicine (The Examination). Edinburgh: Churchill Livingstone. ISBN 978-0-7295-3911-1.
- ^ a b c Kawel, Nadine; Turkbey, Evrim B.; Carr, J. Jeffrey; Eng, John; Gomes, Antoinette S.; Hundley, W. Gregory; Johnson, Craig; Masri, Sofia C.; Prince, Martin R.; van der Geest, Rob J.; Lima, João A.C.; Bluemke, David A. (2012). "Normal Left Ventricular Myocardial Thickness for Middle-Aged and Older Subjects With Steady-State Free Precession Cardiac Magnetic Resonance". Circulation: Cardiovascular Imaging. 5 (4): 500–508. doi:10.1161/CIRCIMAGING.112.973560. ISSN 1941-9651. PMC 3412148. PMID 22705587.
- ^ a b Karna, S.K.; Rohit, M.K.; Wanchu, A. (2015). "Right ventricular thickness as predictor of global myocardial performance in systemic sclerosis: A Doppler tissue imaging study". Indian Heart Journal. 67 (6): 521–528. doi:10.1016/j.ihj.2015.06.021. ISSN 0019-4832. PMC 4699958. PMID 26702679.
- ^ Lang, Roberto M. (1985). "Adverse Cardiac Effects of Acute Alcohol Ingestion in Young Adults". Annals of Internal Medicine. 102 (6): 742–747. doi:10.7326/0003-4819-102-6-742. ISSN 0003-4819. PMID 3994186.
- ^ chfpatients.com > Fractional Shortening (FS) Retrieved on April 7, 2010
- ^ "Left ventricle size – Echocardiography in ICU". Stanford.edu. 2009-06-23. Retrieved 2018-09-21.
- ^ de Simone G, Devereux RB, Roman MJ, Ganau A, Saba PS, Alderman MH, Laragh JH (May 1994). "Assessment of left ventricular function by the midwall fractional shortening/end-systolic stress relation in human hypertension". Journal of the American College of Cardiology. 23 (6): 1444–51. doi:10.1016/0735-1097(94)90390-5. PMID 8176105.
- ^ a b Ugander M, Carlsson M, Arheden H (February 2010). "Short-axis epicardial volume change is a measure of cardiac left ventricular short-axis function, which is independent of myocardial wall thickness". American Journal of Physiology. Heart and Circulatory Physiology. 298 (2): H530-5. doi:10.1152/ajpheart.00153.2009. PMID 19933422.
External links
[edit]- Photo of dissection at uc.edu
- Left Ventricle – Cell Centered Database
- Anatomy photo:20:05-0102 at the SUNY Downstate Medical Center