Recent from talks
Nothing was collected or created yet.
Lactone
View on WikipediaLactones are cyclic carboxylic esters. They are derived from the corresponding hydroxycarboxylic acids by esterification. They can be saturated or unsaturated.[1]
Lactones are formed by lactonization, the intramolecular esterification of the corresponding hydroxycarboxylic acids.[2]
Nomenclature
[edit]Greek prefixes in alphabetical order indicate ring size.
| Ring size (number of atoms in the ring) |
Systematic name | IUPAC name | Parent lactone | Structure, comment |
|---|---|---|---|---|
| 3 | α-lactone | Oxiran-2-one | Acetolactone |  |
| 4 | β-lactone | Oxetan-2-one |
|

|
| 5 | γ-lactone | Oxolan-2-one | γ-Butyrolactone | 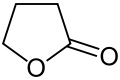
|
| 6 | δ-lactone | Oxan-2-one | 
| |
| 7 | ε-lactone | Oxepan-2-one |
|

|
Lactones are usually named according to the precursor acid molecule (aceto = 2 carbon atoms, propio = 3, butyro = 4, valero = 5, capro = 6, etc.), with a -lactone suffix and a Greek letter prefix that specifies the number of carbon atoms in the heterocycle — that is, the distance between the relevant -OH and the -COOH groups along said backbone. The first carbon atom after the carbon in the -COOH group on the parent compound is labelled α, the second will be labeled β, and so forth. Therefore, the prefixes also indicate the size of the lactone ring: α-lactone = 3-membered ring, β-lactone = 4-membered, γ-lactone = 5-membered, δ-lactone = 6-membered, etc. Macrocyclic lactones are known as macrolactones.[3]
The other suffix used to denote a lactone is -olide, used in substance class names like butenolide, macrolide, cardenolide or bufadienolide.
To obtain the preferred IUPAC names, lactones are named as heterocyclic pseudoketones by adding the suffix 'one', 'dione', 'thione', etc. and the appropriate multiplicative prefixes to the name of the heterocyclic parent hydride.[4]
Etymology
[edit]The name lactone derives from the ring compound called lactide, which is formed from the dehydration of 2-hydroxypropanoic acid (lactic acid) CH3-CH(OH)-COOH. Lactic acid, in turn, derives its name from its original isolation from soured milk (Latin: lac, lactis). The name was coined in 1844 by the French chemist Théophile-Jules Pelouze, who first obtained it as a derivative of lactic acid.[5] An internal dehydration reaction within the same molecule of lactic acid would have produced alpha-propiolactone, a lactone with a 3-membered ring.
In 1880 the German chemist Wilhelm Rudolph Fittig extended the name "lactone" to all intramolecular carboxylic esters.[6]
Occurrence
[edit]
Lactone rings occur widely as building blocks in nature, such as in ascorbic acid, kavain, nepetalactone, gluconolactone, hormones (spironolactone, mevalonolactone), enzymes (lactonase), neurotransmitters (butyrolactone, avermectins), antibiotics (macrolides like erythromycin; amphotericin B), anticancer drugs (vernolepin, epothilones), phytoestrogens (resorcylic acid lactones, cardiac glycosides).
5-Membered γ-lactones and 6-membered δ-lactones are prevalent. β-lactones appear in a number of natural products.[7] α‑Lactones can be detected as transient species in mass spectrometry experiments.[8]
Macrocyclic lactones are also important natural products.[9] Lactones are present in oak wood, and they contribute to the flavour profile of barrel-aged beers.[10]
Synthesis
[edit]
Many methods in ester synthesis can also be applied to that of lactones. Lactonization competes with polymerization for longer hydroxy acids, or the strained β‑lactones. γ‑Lactones, on the other hand, are so stable that 4-hydroxy acids (R-CH(OH)-(CH2)2-CO2H) spontaneously cyclize.
In one industrial synthesis of oxandrolone the key lactone-forming step is an organic reaction – esterification.[11][12]

In halolactonization, an alkene is attacked by a halogen via electrophilic addition with the cationic intermediate captured intramolecularly by an adjacent carboxylic acid.[13]
Specific methods include Yamaguchi esterification, Shiina macrolactonization, Corey-Nicolaou macrolactonization, Baeyer–Villiger oxidation and nucleophilic abstraction.

An alternative radical reaction yielding γ-lactones is the manganese-mediated coupling.
Reactions
[edit]Lactones exhibit the reactions characteristic of esters.
Hydrolysis and aminolysis
[edit]Heating a lactone with a base (sodium hydroxide) will hydrolyse the lactone to its parent compound, the straight chained bifunctional compound. Like straight-chained esters, the hydrolysis-condensation reaction of lactones is a reversible reaction, with an equilibrium. However, the equilibrium constant of the hydrolysis reaction of the lactone is lower than that of the straight-chained ester i.e. the products (hydroxyacids) are less favored in the case of the lactones. This is because although the enthalpies of the hydrolysis of esters and lactones are about the same, the entropy of the hydrolysis of lactones is less than the entropy of straight-chained esters. Straight-chained esters give two products upon hydrolysis, making the entropy change more favorable than in the case of lactones which gives only a single product.
Lactones also react with amines to give the ring-opened alcohol and amide.
Reduction
[edit]Lactones can be reduced to diols using lithium aluminium hydride. For instance, gamma-lactones is reduced to butane-1,4-diol, (CH2(OH)-(CH2)2-CH2(OH).
Polymerization
[edit]Some lactones convert to polyesters:[14][15] For example the double lactone called lactide polymerizes to polylactic acid (polylactide). The resulting polylactic acid has been heavily investigated for commercial applications.[16][17]
Uses
[edit]Flavors and fragrances
[edit]Lactones contribute significantly to the flavor of fruit, and of unfermented and fermented dairy products,[18] and are therefore used as flavors and fragrances.[9] Some examples are γ-decalactone (4-decanolide), which has a characteristic peach flavor;[18] δ-decalactone (5-decanolide), which has a creamy coconut/peach flavour; γ-dodecalactone (4-dodecanolide), which also has a coconut/fruity flavor,[18] a description which also fits γ-octalactone (4-octanolide),[19] although it also has a herbaceous character;[18] γ-nonalactone, which has an intense coconut flavor of this series, despite not occurring in coconut,[20] and γ-undecalactone.
Macrocyclic lactones (cyclopentadecanolide, 15-pentadec-11/12-enolide) have odors similar to macrocyclic ketones of animal origin (muscone, civetone).[9]
Plastics
[edit]Polycaprolactone is an important plastic. Its formation has even been considered in the context of the origin of life.[21]
Dilactones
[edit]- Ellagic acid (Hexahydroxydiphenic acid dilactone)
- Flavogallonic acid dilactone can be found in Rhynchosia volubilis seeds and in Shorea laeviforia
- Lactide
- Tergallic acid dilactone can be found in Rhynchosia volubilis seeds
- Valoneic acid dilactone can be isolated from the heartwood of Shorea laeviforia
- Ethylene brassylate (Musk T), a widely used synthetic musk
See also
[edit]References and notes
[edit]- ^ "lactones", Compendium of Chemical Terminology, 2.3.3, International Union of Pure and Applied Chemistry, 2014-02-24, p. 817
- ^ Francis A. Carey; Robert M. Giuliano (2011), Organic Chemistry (8th ed.), McGraw-Hill, pp. 798–799
- ^ Steven A. Hardinger. "Illustrated Glossary of Organic Chemistry". Department of Chemistry & Biochemistry, UCLA.
- ^ Nomenclature of Organic Chemistry : IUPAC Recommendations and Preferred Names 2013 (Blue Book). Cambridge: The Royal Society of Chemistry. 2014. p. 822. doi:10.1039/9781849733069-00648. ISBN 978-0-85404-182-4.
- ^ Pelouze, J. (9 December 1844). "Mémoire sur l'acide lactique" [Memoir on lactic acid]. Comptes rendus (in French). 19: 1219–1227.
From p. 1223: "Indépendamment de la lactide dont je viens de rappeler l'existence dans les produits de la distllation de l'acide lactique, celui-ci donne encore, par sa décomposition, une autre substance, que je propose d'appeler lactone, parce qu'elle me paraît être à l'acide lactique ce que l'acétone est à l'acide acétique." (Independently of the lactide of which I have just recalled the existence in the products of the distillation of lactic acid, this [i.e., lactic acid] gives further, by its decomposition, another substance, which I propose to call lactone, because it seems to me to be to lactic acid what acetone is to acetic acid.)
- Reprinted: Pelouze, J. (1845). "Mémoire sur l'acide lactique" [Memoir on lactic acid]. Annales de Chimie et de Physique. 3rd series (in French). 13: 257–268. ; see p. 262.
- English translation: Pelouze, J. (January 15, 1845). "Researches on lactic acid". The Chemical Gazette. 3 (54): 29–35. ; see p. 31.
- Menten, Pierre de (2013). Dictionnaire de chimie: Une approche étymologique et historique [Dictionary of Chemistry: an etymological and historical approach] (in French). Brussels, Belgium: de boeck. p. 183. ISBN 9782804181758.
- ^ Fittig, Rudolph (1880). "Untersuchungen über ungesättige Säuren, dritte Abhandlung" [Investigations into unsaturated acids, third article]. Annalen der Chemie und Pharmacie (in German). 200: 1–96. doi:10.1002/jlac.18802000102. From p. 62: "Es ist wünschenswerth, für diese Gruppe von Verbindungen, deren bis jetzt einfachster Repräsentant der im Vorstehenden beschriebene Körper ist, eine allgemeine Bezeichnungsweise zu haben, und da der Name "Lactide" nicht anwendbar ist, weil dann das Lactid κατ εξοχην kein Lactid sein wurde, so schlagen wir als Gruppenbezeichnung den Namen "Lactone" vor". (It's desirable for this group of compounds — whose simplest representative until now has been the substance that's described in the preceding — to have a general designation, and since the name "lactide" isn't applicable because then the archetypal lactide would not be a lactide, we therefore suggest the name "lactone" as the designation of this group [of compounds].)
- ^ Danheiser, Rick L.; Nowick, James S. (1991) [25 July 1990]. "A practical and efficient method for the synthesis of β‑lactones". Journal of Organic Chemistry. 56 (3): 1176–1185. doi:10.1021/jo00003a047.
- ^ Detlef Schröder, Norman Goldberg, Waltraud Zummack, Helmut Schwarz, John C. Poutsma and Robert R. Squires (1997), Generation of α-acetolactone and the acetoxyl diradical •CH2COO• in the gas phase. International Journal of Mass Spectrometry and Ion Processes, Volumes 165-166, November issue, Pages 71-82. doi:10.1016/S0168-1176(97)00150-X
- ^ a b c Karl-Georg Fahlbusch; et al. (2007), "Flavors and Fragrances", Ullmann's Encyclopedia of Industrial Chemistry (7th ed.), Wiley, pp. 74‒78
- ^ Oliver, Garrett. "The Oxford Companion to Beer definition of barrel-aging". Craft Beer and Brewing.
- ^ Development of a Commercial Process to Produce Oxandrolone John E. Cabaj, David Kairys, and Thomas R. Benson Org. Process Res. Dev.; 2007; 11(3) pp 378–388; (Article) doi:10.1021/op060231b
- ^ The complete reaction sequence is bromination to a haloketone (not displayed), elimination reaction with lithium chloride to an enone, organic oxidation by osmium tetroxide and lead tetraacetate with ring-opening and finally reduction of the aldehyde to the alcohol with sodium borohydride and intramolecular lactone formation
- ^ Organic Syntheses, Coll. Vol. 7, p.164 (1990); Vol. 64, p.175 (1986) Article link
- ^ Wilhelm Riemenschneider; Hermann M. Bolt (2007), "Esters, Organic", Ullmann's Encyclopedia of Industrial Chemistry (7th ed.), Wiley
- ^ Chandru, Kuhan; Jia, Tony Z.; Mamajanov, Irena; Bapat, Niraja; Cleaves, H. James (2020-10-16). "Prebiotic oligomerization and self-assembly of structurally diverse xenobiological monomers". Scientific Reports. 10 (1): 17560. Bibcode:2020NatSR..1017560C. doi:10.1038/s41598-020-74223-5. ISSN 2045-2322. PMC 7567815. PMID 33067516.
- ^ R. Auras; L.-T. Lim; S. E. M. Selke; H. Tsuji (2010). Poly(lactic acid): Synthesis, Structures, Properties, Processing, and Applications. Wiley. ISBN 978-0-470-29366-9.
- ^ Odile Dechy-Cabaret; Blanca Martin-Vaca; Didier Bourissou (2004). "Controlled Ring-Opening Polymerization of Lactide and Glycolide". Chem. Rev. 104 (12): 6147–76. doi:10.1021/cr040002s. PMID 15584698.
- ^ a b c d Berger, R.G., ed. (2007). Flavours and fragrances chemistry, bioprocessing and sustainability. Berlin: Springer. ISBN 9783540493396. Retrieved 2 July 2015.
- ^ Mehta, Bhavbhuti M.; Kamal-Eldin, Afaf; Iwanski, Robert Z., eds. (2012). Fermentation effects on food properties. Boca Raton: Taylor & Francis. p. 74. ISBN 9781439853351. Retrieved 2 July 2015.
- ^ Marsili, Ray, ed. (2007). Sensory-directed flavor analysis. Boca Raton, FL: CRC/Taylor & Francis. p. 242. ISBN 9781420017045. Retrieved 2 July 2015.
- ^ Chandru, Kuhan; Mamajanov, Irena; Cleaves, H. James; Jia, Tony Z. (January 2020). "Polyesters as a Model System for Building Primitive Biologies from Non-Biological Prebiotic Chemistry". Life. 10 (1): 6. Bibcode:2020Life...10....6C. doi:10.3390/life10010006. PMC 7175156. PMID 31963928.

