Recent from talks
Nothing was collected or created yet.
Norgestrel
View on Wikipedia
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Opill, others |
| Other names | dl-Norgestrel; DL-Norgestrel; (±)-Norgestrel; WY-3707; SH-70850; SH-850; FH 122-A; rac-13-Ethyl-17α-ethynyl-19-nortestosterone; rac-13-Ethyl-17α-ethynylestr-4-en-17β-ol-3-one |
| AHFS/Drugs.com | Micromedex Detailed Consumer Information |
| MedlinePlus | a602008 |
| License data |
|
| Routes of administration | By mouth |
| Drug class | Progestin |
| ATC code | |
| Legal status | |
| Legal status | |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.026.758 |
| Chemical and physical data | |
| Formula | C21H28O2 |
| Molar mass | 312.453 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
Norgestrel, sold under the brand name Opill among others, is a progestin which is used in birth control pills. It is often combined with the estrogen ethinylestradiol, marketed as Ovral. It is also used in menopausal hormone therapy.[3][4][5][6][7] It is taken by mouth.[5][6]
Side effects of norgestrel include menstrual irregularities, headaches, nausea, and breast tenderness.[8] The most common side effects of the norgestrel include irregular bleeding, headaches, dizziness, nausea, increased appetite, abdominal pain, cramps, or bloating.[2] Norgestrel is a progestin, or a synthetic progestogen, and hence is an agonist of the progesterone receptor, the biological target of progestogens like progesterone.[6] It has weak androgenic activity and no other important hormonal activity.[6]
Norgestrel was patented in 1961 and came into medical use, specifically in birth control pills, in 1966.[9][10][11] It was subsequently introduced for use in menopausal hormone therapy as well.[7] Norgestrel is sometimes referred to as a "second-generation" progestin.[12] It is marketed widely throughout the world.[7][4] Norgestrel is available as a generic medication.[13] In 2022, the version with ethinylestradiol was the 264th most commonly prescribed medication in the United States, with more than 1 million prescriptions.[14][15] In July 2023, the US Food and Drug Administration (FDA) approved norgestrel for over-the-counter sale.[2]
Medical uses
[edit]Norgestrel is used in combination with ethinylestradiol or quinestrol in combined birth control pills, alone in progestogen-only birth control pills, and in combination with estradiol or conjugated estrogens in menopausal hormone therapy.[7] It has also been used as an emergency contraceptive in the Yuzpe regimen.[16]
Side effects
[edit]Pharmacology
[edit]Pharmacodynamics
[edit]Norgestrel is a progestogen, or an agonist of the progesterone receptor.[6] The biological activity of norgestrel lies in the levo enantiomer, levonorgestrel, whereas the dextro isomer is inactive.[6] As such, norgestrel is identical in its hormonal activity to levonorgestrel except that it is half as potent by weight.[6] Levonorgestrel, and by extension norgestrel, have some androgenic activity, but no estrogenic, antimineralocorticoid, or glucocorticoid activity.[6]
| Compound | PR | AR | ER | GR | MR | SHBG | CBG |
|---|---|---|---|---|---|---|---|
| Levonorgestrel | 150–162 | 34a, 45 | 0 | 1–8 | 17–75 | 50 | 0 |
| 5α-Dihydrolevonorgestrel | 50 | 38a | 0 | ? | ? | ? | ? |
| 3α,5α-Tetrahydrolevonorgestrel | ? | ? | 0.4 | ? | ? | ? | ? |
| 3β,5α-Tetrahydrolevonorgestrel | ? | ? | 2.4 | ? | ? | ? | ? |
| Notes: Values are percentages (%). Reference ligands (100%) were promegestone for the PR, metribolone (a = mibolerone) for the AR, E2 for the ER, DEXA for the GR, aldosterone for the MR, DHT for SHBG, and cortisol for CBG. Sources: See template. | |||||||
The ovulation-inhibiting dose of norgestrel appears to be greater than 75 μg/day, as ovulation occurred in 50 to 75% of cycles with this dosage of norgestrel in studies.[17] The ovulation-inhibiting dosage of levonorgestrel, which is twice as potent as norgestrel, is approximately 50 to 60 μg/day.[6][18][17] One review lists the ovulation-inhibiting dose of norgestrel as 100 μg/day.[19] The endometrial transformation dose of norgestrel is listed as 12 mg per cycle and the menstrual delay test dose of norgestrel is listed as 0.5 to 2 mg/day.[19][20]
Pharmacokinetics
[edit]The pharmacokinetics of norgestrel have been reviewed.[21]
Chemistry
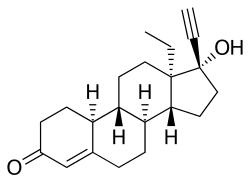
[edit]Norgestrel, also known as rac-13-ethyl-17α-ethynyl-19-nortestosterone or as rac-13-ethyl-17α-ethynylestr-4-en-17β-ol-3-one, is a synthetic estrane steroid and a derivative of testosterone.[3][4] It is a racemic mixture of stereoisomers dextronorgestrel (the C13α isomer; l-norgestrel, L-norgestrel, or (+)-norgestrel) and levonorgestrel (the C13β isomer; d-norgestrel, D-norgestrel, or (–)-norgestrel), the former of which is inactive (making norgestrel exactly half as potent as levonorgestrel).[22][23] Norgestrel is more specifically a derivative of norethisterone (17α-ethynyl-19-nortestosterone) and is a member of the gonane (18-methylestrane) subgroup of the 19-nortestosterone family of progestins.[24]
Synthesis
[edit]Chemical syntheses of norgestrel have been published.[21]
History
[edit]Norgestrel was first introduced, as a birth control pill in combination with ethinylestradiol, under the brand name Eugynon in Germany in 1966.[9][10] It was subsequently marketed as a combined birth control pill with ethinylestradiol in the United States under the brand name Ovral in 1968, and was marketed in many other countries as well.[25][26][7]
The contraceptive efficacy of norgestrel was established in the U.S. with the original approval for prescription use in 1973.[2]
In July 2023, the FDA approved norgestrel for over-the-counter sale.[2][27] The FDA granted the approval to Laboratoire HRA Pharma which was acquired by Perrigo Company plc.[2]
Society and culture
[edit]Generic names
[edit]Norgestrel is the generic name of the drug and its international nonproprietary name, United States Adopted Name, United States Pharmacopeia, British Approved Name, Dénomination Commune Française, Denominazione Comune Italiana, and Japanese Accepted Name.[3][4][5][7] It is also known as dl-norgestrel, DL-norgestrel, or (±)-norgestrel.[3][4][5][7]
Brand names
[edit]Norgestrel is marketed under a variety of brand names including Cyclacur, Cryselle, Cyclo-Progynova, Duoluton, Elinest, Eugynon, Microgynon, Lo/Ovral, Low-Ogestrel, Logynon, Microlut, Minicon, Nordette, Neogest, Opill, Ogestrel, Ovral, Ovran, Ovranette, Ovrette, Planovar, Prempak, Progyluton, and Trinordiol among others.[3][4][7][25]
References
[edit]- ^ "Opill- norgestrel tablet". DailyMed. 4 March 2024. Archived from the original on 11 March 2024. Retrieved 13 March 2024.
- ^ a b c d e f "FDA Approves First Nonprescription Daily Oral Contraceptive". U.S. Food and Drug Administration (FDA) (Press release). 13 July 2023. Archived from the original on 13 July 2023. Retrieved 13 July 2023.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- ^ a b c d e Elks J (14 November 2014). The Dictionary of Drugs: Chemical Data: Chemical Data, Structures and Bibliographies. Springer. pp. 887–. ISBN 978-1-4757-2085-3.
- ^ a b c d e f Index Nominum 2000: International Drug Directory. Taylor & Francis. 2000. pp. 751–. ISBN 978-3-88763-075-1.
- ^ a b c d Morton IK, Hall JM (6 December 2012). Concise Dictionary of Pharmacological Agents: Properties and Synonyms. Springer Science & Business Media. pp. 202–. ISBN 978-94-011-4439-1. Archived from the original on 10 January 2023. Retrieved 10 March 2018.
- ^ a b c d e f g h i Kuhl H (2005). "Pharmacology of estrogens and progestogens: influence of different routes of administration" (PDF). Climacteric. 8 (Suppl 1): 3–63. doi:10.1080/13697130500148875. PMID 16112947. S2CID 24616324. Archived (PDF) from the original on 22 August 2016. Retrieved 10 March 2018.
- ^ a b c d e f g h "Norgestrel - brand name list from". Drugs.com. Archived from the original on 9 January 2021. Retrieved 17 September 2022.
- ^ "Learn more about Opill (0.075mg Oral Norgestrel Tablet)". U.S. Food and Drug Administration (FDA). 13 July 2023. Archived from the original on 9 October 2023. Retrieved 13 March 2024.
- ^ a b Ortiz-Gómez T, Santesmases MJ (22 April 2016). Gendered Drugs and Medicine: Historical and Socio-Cultural Perspectives. Taylor & Francis. pp. 175–. ISBN 978-1-317-12981-3.
The 1966 marketing campaign for Schering's second contraceptive, Eugynon, [...] (Schering AG Berline 1966, 11). [...] In 1970 [Schering] had already conducted an opinion poll among doctors in the run up to the marketing campaign for the newly introduced Neogynon. [...]
- ^ a b Pohl WG (2004). Die wissenschaftliche Welt von gestern: die Preisträger des Ignaz L. Lieben-Preises 1865-1937 und des Richard Lieben-Preises 1912-1928: ein Kapitel österreichischer Wissenschaftsgeschichte in Kurzbiografien. Böhlau Verlag Wien. pp. 150–. ISBN 978-3-205-77303-0. Archived from the original on 12 January 2023. Retrieved 18 April 2018.
[The contraceptive Eugynon is launched in 1966. Neogynon follows in 1970.]
- ^ Fischer J, Ganellin CR (2006). Analogue-based Drug Discovery. John Wiley & Sons. p. 479. ISBN 9783527607495.
- ^ Carp HJ (9 April 2015). Progestogens in Obstetrics and Gynecology. Springer. p. 112. ISBN 978-3-319-14385-9.
- ^ "Generic Lo/Ovral-28 Availability". Archived from the original on 2 March 2019. Retrieved 10 March 2018.
- ^ "The Top 300 of 2022". ClinCalc. Archived from the original on 30 August 2024. Retrieved 30 August 2024.
- ^ "Ethinyl Estradiol; Norgestrel Drug Usage Statistics, United States, 2013 - 2022". ClinCalc. Retrieved 30 August 2024.
- ^ Yuzpe AA, Smith RP, Rademaker AW (April 1982). "A multicenter clinical investigation employing ethinyl estradiol combined with dl-norgestrel as postcoital contraceptive agent". Fertility and Sterility. 37 (4): 508–513. doi:10.1016/s0015-0282(16)46157-1. PMID 7040117.
- ^ a b Endrikat J, Gerlinger C, Richard S, Rosenbaum P, Düsterberg B (December 2011). "Ovulation inhibition doses of progestins: a systematic review of the available literature and of marketed preparations worldwide". Contraception. 84 (6): 549–57. doi:10.1016/j.contraception.2011.04.009. PMID 22078182.
- ^ Schindler AE, Campagnoli C, Druckmann R, Huber J, Pasqualini JR, Schweppe KW, et al. (December 2003). "Classification and pharmacology of progestins". Maturitas. 46 (Suppl 1): S7 – S16. doi:10.1016/j.maturitas.2003.09.014. PMID 14670641.
- ^ a b Knörr K, Knörr-Gärtner H, Beller FK, Lauritzen C (8 March 2013). Geburtshilfe und Gynäkologie: Physiologie und Pathologie der Reproduktion. Springer-Verlag. pp. 583–. ISBN 978-3-642-95583-9. Archived from the original on 11 January 2023. Retrieved 13 August 2022.
- ^ Leidenberger FA, Strowitzki T, Ortmann O (29 August 2009). Klinische Endokrinologie für Frauenärzte. Springer-Verlag. pp. 225, 227. ISBN 978-3-540-89760-6. Archived from the original on 14 July 2023. Retrieved 13 August 2022.
- ^ a b Die Gestagene. Springer-Verlag. 27 November 2013. pp. 16–17, 284–. ISBN 978-3-642-99941-3. Archived from the original on 14 July 2023. Retrieved 19 September 2018.
- ^ Alldredge BK, Corelli RL, Ernst ME (1 February 2012). Koda-Kimble and Young's Applied Therapeutics: The Clinical Use of Drugs. Lippincott Williams & Wilkins. pp. 1072–. ISBN 978-1-60913-713-7. Archived from the original on 12 January 2023. Retrieved 3 August 2017.
- ^ Lavery JP, Sanfilippo JS (6 December 2012). Pediatric and Adolescent Obstetrics and Gynecology. Springer Science & Business Media. pp. 248–. ISBN 978-1-4612-5064-7. Archived from the original on 12 January 2023. Retrieved 3 August 2017.
- ^ Offermanns S, Rosenthal W (14 August 2008). Encyclopedia of Molecular Pharmacology. Springer Science & Business Media. pp. 390–. ISBN 978-3-540-38916-3.
- ^ a b William Andrew Publishing (22 October 2013). Pharmaceutical Manufacturing Encyclopedia (3rd ed.). Elsevier. pp. 2935–. ISBN 978-0-8155-1856-3.
- ^ Marks L (2010). Sexual Chemistry: A History of the Contraceptive Pill. Yale University Press. pp. 73–. ISBN 978-0-300-16791-7.
- ^ "Archived copy" (PDF). Archived (PDF) from the original on 9 March 2024. Retrieved 13 March 2024.
{{cite web}}: CS1 maint: archived copy as title (link)
Norgestrel
View on GrokipediaMedical Applications
Contraceptive Indications
Norgestrel is utilized in progestin-only oral contraceptives (POPs) specifically for the prevention of pregnancy, administered as a daily tablet containing 0.075 mg of the synthetic progestin without an estrogen component. This formulation, exemplified by products like Opill, requires continuous intake at the same time each day to sustain contraceptive effects, with no scheduled pill-free intervals, accommodating users such as lactating women or those intolerant to estrogens.[9][2][10] The primary contraceptive mechanisms of norgestrel at this dose encompass suppression of ovulation in roughly half of cycles through disruption of follicular growth and gonadotropin release, alongside production of thick, viscous cervical mucus that reduces sperm motility and penetration, and modification of the endometrial lining to impair implantation. Clinical evaluations, including ultrasound-monitored cycle studies and mucus scoring assessments, confirm these actions persist across the 28-day cycle, even in ovulatory cycles where mucus remains hostile to sperm.[11][12][7][13] In contrast to combined estrogen-progestin pills, which predominantly rely on consistent ovulation inhibition via dual hormonal action, norgestrel POPs emphasize progestin-driven cervical and endometrial barriers as complementary safeguards, particularly in cycles where ovulation occurs. This progestin-only approach is not approved or recommended for emergency post-coital contraception, unlike levonorgestrel regimens that employ higher single or split doses for that purpose.[10][14]Non-Contraceptive Uses
Norgestrel, typically administered in combination with ethinyl estradiol, has been applied clinically to manage dysfunctional uterine bleeding (DUB) and associated menstrual irregularities through its endometrial stabilizing effects, which promote cessation of excessive hemorrhage and restoration of cyclic function. A 1976 prospective study of 44 patients presenting with spasmodic dysmenorrhea, DUB, or menorrhagia employed an initial regimen of 1.5–2 mg norgestrel (with 0.15–0.2 mg ethinyl estradiol) daily—divided into 3–4 doses—until bleeding halted, followed by standard cyclic dosing of one tablet daily for 21 days with a 7-day interruption; this yielded complete symptom resolution in all dysmenorrhea cases, normalized endometrial histology via biopsy in bleeding disorders, and overall high tolerability with minimal adverse reports.[15] Acute protocols for heavy menstrual bleeding have included 1.2 mg norgestrel (with 120 μg ethinyl estradiol) daily in divided doses until hemostasis, as outlined in management algorithms for abnormal uterine bleeding.[16] For ongoing control of DUB or dysmenorrhea, daily oral dosing of 0.75 mg norgestrel monotherapy has been documented in clinical references, leveraging its progestogenic potency to suppress endometrial proliferation and reduce blood loss volume.[17] Similarly, in menorrhagia, norgestrel-containing combined formulations are noted for efficacy in endometrial stabilization, outperforming less potent progestins in preventing breakthrough hemorrhage.[17] Norgestrel's role extends to endometriosis symptom palliation, where its administration at 0.75 mg daily aids in suppressing ectopic endometrial growth and associated dysmenorrhea by inducing endometrial atrophy.[17] These applications, however, remain secondary to its primary contraceptive indications, with supporting data largely from mid-20th-century trials and pharmacological overviews rather than large randomized controlled trials specific to norgestrel alone.[15][17]Efficacy
Clinical Effectiveness Data
Norgestrel, at a dose of 75 μg daily in progestin-only pills (POPs), exhibits a median Pearl Index of 1.73 pregnancies per 100 woman-years across clinical studies, with a range of 0.00 to 9.00.00448-6/fulltext) This metric primarily captures perfect use efficacy, where adherence is strict, yielding failure rates of approximately 1-2% annually in low-bias trials.[18] Typical use failure rates, accounting for common adherence lapses, are estimated at 9% in the first year, though aggregated data from recent reviews suggest actual observed rates may be lower, around 3-7%.[19][4] A 2021 systematic review of norgestrel 75 μg/day use documented 53 pregnancies among participants, with 26 occurring during perfect use, affirming its high efficacy comparable to combined oral contraceptives under ideal conditions.00375-9/fulltext) Efficacy remains consistent across breastfeeding and non-breastfeeding women, with no significant differences in failure estimates between these groups.[20] Relative to other POPs like desogestrel (75 μg), norgestrel demonstrates superior tolerance for dosing variability due to its longer elimination half-life of 17-19 hours, permitting delays up to 6 hours without elevating pregnancy risk—unlike desogestrel's stricter 3-hour window.[21] This pharmacokinetic advantage contributes to norgestrel's marginally higher real-world effectiveness among POP formulations.00448-6/fulltext)Factors Influencing Performance
Adherence to the daily dosing schedule is a primary determinant of norgestrel's contraceptive performance, as its short half-life requires consistent intake within a narrow window to sustain inhibitory effects on ovulation. Delays exceeding three hours from the usual time necessitate immediate intake of the missed dose followed by backup contraception, such as barrier methods, for the subsequent 48 hours to mitigate failure risk.[2][22] Empirical studies simulating real-world use demonstrate that self-reported adherence rates above 85% correlate with maintained efficacy, underscoring how lapses in routine—often due to forgetfulness or lifestyle disruptions—elevate pregnancy probabilities through inconsistent hormone levels.[23] Gastrointestinal events like vomiting or severe diarrhea occurring within three to four hours post-dose can impair absorption, effectively mimicking a missed dose and requiring supplemental protection until the next scheduled intake confirms resumption.[24][25] Drug interactions further modulate performance, particularly with hepatic enzyme inducers such as phenytoin, carbamazepine, or rifampin, which accelerate norgestrel metabolism via CYP3A4 pathways, thereby reducing circulating levels and contraceptive reliability; users on these medications should employ alternative methods.[26][27] User demographics influence compliance rather than intrinsic pharmacokinetics for norgestrel, with no empirical evidence indicating significant efficacy diminishment from short delays in reproductive-age women across BMI ranges, though obesity may indirectly heighten discontinuation via side effect intolerance. Breastfeeding status does not compromise performance, as progestin-only formulations like norgestrel maintain ovulation suppression without affecting milk production or infant safety. Age-related factors, such as perimenopausal inconsistencies, can affect adherence but not the drug's mechanism when dosed properly.[28][29] In contrast to long-acting reversible contraceptives, norgestrel's pill form amplifies vulnerability to user error, yet exhibits lower typical-failure rates than non-hormonal behavioral methods like condoms, where inconsistent application drives higher unintended pregnancy incidences. Bleeding irregularities, including unscheduled spotting common in the initial months of progestin-only use, represent a verifiable cause of dropout, prompting method switches in users intolerant to cycle disruptions absent in non-user-dependent options.[30][31]Adverse Effects and Risks
Common Side Effects
The most frequently reported adverse reactions to norgestrel, particularly in progestin-only formulations such as the 0.075 mg daily tablet, are menstrual irregularities including breakthrough bleeding, spotting, and changes in menstrual flow or duration.[5][26] These effects arise from progestin-induced endometrial changes and affect up to 70% of users with breakthrough bleeding or spotting in one or more cycles, though incidence often decreases after the first few months of continuous use.[32] Additional common short-term side effects include headaches, nausea, breast tenderness, dizziness, increased appetite, and abdominal cramps or bloating, which are generally mild and self-limiting.[5][33] Clinical data from progestogen-only pill users indicate these symptoms typically manifest early in treatment and resolve without intervention in most cases, distinguishing them from rarer, persistent issues.[32]Serious Adverse Events
Use of norgestrel in progestin-only oral contraceptives is not associated with a significantly elevated risk of venous thromboembolism (VTE), unlike combined estrogen-progestin formulations, where VTE incidence ranges from 7-10 per 10,000 woman-years. Epidemiological data indicate that low-dose oral progestins maintain VTE rates comparable to non-users, with relative risks near 1.0 in multiple cohort studies.[34][35] Contraceptive failure with norgestrel, which occurs in approximately 7-9% of typical users annually, carries an elevated risk of ectopic pregnancy compared to intrauterine pregnancies, attributable to progestins' inhibition of oviductal motility and altered implantation dynamics. Among pregnancies in progestin-only pill users, ectopic rates can reach up to 10-15%, versus 1-2% in the general population; symptoms include severe abdominal pain and spotting, necessitating prompt evaluation.[36][37] Norgestrel is contraindicated in individuals with active or prior breast cancer due to progestins' potential to stimulate hormone-sensitive tumors, as evidenced by observational data showing slightly increased breast cancer incidence during use (relative risk 1.2-1.5). Similarly, severe hepatic impairment or undiagnosed vaginal bleeding precludes use, with post-marketing reports linking progestins to rare cholestatic jaundice or exacerbation of bleeding sources.[38][39] Severe hypersensitivity reactions, including anaphylaxis, have been documented in isolated cases, manifesting as hives, wheezing, or facial edema; immediate discontinuation is required. While hypertension risk remains unincreased relative to non-users, monitoring is advised in predisposed individuals, as progestins may contribute to fluid retention in rare instances.[40][37]Long-Term Health Considerations
Long-term use of norgestrel, a synthetic progestin primarily exerting effects through its levonorgestrel enantiomer, has been linked to elevated breast cancer risk in cohort studies and meta-analyses of progestogen-containing contraceptives. A 2023 UK nested case-control study and meta-analysis reported a relative risk increase of 20% to 30% for breast cancer among current or recent users of hormonal contraceptives, with similar associations observed for progestogen-only formulations comparable to norgestrel. [41] This risk persists for up to 5 years post-discontinuation and aligns with findings from levonorgestrel-based methods, where ever-use of intrauterine systems showed a 26% relative increase. [42] Absolute risks are modest given low baseline incidence in premenopausal women, typically adding 1 to 2 cases per 10,000 woman-years of use among those aged 16-49, though cumulative exposure amplifies incidence without offsetting reductions in overall cancer mortality observed in large registries. [43] Regarding fertility, discontinuation of norgestrel-based oral contraceptives does not appear to delay return to fecundity, with meta-analyses of progestin users showing pregnancy rates of 77% to 86% within 12 months, comparable to non-users. [44] Specific trials with low-dose levonorgestrel equivalents confirmed no significant postponement, as ovulation resumes promptly and endometrial recovery supports conception timelines akin to baseline fertility. [45] Bone mineral density effects from prolonged norgestrel exposure are minimal and reversible, differing from depot progestins like medroxyprogesterone; prospective data on analogous levonorgestrel implants and systems indicate no sustained loss at the lumbar spine or hip after 18-24 months, with accrual resuming post-cessation in adolescents and adults. [46] Metabolic impacts from extended norgestrel use include modest elevations in triglycerides and HDL cholesterol, with variable LDL effects across progestins, but no consistent progression to insulin resistance or weight gain in meta-analyses of oral formulations. [47] These changes lack causal links to long-term cardiovascular events in observational cohorts, though absolute risk increments—such as 5-10% higher triglycerides versus non-users—warrant monitoring in predisposed individuals, without evidence of net mortality benefits from widespread adoption offsetting these exposures. [48] Overall, while risks are small in absolute terms, cohort-derived relative elevations underscore the need for individualized assessment over assumptions of neutrality in prolonged regimens.Pharmacology
Pharmacodynamics
Norgestrel, a racemic mixture of levonorgestrel and dextronorgestrel, exerts its progestogenic effects exclusively through the biologically active levorotatory enantiomer, levonorgestrel, which serves as a potent agonist of the progesterone receptor (PR).[6] [49] Levonorgestrel demonstrates high-affinity binding to the PR, with relative binding affinities (RBA) reported as 125% to rabbit PR, 143% to human uterine PR, and up to 323% relative to progesterone in human steroid receptor assays.[50] [51] This binding induces conformational changes in the PR, promoting dimerization and transcriptional regulation of target genes that mediate progestational responses, including endometrial transformation and inhibition of follicular development.[52] The primary physiological downstream effect involves antigonadotropic activity: levonorgestrel binding to PR in hypothalamic and pituitary tissues suppresses gonadotropin-releasing hormone (GnRH) pulsatility, thereby reducing secretion of follicle-stimulating hormone (FSH) and luteinizing hormone (LH), which prevents the mid-cycle LH surge essential for ovulation.[53] [54] Due to norgestrel's racemic composition, where dextronorgestrel shows no appreciable binding or activity at the PR or other steroid receptors, the effective progestogenic potency of norgestrel is roughly equivalent to half the administered dose when compared to pure levonorgestrel.[49] Levonorgestrel also displays moderate affinity for the androgen receptor (AR), with an RBA of approximately 58% relative to dihydrotestosterone, conferring weak androgenic agonism that can influence sebaceous gland activity and protein anabolism but at lower potency than its progestogenic effects.[55] It exhibits negligible estrogenic activity but antiestrogenic properties in certain contexts, such as opposing estrogen-induced endometrial proliferation, though weaker overall than its PR-mediated actions.[53] Binding to glucocorticoid (GR, RBA ~7.5%) and mineralocorticoid (MR, RBA ~17%) receptors is minimal, distinguishing norgestrel from progestins like medroxyprogesterone acetate that show greater cross-reactivity and associated metabolic effects.[55] [56]Pharmacokinetics
Norgestrel is rapidly absorbed following oral administration, with peak serum progestin levels occurring about 2 hours after dosing, followed by rapid distribution.[10] Its bioavailability is approximately 90%, indicating minimal first-pass effect and efficient systemic uptake.[57] Plasma concentrations decline bi-exponentially, reflecting distribution into tissues and subsequent elimination. The elimination half-life of norgestrel in plasma is approximately 15 to 17 hours, supporting once-daily dosing with some flexibility for timing variations.[57] [58] Steady-state levels are typically reached after 1 to 2 weeks of continuous daily administration, due to enterohepatic recirculation which prolongs exposure by recycling conjugated metabolites.[59] Norgestrel undergoes primary hepatic metabolism via cytochrome P450 3A4 (CYP3A4) enzymes, forming hydroxylated metabolites that are subsequently conjugated to glucuronides and sulfates.[59] Excretion occurs mainly as metabolites, with about 43% eliminated in urine over 5 days and the remainder via feces, including through biliary routes.[3] Relative to other progestin-only pills like those containing norethisterone (half-life ~8 hours), norgestrel's extended half-life contributes to sustained plasma levels, reducing the immediacy of ovulation risk after a missed dose.[60]Chemistry
Chemical Properties and Structure
Norgestrel is a synthetic progestin with the molecular formula C₂₁H₂₈O₂ and a molecular weight of 312.45 g/mol.[6] It exists as a racemic mixture of two enantiomers: dextronorgestrel (the inactive (+)-form) and levonorgestrel (the active (-)-eutomer).[6] The core structure is a 18,19-dinorpregna-4-en-3-one gonane derivative featuring a 13-ethyl substituent, a 17α-ethynyl group, a 17β-hydroxy group, and a Δ¹⁵ double bond.[3] This configuration distinguishes it from norethindrone, from which it is derived by replacing the 13-methyl group with an ethyl group, altering its steric properties and bioactivity.[61]
The IUPAC name for the levonorgestrel enantiomer is (8R,9R,10R,13S,14R,17R)-13-ethyl-17-ethynyl-17-hydroxy-18,19-dinorpregna-4,15-diene-3-one.[3] Norgestrel's lipophilic nature is evidenced by a predicted octanol-water partition coefficient (logP) of 3.25–3.66, promoting passive diffusion across lipid membranes.[6] Its aqueous solubility is low, approximately 0.006 mg/mL at physiological pH, classifying it as practically insoluble in water and requiring pharmaceutical formulations like micronized powders or lipid-based vehicles for oral bioavailability.[6][3]
Norgestrel demonstrates chemical stability under physiological conditions, with no readily hydrolyzable functional groups, enabling intact absorption from the gastrointestinal tract following oral administration.[3] This stability is critical for its formulation in tablets, where it resists degradation in acidic environments without significant excipient interactions under standard storage.[62]

