Recent from talks
Nothing was collected or created yet.
Monoglyceride
View on Wikipedia

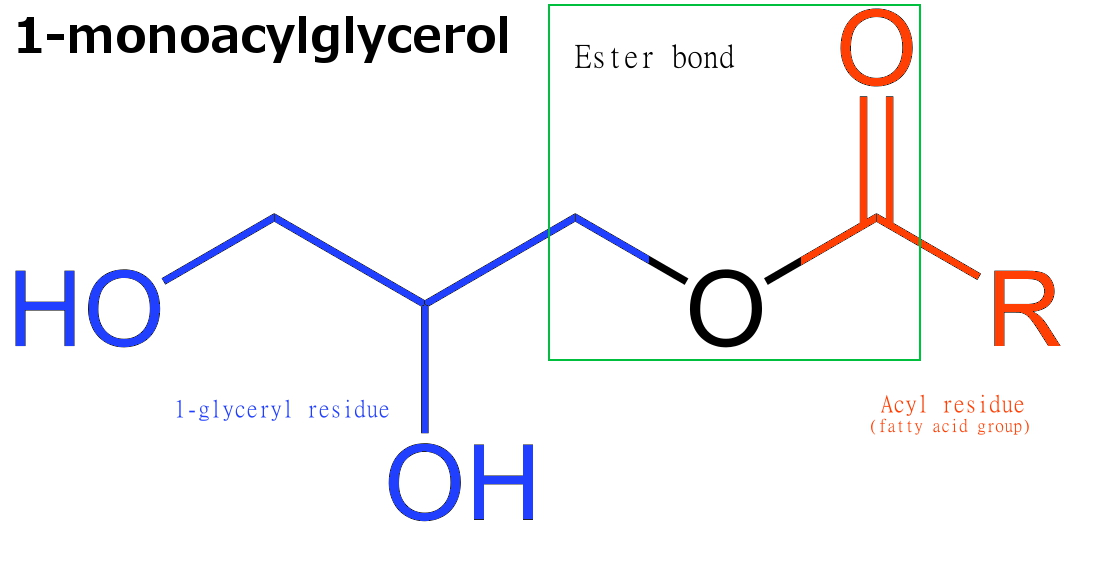
Monoglycerides (also: acylglycerols or monoacylglycerols) are a class of glycerides which are composed of a molecule of glycerol linked to a fatty acid via an ester bond.[1] As glycerol contains both primary and secondary alcohol groups two different types of monoglycerides may be formed; 1-monoacylglycerols where the fatty acid is attached to a primary alcohol, or a 2-monoacylglycerols where the fatty acid is attached to the secondary alcohol.
Synthesis
[edit]Monoglycerides are produced both biologically and industrially. They are naturally present at very low levels (0.1-0.2%) in some seed oils such as olive oil, rapeseed oil and cottonseed oil.[2] They are biosynthesized by the enzymatic hydrolysis of triglycerides by lipoprotein lipase and the enzymatic hydrolysis of diglycerides by diacylglycerol lipase; or as an intermediate in the alkanoylation of glycerol to form fats. Several monoglycerides are pharmacologically active (e.g. 2-oleoylglycerol, 2-arachidonoylglycerol[3]).
Industrial production is primarily achieved by a glycerolysis reaction between triglycerides and glycerol.[4] The commercial raw materials for the production of monoacylglycerols may be either vegetable oils or animal fats.
Uses
[edit]Monoglycerides are primarily used as surfactants, usually in the form of emulsifiers. Together with diglycerides, monoglycerides are commonly added to commercial food products in small quantities as "E471" (s.a. Mono- and diglycerides of fatty acids), which helps to prevent mixtures of oils and water from separating. They are also often found in bakery products, beverages, ice cream, chewing gum, shortening, whipped toppings, margarine, spreads, and peanut butter,[5] and confections.[6] In bakery products, monoglycerides are useful in improving loaf volume and texture, and as antistaling agents.[7][8] Monoglycerides are used to enhance the physical stability towards creaming in milk beverages.[9]
Examples
[edit]-
Monolaurin, commonly used in cosmetics and as a food additive
-
Glycerol monostearate, used in foods as a thickening, emulsifying, and preservative agent
-
Glyceryl hydroxystearate, found in a variety of cosmetic and skin care products.
See also
[edit]References
[edit]- ^ IUPAC, Compendium of Chemical Terminology, 5th ed. (the "Gold Book") (2025). Online version: (2006–) "glycerides". doi:10.1351/goldbook.G02647
- ^ Flickinger, Brent D.; Matsuo, Noboru (February 2003). "Nutritional characteristics of DAG oil". Lipids. 38 (2): 129–132. doi:10.1007/s11745-003-1042-8. PMID 12733744. S2CID 4061326.
- ^ Hansen, KB; Rosenkilde, MM; Knop, FK; Wellner, N; Diep, TA; Rehfeld, JF; Andersen, UB; Holst, JJ; Hansen, HS (September 2011). "2-Oleoyl glycerol is a GPR119 agonist and signals GLP-1 release in humans". The Journal of Clinical Endocrinology and Metabolism. 96 (9): E1409–17. doi:10.1210/jc.2011-0647. PMID 21778222.
- ^ Sonntag, Norman O. V. (1982). "Glycerolysis of fats and methyl esters — Status, review and critique". Journal of the American Oil Chemists' Society. 59 (10): 795A – 802A. doi:10.1007/BF02634442. ISSN 0003-021X. S2CID 84808531.
- ^ Jain, Niharika (27 September 2021). "Using Distilled Monoglycerides (DMG) in Peanut Butter". Source Good Food. Retrieved 27 September 2021.
- ^ Zdzislaw Z. E. Sikorski; Anna Kolakowska (30 July 2002). Chemical and Functional Properties of Food Lipids. CRC Press. pp. 218–. ISBN 978-1-4200-3199-7.
- ^ Y. H. Hui (15 February 2008). Bakery Products: Science and Technology. John Wiley & Sons. pp. 350–. ISBN 978-0-470-27632-7.
- ^ Gerard L. Hasenhuettl; Richard W. Hartel (1 January 1997). Food Emulsifiers and Their Applications. Springer. ISBN 978-0-412-07621-3.
- ^ Loi, Chia Chun; Eyres, Graham T.; Birch, E. John (2019). "Effect of mono- and diglycerides on physical properties and stability of a protein-stabilised oil-in-water emulsion". Journal of Food Engineering. 240: 56–64. doi:10.1016/j.jfoodeng.2018.07.016. ISSN 0260-8774. S2CID 106021441.