Recent from talks
Nothing was collected or created yet.
Inverted sugar syrup
View on Wikipedia
| |||
| Identifiers | |||
|---|---|---|---|
| ChEMBL | |||
| ChemSpider |
| ||
PubChem CID
|
|||
| UNII | |||
| Properties | |||
| Molar mass | 360.312 g/mol | ||
| Pharmacology | |||
| C05BB03 (WHO) | |||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||

Inverted sugar syrup is a syrup mixture of the monosaccharides glucose and fructose, made by splitting the disaccharide sucrose. This mixture's optical rotation is opposite to that of the original sugar, which is why it is called an invert sugar. Splitting is completed through hydrolytic saccharification.
It is 30% sweeter than table sugar,[1] and foods that contain invert sugar retain moisture better and crystallize less easily than those that use table sugar instead. Bakers, who call it invert syrup, may use it more than other sweeteners.[2]
Other names include invert sugar,[3] simple syrup, sugar syrup, sugar water, bar syrup, and sucrose inversion.
Production
[edit]Additives
[edit]Commercially prepared enzyme-catalyzed solutions are inverted at 60 °C (140 °F). The optimum pH for inversion is 5.0. Invertase is added at a rate of about 0.15% of the syrup's weight, and inversion time will be about 8 hours. When completed the syrup temperature is raised to inactivate the invertase, but the syrup is concentrated in a vacuum evaporator to preserve color.[4]
Though inverted sugar syrup can be made by heating table sugar in water alone, the reaction can be sped up by adding lemon juice, cream of tartar, or other catalysts, often without changing the flavor noticeably.[citation needed] Common sugar can be inverted quickly by mixing sugar and citric acid or cream of tartar at a ratio of about 1000:1 by weight and adding water. If lemon juice, which is about five percent citric acid by weight, is used instead then the ratio becomes 50:1. Such a mixture, heated to 114 °C (237 °F)[5] and added to another food, prevents crystallization without tasting sour.
Commercially prepared hydrochloric acid-catalyzed solutions may be inverted at the relatively low temperature of 50 °C (122 °F). The optimum pH for acid-catalyzed inversion is 2.15. As the inversion temperature is increased, the inversion time decreases.[4] They are then given a pH neutralization when the desired level of inversion is reached.[6][7]
In confectionery and candy making, cream of tartar is commonly used as the acidulant, with typical amounts in the range of 0.15–0.25% of the sugar's weight.[8] The use of cream of tartar imparts a honey-like flavor to the syrup.[7] After the inversion is completed, it may be neutralized with baking soda using a weight of 45% of the cream of tartar's weight.[9][10]
For fermentation
[edit]All constituent sugars (sucrose, glucose, and fructose) support fermentation, so invert sugar solutions of any composition can be fermented.
Syrup is used to feed microbiological life, which requires oxygen found in the water. For example, kombucha is produced by fermenting inverted sugar syrup with tea using a symbiotic culture of bacteria and yeast (SCOBY), and yeast in winemaking is used for ethanol fermentation. Cold water can hold more dissolved oxygen than warm water, but granulated sugar does not dissolve easily in cold water.
Water in a container with wide bottom surface area allows for faster dissolving of the sucrose, which only has to be mixed a few times periodically to form a homogeneous solution. Also, a mixer or blender may be used to rotate the sugar, in turns, if necessary.
In other foods and products
[edit]
- Honey which is mostly a mixture of glucose and fructose, being similar to invert syrup therefore, can remain a liquid for longer periods of time.
- Jam contains invert sugar formed by the heating process and the acid content of the fruit. This sugar preserves the jam for long periods of time.
- Golden syrup is a syrup of about 55% invert syrup and 45% table sugar (sucrose).
- Fondant filling for chocolates is unique in that the conversion enzyme is added, but not activated by acidification (microenvironment pH adjustment) or cofactor addition depending on the enzymes, before the filling is enrobed with chocolate. The very viscous (and thus formable) filling then becomes less viscous with time, giving the creamy consistency desired. This results from the sub-optimal enzymes conditions purposely created by withholding activation factors, which allows only a fraction of the enzymes to be active, or allows all enzymes to proceed at only a fraction of the biological rate [biologically, it's realistically a combination of both: a reduced number of functional enzymes, with the ones that do function having reduced catalytic kinetics/rates].
- Cadbury Creme Eggs are filled with inverted sugar syrup produced by processing fondant with invertase.[11][12]
- Sour Patch Kids also contain inverted sugar to add sweet flavor.
Sweetened beverages
[edit]Inverted sugar syrup is the basis in sweetened beverages.
- Sweet reserve is a wine term referring to a portion of selected unfermented grape must, free of microorganisms, to be added to wine as a sweetening component. When wine ferments, glucose is fermented at a faster rate than fructose. Thus, arresting fermentation after a significant portion of the sugars have fermented results in a wine where the residual sugar consists mainly of fructose, while the use of sweet reserve will result in a wine where the sweetness comes from a mixture of glucose and fructose.
- Alcoholic beverage manufacturers often add invert sugar in the production of drinks like gin, beer, and sparkling wines for flavoring. Candi sugar, similar to invert sugar, is used in the brewing of Belgian-style beers to boost alcohol content without drastically increasing the body of the beer; it is frequently found in the styles of beer known as dubbel and tripel.[7]
Chemistry
[edit]This section needs additional citations for verification. (November 2019) |
Table sugar (sucrose) is converted to invert sugar by hydrolysis. Heating a mixture or solution of table sugar and water breaks the chemical bond that links together the two simple-sugar components.
The balanced chemical equation for the hydrolysis of sucrose into glucose and fructose is:
- C12H22O11 (sucrose) + H2O (water) → C6H12O6 (glucose) + C6H12O6 (fructose)
Optical rotation
[edit]After a sucrose solution has had some of its sucrose turned into glucose and fructose the solution is no longer said to be pure. The gradual decrease in purity of a sucrose solution as it is hydrolyzed affects a chemical property of the solution called optical rotation that can be used to figure out how much of the sucrose has been hydrolyzed and therefore whether the solution has been inverted or not.
Definition and measurement
[edit]Plane-polarized light can be shone through a sucrose solution as it is heated up for hydrolysis. Such light has an 'angle' that can be measured using a tool called a polarimeter. When such light is shone through a solution of pure sucrose it comes out the other side with a different angle than when it entered, which is proportional to both the concentration of the sugar and the length of the path of light through the solution; its angle is therefore said to be 'rotated' and how many degrees the angle has changed (the degree of its rotation or its 'optical rotation') is given a letter name, (alpha). When the rotation between the angle the light has when it enters and when it exits is in the clockwise direction, the light is said to be 'rotated right' and is given to have a positive angle such as 64°. When the rotation between the angle the light has when it enters and when it exits is in the counterclockwise direction, the light is said to be 'rotated left' and is given a negative angle such as −39°.
Definition of the inversion point
[edit]When plane-polarized light passes through a solution of pure sucrose, its angle is rotated clockwise. As the sucrose is heated and hydrolyzed, the amount of glucose and fructose in the mixture increases, causing the optical rotation to decrease. After passes zero and becomes a negative optical rotation, meaning that the rotation between the angle the light has when it enters and when it exits is in the counter clockwise direction, it is said that the optical rotation has 'inverted' its direction. This leads to the definition of an 'inversion point' as the percentage of sucrose that has to be hydrolyzed before equals zero. Any solution which has passed the inversion point (and therefore has a negative value of ) is said to be 'inverted'.
Chirality and specific rotation
[edit]
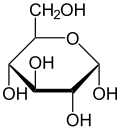
Glucose and fructose are chiral molecules, meaning they can't be rotated to superimpose with their mirror image (like how a right hand cannot be rotated to become a left hand). Because they are chiral, the plane of linearly polarized light is rotated as it passes through solutions of glucose and fructose. The cyclic forms of glucose and fructose have two major anomeric forms which rotate light differently: the α-anomer and the β-anomer (see image).
The anomers of glucose and fructose rapidly interconvert into one another until they reach an equilibrium mixture. At room temperature, the equilibrium mixture for glucose corresponds to roughly one third of the glucose molecules being the α-anomer, and two thirds the β-anomer.
Because the anomers rotate light differently, the overall rotation of light as it passes through a solution of glucose or fructose is the average of the rotation from all of the anomers. This average is called the sugar’s specific rotation, denoted , and is a characteristic physical property measured under defined conditions.
At 20 °C, the specific optical rotation of sucrose is 66.6°, glucose is 52.2°, and fructose is −92.4°.[13]
Effects of water
[edit]Water molecules do not have chirality, therefore they do not have any effect on the measurement of optical rotation. When plane-polarized light enters a body of pure water its angle is no different from when it exits. Thus, for water, = 0°. Chemicals that, like water, have specific rotations that equal zero degrees are called 'optically inactive' chemicals and like water, they do not need to be considered when calculating optical rotation, outside of the concentration and path length.
Mixtures in general
[edit]The overall optical rotation of a mixture of chemicals can be calculated if the proportion of the amount of each chemical in the solution is known. If there are -many optically active different chemicals ('chemical species') in a solution and the molar concentration (the number of moles of each chemical per liter of liquid solution) of each chemical in the solution is known and written as (where is a number used to identify the chemical species); and if each species has a specific rotation (the optical rotation of that chemical were it made as a pure solution) written as , then the mixture has the overall optical rotationWhere is the mole fraction of the species.
Fully hydrolyzed sucrose
[edit]Assuming no extra chemical products are formed by accident (that is, there are no side reactions) a completely hydrolyzed sucrose solution no longer has any sucrose and is a half-and-half mixture of glucose and fructose. This solution has the optical rotation
Partly hydrolyzed sucrose
[edit]If a sucrose solution has been partly hydrolyzed, then it contains sucrose, glucose, and fructose and its optical rotation angle depends on the relative amounts of each for the solution;Where , , and stand for sucrose, glucose, and fructose.
The particular values of do not need to be known to make use of this equation as the inversion point (per cent amount of sucrose that must be hydrolyzed before the solution is inverted) can be calculated from the specific rotation angles of the pure sugars. The reaction stoichiometry (the fact that hydrolyzing one sucrose molecule makes one glucose molecule and one fructose molecule) shows that when a solution begins with moles of sucrose and no glucose nor fructose and moles of sucrose are then hydrolyzed the resulting solution has moles of sucrose, moles of glucose and moles of fructose. The total number of moles of sugars in the solution is therefore and the reaction progress (per cent completion of the hydrolysis reaction) equals . It can be shown that the solution's optical rotation angle is a function of (explicitly depends on) this per cent reaction progress. When the quantity is written as and the reaction is done, the optical rotation angle is
By definition, equals zero degrees at the 'inversion point'; to find the inversion point, therefore, alpha is set equal to zero and the equation is manipulated to find . This givesThus it is found that a sucrose solution is inverted once at least of the sucrose has been hydrolyzed into glucose and fructose.
Monitoring reaction progress
[edit]Holding a sucrose solution at temperatures of 50–60 °C (122–140 °F) hydrolyzes no more than about 85% of its sucrose. Finding when r = 0.85 shows that the optical rotation of the solution after hydrolysis is done is −12.7° this reaction is said to invert the sugar because its final optical rotation is less than zero. A polarimeter can be used to figure out when the inversion is done by detecting whether the optical rotation of the solution at an earlier time in its hydrolysis reaction equals −12.7°.
See also
[edit]References
[edit]- ^ "Making simple syrup is an exercise in chemical reactions". A Word from Carol Kroskey. Archived from the original on July 14, 2007. Retrieved May 1, 2006.
In addition to increased moisture retention ability, converting sucrose to invert syrup has two other interesting results: increased sweetness and better solubility. On a sweetness scale where sucrose is set at 100, invert syrup ranks about 130.
- ^ Schiweck, Hubert; Clarke, Margaret; Pollack, Günter (2007). "Sugar". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a25_345.pub2. ISBN 978-3527306732.
- ^ "What are the types of sugar?". The Sugar Association. Archived from the original on March 1, 2009.
- ^ a b W. Minifie, Bernard (1989). Chocolate, Cocoa and Confectionery: Science and Technology (3rd ed.). Aspen Publishers, Inc. p. 246. ISBN 083421301X. Retrieved July 3, 2014 – via Google Books.
- ^ Van Damme, Eddy. "Invert sugar recipe". Retrieved September 27, 2012.
- ^ Ranken, Michael D.; Kill, R.C.; Baker, C., eds. (1997). Food Industries Manual (24th ed.). London: Blackie Academic & Professional. pp. 407–408. ISBN 0751404047. Retrieved June 30, 2014 – via Google Books.
Commercially, invert sugar is prepared as a syrup of about 70% soluble solids concentration. Invert sugar can be produced by holding a 65% sucrose solution containing 0.25% hydrochloric acid at 50°C (122°F) for one hour. Sodium bicarbonate should then be added to neutralize the acid.
- ^ a b c "The Sugar Beet". The Sugar Beet. Vol. 25, no. 10. Philadelphia: H.C. Baird & Company. 1904. pp. 171–172. Retrieved July 4, 2014 – via Google Books.
- ^ Lean, Michael E.J. (2006). Fox and Cameron's Food Science, Nutrition & Health (7th ed.). Boca Raton, FL: CRC Press. p. 110. ISBN 9780340809488. Retrieved July 1, 2014 – via Google Books.
- ^ Morrison, Abraham Cressy (1904). The Baking Powder Controversy. Vol. 1. New York: The American Baking Powder Association. p. 154. Retrieved July 2, 2014 – via Google Books.
The best cream of tarter baking powder on the market contains about 28 per cent of bicarbonate of soda. To neutralize this quantity ... 62.6 per cent of cream of tartar is required. This quantity will leave in the food 70 per cent of anhydrous Rochelle Salts.
- ^ Maga, Joseph A.; Tu, Anthony T., eds. (1995). Food Additive Toxicology. New York: Marcel Dekker. p. 71, table 24. ISBN 0824792459. Retrieved July 3, 2014 – via Google Books.
- ^ "Creme Egg". Cadbury. Archived from the original on December 16, 2014. Retrieved April 10, 2015.
- ^ LaBau, Elizabeth. "What is Invertase?". About.com. Archived from the original on April 6, 2015. Retrieved April 10, 2015.
- ^ Li, D.; Weng, C.; Ruan, Y.; Li, K.; Cai, G.; Song, C.; Lin, Q. (2021). "An Optical Chiral Sensor Based on Weak Measurement for the Real-Time Monitoring of Sucrose Hydrolysis". Sensors (Basel, Switzerland). 21 (3): 1003. Bibcode:2021Senso..21.1003L. doi:10.3390/s21031003. PMC 7867249. PMID 33540721.
External links
[edit]- "Invertase". Greenwood Health Systems. Archived from the original on May 29, 2017. Retrieved November 27, 2012.
Inverted sugar syrup
View on GrokipediaC12H22O11 + H2O → C6H12O6 (glucose) + C6H12O6 (fructose)
Partial inversion can occur naturally in processes like honey production by bee enzymes, but commercial syrup is fully inverted for consistency.[3] Key properties include greater sweetness than sucrose (due to fructose's higher sweetening power), higher solubility in water than sucrose at room temperature, and resistance to crystallization, making it ideal for applications requiring a smooth texture.[1] It also functions as a humectant, retaining moisture in products, and lowers freezing points, which prevents ice crystal formation.[1] In the food industry, inverted sugar syrup is widely used in confectionery to produce soft, non-grainy candies and fondants; in baking to enhance tenderness; in beverages for clarity and stability; and in frozen desserts like ice cream to improve creaminess.[1] It serves as a key ingredient in brewing for easy yeast fermentation and in pharmaceuticals as a syrup base, offering advantages over sucrose in solubility and shelf life. Nutritionally, it is similar to sucrose, but its liquid form facilitates precise dosing in formulations.[3]
Definition and Composition
Basic Definition
Inverted sugar syrup is a liquid sweetener produced by the hydrolysis of sucrose, resulting in a mixture of approximately equal parts glucose and fructose monosaccharides.[1] This composition makes it sweeter than sucrose and more soluble in water, commonly used in food and beverage applications.[4] It is known by several common names, including invert sugar, invert syrup, and in culinary contexts, as an inverted variant of simple syrup for enhanced moisture retention and texture.[4] The term "inverted" derives from the inversion of the solution's optical rotation during hydrolysis: sucrose rotates plane-polarized light to the right (dextrorotatory), while the resulting glucose-fructose mixture rotates it to the left (levorotatory).[4] The term originated in the 19th century based on polarimetry observations.Chemical Makeup
Inverted sugar syrup is produced through the hydrolysis of sucrose, a disaccharide with the molecular formula , which breaks down into its constituent monosaccharides, D-glucose and D-fructose, both with the formula .[2][5] In a fully inverted syrup, this breakdown occurs in a 1:1 molar ratio, resulting in approximately equal proportions of glucose and fructose by weight, typically around 50% each in the dry matter.[2][1] Commercial inverted sugar syrup is an aqueous solution, with water comprising 20-30% by weight to maintain its liquid form and prevent crystallization.[1] The exact water content can vary depending on the intended application, but this range ensures stability and ease of handling in industrial settings.[2] The composition of inverted sugar syrup can differ based on the degree of inversion achieved during production. Fully inverted syrup contains 100% glucose and fructose with no residual sucrose, while partially inverted forms, common in many commercial products, feature 50-90% inversion, leaving 10-50% unhydrolyzed sucrose alongside the monosaccharides.[1] These variations allow for tailored sweetness and functionality in different uses. Incomplete hydrolysis in the production process may introduce minor impurities or byproducts, such as organic acids and degradation products like hydroxymethylfurfural (HMF), which can arise from side reactions during acid-catalyzed breakdown.[1] Enzymatic methods tend to minimize these impurities, resulting in a purer syrup composition.[6]Production Processes
Acid-Catalyzed Hydrolysis
The acid-catalyzed hydrolysis represents the conventional industrial approach to producing inverted sugar syrup, leveraging the chemical breakdown of sucrose into its monosaccharide components under acidic conditions. The process begins with dissolving granulated sucrose in water to create a concentrated syrup, typically at 60-70% solids content, which provides an optimal medium for the reaction. An acid catalyst is then added, commonly citric acid, tartaric acid, or hydrochloric acid, to lower the pH and initiate hydrolysis; food-grade organic acids like citric are preferred in applications requiring mild flavors, while inorganic acids such as hydrochloric are used for efficiency in large-scale operations.[7][8] The mixture is heated to 50-80°C, with the exact temperature varying by acid type—for instance, hydrochloric acid reactions often occur at 70-80°C—while maintaining a pH of 2-4 to accelerate inversion without excessive degradation. The reaction typically requires 30-60 minutes to achieve near-complete conversion, during which the solution is agitated to ensure uniform heating and catalysis by hydrogen ions. This controlled environment minimizes side reactions, such as the formation of colored by-products like hydroxymethylfurfural, which can arise from over-acidification or overheating leading to caramelization.[9][7][10] The underlying hydrolysis reaction follows the equation: This cleaves the glycosidic bond in sucrose, yielding an equimolar mixture of glucose and fructose that imparts the syrup's characteristic properties.[11] Upon completion, the acid catalyst is neutralized by adding a base such as sodium bicarbonate, raising the pH to 4.5-6 to halt the reaction and remove residual acidity, thereby producing a stable, clear syrup suitable for further processing or direct use.[12][10] In industrial settings, this method is conducted in large batches or continuous flow systems, often integrated into cane sugar refining, offering cost-effective scalability for food manufacturing due to the simplicity and low cost of acid catalysts compared to alternatives.[10]Enzymatic Methods
Enzymatic methods for producing inverted sugar syrup rely on the enzyme invertase, also known as β-fructofuranosidase, sourced from yeast such as Saccharomyces cerevisiae or other microbial strains, to catalyze the hydrolysis of sucrose into an equimolar mixture of glucose and fructose.[13] This approach operates at pH 4.5-5.5 and mild temperatures of 40-60°C, enabling the reaction to proceed efficiently without the need for harsh conditions.[14] The process typically begins with the immobilization of invertase onto solid supports, such as gelatine beads, corn grits, or other matrices, to allow for enzyme reusability and easy recovery.[15][16] A sucrose solution, often at concentrations of 50-70%, is then incubated with the immobilized enzyme for several hours in batch processes or minutes in continuous setups to ensure near-complete inversion, followed by filtration to remove the enzyme.[14][15] This batch or continuous setup contrasts with faster but more aggressive traditional alternatives by prioritizing controlled, low-energy operation.[16] The underlying hydrolysis reaction is: The enzyme's kinetics follow the Michaelis-Menten model, , where values for sucrose typically range from 10 to 30 mM, indicating moderate substrate affinity suitable for industrial-scale syrup production.[17][13] These methods offer significant advantages, including the preservation of delicate flavors and aromas due to the absence of acid byproducts or high-heat degradation.[14][16] Furthermore, invertase hydrolysis can be sequentially combined with glucose isomerase treatment to generate high-fructose syrup variants with enhanced sweetness for specialized applications. Commercial enzymatic production of inverted sugar syrup, leveraging immobilized invertase, emerged in the 1970s as one of the earliest industrial biocatalysis successes and remains prevalent in organic and premium product lines for its superior purity and quality control.[19][15]Physical and Sensory Properties
Solubility and Viscosity
Inverted sugar syrup exhibits higher solubility in water compared to sucrose, primarily due to its fructose content, allowing up to approximately 77% solids concentration in stable solutions at room temperature without crystallization. This enhanced solubility stems from the greater water affinity of fructose (solubility exceeding 375 g/100 ml at 20°C) over glucose, enabling the syrup to remain fully dissolved and prevent crystal formation in high-sugar mixtures.[2][20][21] The viscosity of inverted sugar syrup is significantly higher than that of water, typically ranging from 100 to 500 cP at 20°C depending on concentration, with values around 500 mPas for 70-73% solids syrups. This thickness provides a smooth, pourable consistency but decreases with increasing temperature or dilution, facilitating easier handling in warmer conditions or lower-solid formulations. Higher concentrations of solids lead to elevated viscosity and reduced pourability, making the syrup more gel-like at levels above 80% solids.[22] Due to its hygroscopic nature, inverted sugar syrup readily absorbs moisture from the air, which enhances its role as a humectant in applications but necessitates storage in sealed containers to prevent dilution or microbial growth. The density of typical 70-80% solids syrups ranges from 1.3 to 1.4 g/cm³ at 20°C, reflecting the concentrated aqueous nature of the product.[23][22]Taste and Stability
Inverted sugar syrup is approximately 1.3 times sweeter than sucrose on a weight basis, owing to the enhanced sweetness intensity of its fructose component compared to glucose or sucrose alone.[1] This composition delivers a clean, rapid sweetness onset with a harmonious, mild flavor profile that enhances fruit notes without bitterness.[24] In sensory evaluations, it exhibits less aftertaste than high-fructose corn syrup, attributed to its balanced 1:1 glucose-fructose ratio versus the higher fructose content in HFCS.[1] A key advantage of inverted sugar syrup is its exceptional stability against crystallization; the equimolar mixture of glucose and fructose disrupts sucrose crystal formation, allowing the syrup to remain liquid indefinitely under appropriate storage conditions, in contrast to sucrose solutions that crystallize readily.[4] This property stems from the monosaccharides' interference with nucleation sites, providing consistent liquidity for extended periods.[24] The shelf life of inverted sugar syrup typically reaches up to 12 months in unopened containers stored at around 20°C, with potential extension beyond this if kept below 25°C in a clean, dry environment to minimize risks.[24] However, contamination can lead to fermentation by yeasts or bacteria, though the syrup's high solute concentration results in a low water activity (aw < 0.85), which inherently limits microbial growth and bolsters stability.[25] Inverted sugar syrup maintains a pH range of 3.5 to 5.0, conferring a mildly acidic character that further aids preservation by creating an unfavorable environment for many spoilage organisms.[26] This acidity, combined with low aw, synergistically extends usability without additional preservatives.[24]Chemical Properties
Hydrolysis Reaction
The hydrolysis of sucrose to form inverted sugar syrup involves the reversible cleavage of the glycosidic bond in sucrose (C₁₂H₂₂O₁₁), a disaccharide composed of α-D-glucose and β-D-fructose, yielding equimolar amounts of D-glucose and D-fructose monosaccharides. The reaction is represented as: This process is a first-order reversible reaction with respect to sucrose concentration under typical conditions, where the forward hydrolysis predominates due to a large equilibrium constant (K_eq ≈ 4.4 × 10⁴ at 25°C).[27] The kinetics of the reaction follow first-order rate laws, with the rate constant exhibiting temperature dependence described by the Arrhenius equation: , where is the pre-exponential factor, is the activation energy, is the gas constant, and is the absolute temperature. The rate increases with rising temperature, higher acid concentration in catalyzed pathways, and greater water activity, as these factors lower the energy barrier and enhance molecular collisions. For the acid-catalyzed pathway, the activation energy is approximately 107–109 kJ/mol, while the enzymatic pathway using invertase has a lower activation energy of about 25–36 kJ/mol, enabling milder conditions.[28][29] At equilibrium, the degree of inversion—defined as the percentage of sucrose hydrolyzed—approaches nearly 100% under standard aqueous conditions, as the high K_eq results in negligible residual sucrose (typically <0.1% for initial concentrations around 1 M). This near-complete conversion is foundational to the production of inverted sugar syrup, where process conditions are adjusted to accelerate attainment of equilibrium.[27] Under extreme conditions, such as prolonged high-temperature or high-acid exposure, minor byproducts may form, such as di-fructose dianhydrides via fructose condensation and 5-hydroxymethylfurfural from fructose dehydration, though these constitute less than 1–2% of the total saccharides.[30]Optical Rotation Characteristics
Inverted sugar syrup exhibits distinct optical rotation characteristics due to the chiral nature of its constituent sugars, which arise from the hydrolysis of sucrose. Sucrose itself has a positive specific rotation of +66.5° at 20°C using the sodium D-line, while the hydrolysis products, D-glucose and D-fructose, possess specific rotations of +52.7° and -92°, respectively. The dominant levorotatory effect of fructose causes the overall rotation of the mixture to invert from positive to negative as hydrolysis proceeds.[31] The inversion point, defined as the stage where the net optical rotation of the solution is zero, occurs at approximately 77% hydrolysis under standard conditions, corresponding to Fehling's method adjusted for temperature variations in polarimetric analysis. At this point, the remaining unhydrolyzed sucrose's positive rotation is exactly balanced by the negative rotation from the equimolar glucose-fructose mixture produced. Beyond this, further hydrolysis results in a levorotatory solution, with fully inverted sugar syrup typically exhibiting a specific rotation of approximately -20° at equilibrium.[32][33] Optical rotation is measured using a polarimeter, which quantifies the angle through which plane-polarized light is rotated by the sample. The specific rotation is calculated as , where is the observed rotation in degrees, is the concentration in g/mL, and is the path length in decimeters. This measurement allows precise tracking of hydrolysis progress, as the rotation shifts from the initial +66° for sucrose solutions to -20° for fully inverted syrup over time.[34][35] All components in inverted sugar syrup—sucrose, D-glucose, and D-fructose—are chiral D-sugars, with multiple asymmetric carbons contributing to their optical activity. Post-hydrolysis, mutarotation occurs as the α- and β-anomers of glucose and fructose equilibrate in solution, stabilizing the specific rotation values (e.g., fructose shifts from an initial -132° for the α-anomer to -92° at equilibrium). This anomeric interconversion via ring opening and closing slightly influences the observed rotation immediately after hydrolysis but reaches steady state rapidly.[36][32] Dilution of the syrup with water shifts the hydrolysis equilibrium slightly toward further inversion, per Le Chatelier's principle, as the reaction consumes water to produce the monosaccharides; however, this effect is minor in typical syrup concentrations. Partial inversion typically yields rotations between 0° and -20°, enabling quality control assessments in production by monitoring the polarimetric change from +66° to the equilibrium value.[30][35]Culinary and Industrial Applications
In Baking and Confectionery
Inverted sugar syrup plays a crucial role in baking and confectionery by preventing undesirable sugar crystallization in products such as icings, fondants, and creams. The monosaccharides glucose and fructose in the syrup interfere with sucrose crystal formation, resulting in smoother, more stable textures that enhance machinability and sensory appeal.[37] In fondants, enzymatic hydrolysis using invertase converts sucrose to inverted sugar, effectively retarding crystallization while achieving the necessary solids content for microbial stability.[37] As a humectant, inverted sugar syrup retains moisture in baked goods like cookies and cakes, promoting prolonged softness and tenderness by binding water molecules and reducing drying out.[38] This property allows for partial replacement of sucrose in formulations to maintain optimal texture.[39] In confectionery applications, inverted sugar syrup is employed in fruit preserves and jellies to inhibit crystallization.[39] It also contributes to smoother textures in caramel production by acting as an interfering agent that limits sucrose recrystallization during cooking.[40] These applications leverage the syrup's stability to extend shelf life.In Beverages and Fermentation
Inverted sugar syrup is widely utilized in the production of soft drinks and cordials due to its enhanced solubility, which promotes clarity and prevents the settling of sugar crystals that can occur with sucrose.[4] This property ensures a more uniform distribution of sweetness throughout the beverage, avoiding gritty residues at the bottom of containers.[41] In fermentation processes, such as brewing and winemaking, inverted sugar syrup is preferred over sucrose because its monosaccharides—glucose and fructose—are directly fermentable by yeast, eliminating the need for enzymatic inversion during the initial stages of metabolism.[42] This direct utilization leads to more efficient yeast activity and faster fermentation rates compared to disaccharide-based sweeteners.[43] It is frequently combined with preservatives in beverage formulations to maintain stability and inhibit microbial growth.[44] In beer production, partial invert syrups, which retain some sucrose alongside glucose and fructose, are employed to boost alcohol yields by enhancing fermentable content without introducing off-flavors or unwanted byproducts.[43] On an industrial scale, inverted sugar has been a key component in soda production throughout the 20th century, contributing to consistent sweetness and texture in carbonated beverages.[45] For instance, it is incorporated into energy drinks to provide rapid energy through its simple sugars, which are quickly absorbed by the body for immediate metabolic support.[46] A notable challenge in using inverted sugar syrup in heated beverages arises from its high fructose content, a reducing sugar that can participate in the Maillard reaction with amino acids, leading to undesirable browning if temperature and processing conditions are not carefully controlled.[47]Advantages and Comparisons
Versus Regular Sucrose
Inverted sugar syrup offers greater sweetness per unit weight than regular sucrose, primarily due to the higher sweetness intensity of its fructose component, which is approximately 1.2–1.8 times sweeter than sucrose on a molar basis.[48] This allows for reduced usage in formulations to achieve equivalent sweetness levels, but when matching the bulk volume provided by granulated sucrose—such as in recipes requiring structural fill—inverted sugar syrup demands a larger volume because it is a liquid with lower density compared to the crystalline solid form of sucrose.[49] In contrast, sucrose readily crystallizes upon cooling or concentration, forming a stable granular structure, whereas inverted sugar syrup remains fluid and resists crystallization, making it unsuitable for applications where a crystalline texture is desired.[48] The production of inverted sugar syrup incurs higher costs than sucrose, often due to the additional energy-intensive hydrolysis step, rendering it more expensive—typically perceived as significantly costlier in market analyses—though it can offset expenses by eliminating the need for separate anti-crystallization agents in formulations.[50][11] Sucrose, derived directly from cane or beet extraction without inversion, benefits from simpler processing and lower overall production expenses.[11] In terms of functionality, inverted sugar syrup lowers water activity in food products more effectively than sucrose, which contributes to extended shelf life by inhibiting microbial growth and moisture migration.[45][49] For instance, sucrose excels in providing structural rigidity through controlled crystallization in hard candies and fondants, where its ability to form large, stable crystals defines the product's texture.[51] However, inverted sugar syrup's humectant properties enhance moisture retention, preventing drying out in soft confections. During processing, inverted sugar syrup integrates more rapidly into mixtures owing to its pre-dissolved, low-viscosity state, unlike sucrose which requires time to dissolve and may lead to uneven distribution if not fully solubilized.[51] In jam production, for example, using inverted sugar syrup results in smoother, more homogeneous spreads with reduced graininess, as its resistance to recrystallization yields a gel-like consistency superior to the potentially gritty texture from sucrose.[45] Both inverted sugar syrup and sucrose originate from similar plant sources like sugarcane or sugar beets, but the inversion process for the former involves additional energy for acid or enzymatic hydrolysis, increasing the overall environmental footprint through higher thermal and electrical demands compared to sucrose refining.[15][11]Nutritional and Health Considerations
Inverted sugar syrup provides approximately 3.2 to 3.4 kcal per gram, comparable to sucrose, as it consists of roughly equal parts glucose and fructose, both of which yield about 4 kcal per gram in their pure form but are typically present in a 70-80% solids solution.[41][52] The glucose component offers rapid energy absorption due to its direct uptake by cells for immediate metabolism, while the fructose is primarily metabolized in the liver, where it is converted to energy or stored as glycogen or fat.[41][53] The glycemic index of inverted sugar syrup is estimated at 50-60, lower than sucrose's 65, primarily because fructose has a low glycemic impact (around 19) that tempers the high index of glucose (100), though excessive consumption can still lead to blood sugar spikes due to the overall carbohydrate load.[54] Health concerns associated with inverted sugar syrup mirror those of other added sugars, particularly the fructose content, which in high amounts is linked to metabolic issues such as nonalcoholic fatty liver disease through increased hepatic lipogenesis and ATP depletion in liver cells.[53][55] Unlike some sweeteners, inverted sugar syrup contains no unique additives beyond its base monosaccharides, but overconsumption contributes to broader risks like insulin resistance when exceeding recommended limits.[56] The U.S. Food and Drug Administration classifies inverted sugar as generally recognized as safe (GRAS) for use as a nutritive sweetener in food products.[57] Daily intake recommendations for inverted sugar syrup align with those for added sugars, with the World Health Organization advising less than 10% of total caloric intake from free sugars to minimize health risks.[58] Post-2020 research highlights associations between monosaccharides, such as those found in inverted sugar syrup, and altered gut microbial diversity, potentially influencing insulin resistance.[56]References
- https://www.[researchgate](/page/ResearchGate).net/publication/229083869_Fructose_Syrup_A_Biotechnology_Asset



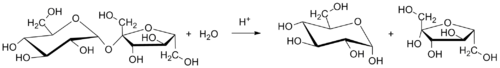

![{\displaystyle [\alpha ]}](https://wikimedia.org/api/rest_v1/media/math/render/svg/f53a93c296d4695d3466a11d6aa93650ebd86c3e)



![{\displaystyle [\alpha ]_{i}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/a9dad4ac16675fce62b84e8db9af3212c06fc8eb)
![{\displaystyle \displaystyle \alpha ={\frac {\sum _{i=1}^{N}C_{i}[\alpha ]_{i}}{\sum _{i=1}^{N}C_{i}}}=\sum _{i=1}^{N}\left({\frac {C_{i}}{\sum _{i=1}^{N}C_{i}}}\right)[\alpha ]_{i}=\sum _{i=1}^{N}\chi _{i}[\alpha ]_{i}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/e57d93862ff6297340de521fe683f600034d0e28)


![{\displaystyle \displaystyle \alpha ={\frac {1}{2}}[\alpha ]_{\text{glucose}}+{\frac {1}{2}}[\alpha ]_{\text{fructose}}={\frac {1}{2}}(52.7^{\circ }-92.0^{\circ })=-19.7^{\circ }}](https://wikimedia.org/api/rest_v1/media/math/render/svg/8fa344a5b36e0f6b146ec72ac428090eb829f285)
![{\displaystyle \displaystyle \alpha =\chi _{s}[\alpha ]_{s}+\chi _{g}[\alpha ]_{g}+\chi _{f}[\alpha ]_{f}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/d3071aefface597dd764b9ede0c40f55a7a0d350)












![{\displaystyle \displaystyle \alpha _{r}={\frac {(x_{0}-x)[\alpha ]_{s}+x[\alpha ]_{g}+x[\alpha ]_{f}}{x_{0}+x}}={\frac {1}{1+r}}\left([\alpha ]_{s}+([\alpha ]_{g}+[\alpha ]_{f}-[\alpha ]_{s})r\right)}](https://wikimedia.org/api/rest_v1/media/math/render/svg/916d0ae30349c3aa322c0450ff487aa324d4ff43)
![{\displaystyle \displaystyle r_{\text{inversion}}={\frac {[\alpha ]_{s}}{[\alpha ]_{s}-[\alpha ]_{g}-[\alpha ]_{f}}}=0.629}](https://wikimedia.org/api/rest_v1/media/math/render/svg/11554362cb86f8b98b79202e2e580d1528c2f7b1)
