Recent from talks
Contribute something
Nothing was collected or created yet.
Combined oral contraceptive pill
View on Wikipedia
| Combined oral contraceptive pill | |
|---|---|
 | |
| Background | |
| Type | Hormonal |
| First use | 1960 (United States) |
| Failure rates (first year) | |
| Perfect use | 0.3%[1] |
| Typical use | 9%[1] |
| Usage | |
| Duration effect | 1–4 days |
| Reversibility | Yes |
| User reminders | Taken within same 24-hour window each day |
| Clinic review | 6 months |
| Advantages and disadvantages | |
| STI protection | No |
| Periods | Regulated, and often lighter and less painful |
| Weight | No proven effect |
| Benefits | Evidence for reduced mortality risk and reduced death rates in all cancers.[2] Possible reduced ovarian and endometrial cancer risks.[3][citation needed] May treat acne, PCOS, PMDD, endometriosis[citation needed] |
| Risks | Increase in some cancers.[4][5] Increase in DVTs; stroke,[6] cardiovascular disease[7] |
| Medical notes | |
| Affected by the antibiotic rifampicin,[8] the herb Hypericum (St John's wort) and some anti-epileptics, also vomiting or diarrhea. Caution if history of migraines. | |
The combined oral contraceptive pill (COCP), often referred to as the birth control pill or colloquially as "the pill", is a type of birth control that is designed to be taken orally by women. It is the oral form of combined hormonal contraception. The pill contains two important hormones: a progestin (a synthetic form of the hormone progestogen/progesterone) and estrogen (usually ethinylestradiol or 17β estradiol).[9][10][11][12] When taken correctly, it alters the menstrual cycle to eliminate ovulation and prevent pregnancy.
Combined oral contraceptive pills were first approved for contraceptive use in the United States in 1960, and remain a very popular form of birth control. They are used by more than 100 million women worldwide [13][14] including about 9 million women in the United States.[15][16] From 2015 to 2017, 12.6% of women aged 15–49 in the US reported using combined oral contraceptive pills, making it the second most common method of contraception in this age range (female sterilization is the most common method).[17] Use of combined oral contraceptive pills, however, varies widely by country,[18] age, education, and marital status. For example, one third of women aged 16–49 in the United Kingdom use either the combined pill or progestogen-only pill (POP),[19][20] compared with less than 3% of women in Japan (as of 1950–2014).[21]
Combined oral contraceptives are on the World Health Organization's List of Essential Medicines.[22] The pill was a catalyst for the sexual revolution.[23]
Background
[edit]Oral contraceptives
[edit]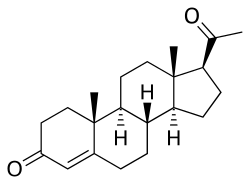
Hormonal oral contraceptives are preventive medications taken orally by females to avoid pregnancy by manipulating their sex hormones. The first oral contraceptive was approved by the US Food and Drug Administration (FDA) and sold to the market in 1960. There are two types of hormonal oral contraceptives, namely Combined Oral Contraceptives and Progesterone Only Pills. Oral contraceptives, be it combined or progesterone-only, can effectively prevent pregnancy by regulating hormonal changes in the menstrual cycle, inhibiting ovulation, and altering cervical mucus to impede sperm mobility; combined pills have extra effects in menstrual cycle regulation and menstrual pain relief. Common off-label uses include menstrual suppression and acne relief, with Combined Oral Contraceptives having additional benefits in relieving menstrual migraine.
Variants
[edit]Progesterone-only pills (POPs) utilise progestin, the synthetic form of progesterone, as the only active pharmaceutical ingredient in the formulation.[24][25] In the US, drospirenone and norethindrone are the most commonly used compounds in formulations.[26]
Combined oral contraceptives (COCs) are commonly classified into generations, referring to their order of development in history.[27] This discussion may also help identify some key features in a variety of products. According to EMA, the first generation of combined oral contraceptives, which made use of a high concentration of oestrogen only, were those invented in the 1960s.[27] In the second generation of products, progestogens were introduced into the formulation while the concentration of oestrogen was reduced.[27] Starting from the 1990s, the progression in the development of combined oral contraceptives has been directed towards varying the type of progestogen incorporated.[27] These products are referred as the third and fourth generation.[27]
Oestrogen ingredients: estradiol, ethinylestradiol, estetrol.[24]
1st generation progestin: norethindrone acetate, ethynodiol diacetate, lynestrenol, norethynodrel.[24]
2nd generation progestin: levonorgestrel, dl-norgestrel.[24]
3rd generation progestin: norgestimate, gestodene, desogestrel.[24]
The menstrual cycle
[edit]
Hormonal oral contraceptives (HOCs) interact with hormonal changes in the menstrual cycle in females to prevent ovulation, and hence achieve contraception.[24] In a 28-day menstrual cycle, there are the proliferative phase, ovulation, and then the secretory phase.[28]
Menstruation marks the beginning of proliferative phase in day 1-14.[28] In this period, the pituitary gland located near the brain secretes follicle-stimulating hormone (FSH) into the bloodstream to signal the development of follicle in ovary in the female reproductive system.[28] While follicle serves as the chamber of ovum development, it secretes Oestrogen, a hormone that not only triggers the thickening of uterine lining in preparation for implantation, but also inhibits the secretion of FSH in pituitary via a negative feedback mechanism.[28]
Specifically in ovulation, transient positive feedback by Oestrogen on FSH and Luteinising Hormone (LH) secretion from pituitary is permitted so that the release of mature ovum from follicle is triggered.[28]
In secretory phase on day 14-28, this follicle then transforms into corpus luteum and continues releasing Oestrogen with Progesterone into bloodstream.[28] While Oestrogen and Progesterone primarily aid the maintenance of thickness in uterine lining,[28] the negative feedback in pituitary allows them to inhibit FSH and LH secretion.[28] In the absence of LH, corpus luteum degenerates and ultimately causes blood Oestrogen and Progesterone levels to decline.[28] Without these thickness maintaining agents, uterine lining breaks down and hence the presentation of menstruation.[28]
Mechanism of action
[edit]Progesterone and Oestrogen, either in combination or with Progesterone-only, are the active pharmaceutical ingredients found in a hormonal oral contraceptive formulation.[29] These medications are orally administered for systemic absorption to exert their effects.[29] An artificially enhanced level of Progesterone throughout the menstrual cycle inhibits the pituitary secretion of FSH and LH such that their actions in stimulating follicular development and ovulation are prevented.[24] Similarly, a boosted Oestrogen level activates the negative feedback mechanism in reducing FSH secretion from pituitary and therefore prevents follicular development.[24] In the absence of a developed follicle, ovulation cannot occur so that fertilisation is made impossible and contraception is achieved.[29] In comparison, Progesterone is more efficacious than Oestrogen not only because of its additional action in impeding LH, but also its ability to modulate the cervical mucus into sperm-repellent.[24]
Combined oral contraceptive pills were developed to prevent ovulation by suppressing the release of gonadotropins. Combined hormonal contraceptives, including combined oral contraceptive pills, inhibit follicular development and prevent ovulation as a primary mechanism of action.[30][31][32][33]
Under normal circumstances, luteinizing hormone (LH) stimulates the theca cells of the ovarian follicle to produce androstenedione. The granulosa cells of the ovarian follicle then convert this androstenedione to estradiol. This conversion process is catalyzed by aromatase, an enzyme produced as a result of follicle-stimulating hormone (FSH) stimulation.[34] In individuals using oral contraceptives, progestogen negative feedback decreases the pulse frequency of gonadotropin-releasing hormone (GnRH) release by the hypothalamus, which decreases the secretion of FSH and greatly decreases the secretion of LH by the anterior pituitary. Decreased levels of FSH inhibit follicular development, preventing an increase in estradiol levels. Progestogen negative feedback and the lack of estrogen positive feedback on LH secretion prevent a mid-cycle LH surge. Inhibition of follicular development and the absence of an LH surge prevent ovulation.[30][31][32]
Estrogen was originally included in oral contraceptives for better cycle control (to stabilize the endometrium and thereby reduce the incidence of breakthrough bleeding), but was also found to inhibit follicular development and help prevent ovulation. Estrogen negative feedback on the anterior pituitary greatly decreases the secretion of FSH, which inhibits follicular development and helps prevent ovulation.[30][31][32]
Another primary mechanism of action of all progestogen-containing contraceptives is inhibition of sperm penetration through the cervix into the upper genital tract (uterus and fallopian tubes) by decreasing the water content and increasing the viscosity of the cervical mucus.[30]
The estrogen and progestogen in combined oral contraceptive pills have other effects on the reproductive system, but these have not been shown to contribute to their contraceptive efficacy:[30]
- Slowing tubal motility and ova transport, which may interfere with fertilization.
- Endometrial atrophy and alteration of metalloproteinase content, which may impede sperm motility and viability, or theoretically inhibit implantation.
- Endometrial edema, which may affect implantation.
Insufficient evidence exists on whether changes in the endometrium could actually prevent implantation. The primary mechanisms of action are so effective that the possibility of fertilization during combined oral contraceptive pill use is very small. Since pregnancy occurs despite endometrial changes when the primary mechanisms of action fail, endometrial changes are unlikely to play a significant role, if any, in the observed effectiveness of combined oral contraceptive pills.[30]
Formulations
[edit]Oral contraceptives come in a variety of formulations, some containing both estrogen and progestins, and some only containing progestin. Doses of component hormones also vary among products, and some pills are monophasic (delivering the same dose of hormones each day) while others are multiphasic (doses vary each day). combined oral contraceptive pills can also be divided into two groups, those with progestins that possess androgen activity (norethisterone acetate, etynodiol diacetate, levonorgestrel, norgestrel, norgestimate, desogestrel, gestodene) or antiandrogen activity (cyproterone acetate, chlormadinone acetate, drospirenone, dienogest, nomegestrol acetate).
Combined oral contraceptive pills have been somewhat inconsistently grouped into "generations" in the medical literature based on when they were introduced.[35][36]
- First generation combined oral contraceptive pills are sometimes defined as those containing the progestins noretynodrel, norethisterone, norethisterone acetate, or etynodiol acetate;[35] and sometimes defined as all combined oral contraceptive pills containing ≥ 50 μg ethinylestradiol.[36]
- Second generation combined oral contraceptive pills are sometimes defined as those containing the progestins norgestrel or levonorgestrel;[35] and sometimes defined as those containing the progestins norethisterone, norethisterone acetate, etynodiol acetate, norgestrel, levonorgestrel, or norgestimate and < 50 μg ethinylestradiol.[36]
- Third generation combined oral contraceptive pills are sometimes defined as those containing the progestins desogestrel or gestodene;[36] and sometimes defined as those containing desogestrel, gestodene, or norgestimate.[35]
- Fourth generation combined oral contraceptive pills are sometimes defined as those containing the progestin drospirenone;[35] and sometimes defined as those containing drospirenone, dienogest, or nomegestrol acetate.[36]
Medical use
[edit]
Contraceptive use
[edit]Combined oral contraceptive pills are a type of oral medication that were originally designed to be taken every day at the same time of day in order to prevent pregnancy.[26][37] There are many different formulations or brands, but the average pack is designed to be taken over a 28-day period (also known as a cycle).[citation needed] For the first 21 days of the cycle, users take a daily pill that contains two hormones, estrogen and progestogen.[citation needed] During the last 7 days of the cycle, users take daily placebo (biologically inactive) pills and these days are considered hormone-free days.[citation needed] Although these are hormone-free days, users are still protected from pregnancy during this time.[medical citation needed]
Some combined oral contraceptive pill packs only contain 21 pills and users are advised to take no pills for the last 7 days of the cycle.[9] Other combined oral contraceptive pill formulations contain 91 pills, consisting of 84 days of active hormones followed by 7 days of placebo (Seasonale).[26] Combined oral contraceptive pill formulations can contain 24 days of active hormone pills followed by 4 days of placebo pills (e.g. Yaz 28 and Loestrin 24 Fe) as a means to decrease the severity of placebo effects.[9] These combined oral contraceptive pills containing active hormones and a placebo/hormone-free period are called cyclic combined oral contraceptive pills. Once a pack of cyclical combined oral contraceptive pill treatment is completed, users start a new pack and new cycle.[38]
Most monophasic combined oral contraceptive pills can be used continuously such that patients can skip placebo days and continuously take hormone active pills from a combined oral contraceptive pill pack.[9] One of the most common reasons users do this is to avoid or diminish withdrawal bleeding. The majority of women on cyclic combined oral contraceptive pills have regularly scheduled withdrawal bleeding, which is vaginal bleeding mimicking users' menstrual cycles with the exception of lighter menstrual bleeding compared to bleeding patterns prior to combined oral contraceptive pill commencement. As such, a study reported that out of 1003 women taking combined oral contraceptive pills approximately 90% reported regularly scheduled withdrawal bleeds over a 90-day standard reference period.[9] Withdrawal bleeding usually occurs during the placebo, hormone-free days.[medical citation needed] Therefore, avoiding placebo days can diminish withdrawal bleeding among other placebo effects.[medical citation needed]
Regimen
[edit]This section demonstrates the overall rationalisation of dosing route and intervals of hormonal oral contraceptives, please seek advice and follow instructions from healthcare professionals in administering specific hormonal oral contraceptives. Considering the menstrual cycle as a 28-day cycle, hormonal oral contraceptives are available in packages of 21, 28, or 91 tablets.[29] These pills have typically undergone unit dose optimisation so that they follow the administration pattern of once daily, every day or almost every day on a regular basis.[29] Since they are formulated into daily doses, it is recommended that the medication should be taken at the same time every day to maximise efficacy.[29]

For 21-tablet packs, the general instruction is to take one tablet daily for 21 days, followed by a 7-day blank interval without taking hormonal oral contraceptives before initiating another 21-tablet pack.[29] For 28-tablet packs, the 1st tablet from a new pack should be taken on the next day when the 28th tablet from an old pack was finished.[29] While the 7-day blank period does not apply to 28-tablet packs, they will likely include tablets in distinctive colours indicating that they have an alternate amount of active ingredients, otherwise inactive ingredient or folate supplement only.[29] The instruction for 91-tablet pack follows that of 28-tablet packs with some colour-distinguishable tablets which contain different amounts of medicine or supplement.[29]
To acquire immediate contraceptive effects, the initiation of hormonal oral contraceptive dosing is recommended within the 1st-5th day from menstruation in order to discard other means of contraception.[39] Specific to Progesterone only pills, even if dosing is initiated within five days, backup contraception is suggested in the first 48 hours since the first pill.[24] In the case of dosing initiated after the 5th day from menstruation, effects usually take place after seven days and other contraceptive methods should remain in place until then.[39]
Effectiveness
[edit]If used exactly as instructed, the estimated risk of getting pregnant is 0.3% which means that about 3 in 1000 women on combined oral contraceptive pills will become pregnant within one year.[40] However, typical use of combined oral contraceptive pills by users often consists of timing errors, forgotten pills, or unwanted side effects. With typical use, the estimated risk of getting pregnant is about 9% which means that about 9 in 100 women on combined oral contraceptive pills will become pregnant in one year.[41] The perfect use failure rate is based on a review of pregnancy rates in clinical trials, and the typical use failure rate is based on a weighted average of estimates from the 1995 and 2002 US National Surveys of Family Growth (NSFG), corrected for underreporting of abortions.[42][43]
Several factors account for typical use effectiveness being lower than perfect use effectiveness:
- Mistakes on part of those providing instructions on how to use the method
- Mistakes on part of the user
- Conscious user non-compliance with instructions
For instance, someone using combined oral contraceptive pills might have received incorrect information by a health care provider about medication frequency, forgotten to take the pill one day or not gone to the pharmacy in time to renew a combined oral contraceptive pill prescription.
Combined oral contraceptive pills provide effective contraception from the very first pill if started within five days of the beginning of the menstrual cycle (within five days of the first day of menstruation). If started at any other time in the menstrual cycle, combined oral contraceptive pills provide effective contraception only after 7 consecutive days of use of active pills, so a backup method of contraception (e.g. condoms) must be used in the interim.[44][45]
The effectiveness of combined oral contraceptive pills appears to be similar whether the active pills are taken continuously or if they are taken cyclically.[46] Contraceptive efficacy, however, could be impaired by numerous means. Factors that may contribute to a decrease in effectiveness:[44]
- Missing more than one active pill in a packet,
- Delay in starting the next packet of active pills (i.e., extending the pill-free, inactive pill or placebo pill period beyond 7 days),
- Intestinal malabsorption of active pills due to vomiting or diarrhea,
- Drug-drug interactions among combined oral contraceptive pills and other medications of the user that decrease contraceptive estrogen and/or progestogen levels.[44]
In any of these instances, a backup contraceptive method should be used until hormone active pills have been consistently taken for 7 consecutive days or drug-drug interactions or underlying illnesses have been discontinued or resolved.[44] According to the US Centers for Disease Control and Prevention (CDC) guidelines, a pill is considered "late" if a user takes the pill after the user's normal medication time, but no longer than 24 hours after this normal time. If 24 hours or more have passed since the time the user was supposed to take the pill, then the pill is considered "missed".[40] CDC guidelines discuss potential next steps for users who missed their pill or took it late.[47]
Role of placebo pills
[edit]The role of the placebo pills is two-fold: to allow the user to continue the routine of taking a pill every day and to simulate the average menstrual cycle. By continuing to take a pill every day, users remain in the daily habit even during the week without hormones. Failure to take pills during the placebo week does not impact the effectiveness of the pill, provided that daily ingestion of active pills is resumed at the end of the week.[48]
The placebo, or hormone-free, week in the 28-day pill package simulates an average menstrual cycle, though the hormonal events during a pill cycle are significantly different from those of a normal ovulatory menstrual cycle. Because the pill suppresses ovulation (to be discussed more in the Mechanism of action section), birth control users do not have true menstrual periods. Instead, it is the lack of hormones for a week that causes a withdrawal bleed.[37] The withdrawal bleeding that occurs during the break from active pills has been thought to be reassuring, a physical confirmation of not being pregnant.[49] The withdrawal bleeding is also predictable. Unexpected breakthrough bleeding can be a possible side effect of longer term active regimens.[50]
Since it is not uncommon for menstruating women to become anemic, some placebo pills may contain an iron supplement.[51][52] This replenishes iron stores that may become depleted during menstruation. As well, birth control pills, such as combined oral contraceptive pills, are sometimes fortified with folic acid as it is recommended to take folic acid supplementation in the months prior to pregnancy to decrease the likelihood of neural tube defect in infants.[53][54]
No or less frequent placebos
[edit]If the pill formulation is monophasic, meaning each hormonal pill contains a fixed dose of hormones, it is possible to skip withdrawal bleeding and still remain protected against conception by skipping the placebo pills altogether and starting directly with the next packet. Attempting this with bi- or tri-phasic pill formulations carries an increased risk of breakthrough bleeding and may be undesirable. It will not, however, increase the risk of getting pregnant.
Starting in 2003, women have also been able to use a three-month version of the pill.[55] Similar to the effect of using a constant-dosage formulation and skipping the placebo weeks for three months, Seasonale gives the benefit of less frequent periods, at the potential drawback of breakthrough bleeding. Seasonique is another version in which the placebo week every three months is replaced with a week of low-dose estrogen.
A version of the combined pill has also been packaged to eliminate placebo pills and withdrawal bleeds. Marketed as Anya or Lybrel, studies have shown that after seven months, 71% of users no longer had any breakthrough bleeding, the most common side effect of going longer periods of time without breaks from active pills.
While more research needs to be done to assess the long term safety of using combined oral contraceptive pills continuously, studies have shown there may be no difference in short term adverse effects when comparing continuous use versus cyclic use of birth control pills.[46]
Non-contraceptive use
[edit]The hormones in the pill have also been used to treat other medical conditions, such as polycystic ovary syndrome (PCOS), endometriosis, adenomyosis, acne, hirsutism, amenorrhea, menstrual cramps, menstrual migraines, menorrhagia (excessive menstrual bleeding), menstruation-related or fibroid-related anemia and dysmenorrhea (painful menstruation).[41][56] Besides acne, no oral contraceptives have been approved by the US FDA for the previously mentioned uses despite extensive use for these conditions.[57]
PCOS
[edit]The cause of PCOS, or polycystic ovary syndrome, is multifactorial and not well-understood. Women with PCOS often have higher than normal levels of luteinizing hormone (LH) and androgens that impact the normal function of the ovaries.[58] While multiple small follicles develop in the ovary, none are able to grow in size enough to become the dominant follicle and trigger ovulation.[59] This leads to an imbalance of LH, follicle stimulating hormone, estrogen, and progesterone. Without ovulation, unopposed estrogen can lead to endometrial hyperplasia, or overgrowth of tissue in the uterus.[60] This endometrial overgrowth is more likely to become cancerous than normal endometrial tissue.[61] Thus, although the data varies, it is generally agreed upon by most gynecological societies that due to the unopposed estrogen, women with PCOS are at higher risk for endometrial cancer.[62]
To reduce the risk of endometrial cancer, it is often recommended that women with PCOS who do not desire pregnancy take hormonal contraceptives to prevent the effects of unopposed estrogen. Both combined oral contraceptive pills and progestin-only methods are recommended.[citation needed] It is the progestin component of combined oral contraceptive pills that protects the endometrium from hyperplasia, and thus reduces a woman with PCOS's endometrial cancer risk.[63] Combined oral contraceptive pills are preferred to progestin-only methods in women who also have uncontrolled acne, symptoms of hirsutism, and androgenic alopecia, because combined oral contraceptive pills can help treat these symptoms.[37]
Acne and hirsutism
[edit]Combined oral contraceptive pills are sometimes prescribed to treat symptoms of androgenization, including acne and hirsutism.[64] The estrogen component of combined oral contraceptive pills appears to suppress androgen production in the ovaries. Estrogen also leads to increased synthesis of sex hormone binding globulin, which causes a decrease in the levels of free testosterone.[65]
Ultimately, the drop in the level of free androgens leads to a decrease in the production of sebum, which is a major contributor to development of acne.[citation needed] Four different oral contraceptives have been approved by the US FDA to treat moderate acne if the patient is at least 14 or 15 years old, has already begun menstruating, and needs contraception. These include Ortho Tri-Cyclen, Estrostep, Beyaz, and YAZ.[66][67][68]
Hirsutism is the growth of coarse, dark hair where women typically grow only fine hair or no hair at all.[69] This hair growth on the face, chest, and abdomen is also mediated by higher levels or action of androgens. Therefore, combined oral contraceptive pills also work to treat these symptoms by lowering the levels of free circulating androgens.[70]
Studies have shown that combined oral contraceptives are effective in reducing both inflammatory and non-inflammatory facial acne lesions.[71] However, comparisons between different combined oral contraceptives have not been studied to understand if any brand is superior than the others.[71] Oestrogen decreases sebum production by shrinking the sebaceous gland, increasing Sex hormone-binding globulin (SHBG) production to reduce unbound testosterone, and regulating LH and FSH levels.[72] Studies have not shown that POPs are effective against acne lesions.[citation needed]
Endometriosis
[edit]For pelvic pain associated with endometriosis, combined oral contraceptive pills are considered a first-line medical treatment, along with NSAIDs, GnRH agonists, and aromatase inhibitors.[73] Combined oral contraceptive pills work to suppress the growth of the extra-uterine endometrial tissue. This works to lessen its inflammatory effects.[37] Combined oral contraceptive pills, along with the other medical treatments listed above, do not eliminate the extra-uterine tissue growth, they just reduce the symptoms. Surgery is the only definitive treatment. Studies looking at rates of pelvic pain recurrence after surgery have shown that continuous use of combined oral contraceptive pills is more effective at reducing the recurrence of pain than cyclic use.[74]
Adenomyosis
[edit]Similar to endometriosis, adenomyosis is often treated with combined oral contraceptive pills to suppress the growth the endometrial tissue that has grown into the myometrium. Unlike endometriosis however, levonorgestrel containing IUDs are more effective at reducing pelvic pain in adenomyosis than combined oral contraceptive pills.[37]
Menorrhagia
[edit]In the average menstrual cycle, a woman typically loses 35 to 40 milliliters of blood.[75] However, up to 20% of women experience much heavier bleeding, or menorrhagia.[76] This excess blood loss can lead to anemia, with symptoms of fatigue and weakness, as well as disruption in their normal life activities.[77] Combined oral contraceptive pills contain progestin, which causes the lining of the uterus to be thinner, resulting in lighter bleeding episodes for those with heavy menstrual bleeding.[78]
Amenorrhea
[edit]Although the pill is sometimes prescribed to induce menstruation on a regular schedule for women bothered by irregular menstrual cycles, it actually suppresses the normal menstrual cycle and then mimics a regular 28-day monthly cycle.
Women who are experiencing menstrual dysfunction due to female athlete triad are sometimes prescribed oral contraceptives as pills that can create menstrual bleeding cycles.[79] However, the condition's underlying cause is energy deficiency and should be treated by correcting the imbalance between calories eaten and calories burned by exercise. Oral contraceptives should not be used as an initial treatment for female athlete triad.[79]
Menstrual suppression
[edit]Menstrual bleeding is not necessary in women who do not wish to conceive, therefore menstrual suppression may be implemented in women who do not want to have menstrual bleeding for convenience, gynecologic disorders, bleeding disorders or other medical conditions.[80]
In the two types of hormonal oral contraceptives, only combined oral contraceptives can achieve amenorrhea, while POPs can only reduce the amount of blood.[81] The method of using combined oral contraceptives for menstrual suppression is to skip the 7 placebo pills and continue taking active pills after the 21 active pills.[82] This can be used in extended method or continuous method.[82] For extended method, patients who take active pills for 3, 4, or 6 months and then take placebo pills for a period of time will more likely experience withdrawal bleeding.[82] The interval can be decided by the patients according to their own preferences.[82] For continuous method, people can take combined oral contraceptives for a year continuously without any placebo pills.[82] In the first few months of extended or continuous use of combined oral contraceptives, unscheduled bleeding or spotting may occur.[83] However, the bleeding or spotting is expected to resolve after a few months of use.[83]
Menstrual suppression is commonly used for convenience especially when women go on vacation.[80] It is also used for gynecologic disorders such as dysmenorrhea (commonly known as menstrual pain), symptoms related to premenstrual hormone change and excessive bleeding related to uterine fibroids. Patients can also benefit from menstrual suppression for bleeding disorders or chronic anemia.[80]
Menstrual migraine
[edit]Patients with menstrual Oestrogen-related migraine, but without aura and additional risk factors to stroke, can be benefited from combined oral contraceptives.[84][85] However, older women and those with a strong family history of problematic headaches may find that using hormonal oral contraceptives worsens their headache.[84][85]
Benefits
[edit]The distinctive feature of hormonal oral contraceptives when compared to other contraceptive methods is that they are less invasive and do not interfere with sex.[39] Conclusive data suggest that the failure rate of contraception in using hormonal oral contraceptives for the first year is 9% in typical use which allows missed doses, and <1% in perfect use.[24][39] The efficacy of hormonal oral contraceptives in preventing pregnancy is high overall.[24] Furthermore, the regular use of hormonal oral contraceptive tends to not only ease premenstrual syndrome, but also allow lighter and less painful menstruation.[39] In addition, the association between a suppressed risk of developing ovarian cancer and hormonal oral contraceptive use is proven.[86][87]
Contraindications
[edit]While combined oral contraceptives are generally considered to be a relatively safe medication, they are contraindicated for those with certain medical conditions. The World Health Organization and the US Centers for Disease Control and Prevention publish guidance, called medical eligibility criteria, on the safety of birth control in the context of medical conditions.[88][41]
In terms of protection in sexual intercourse, a sole reliance on hormonal oral contraceptives does not defend one from sexually transmitted infections such as HPV.[24][39] Additionally, breakthrough bleeding and spotting are exceptionally prevalent in the early stage of using hormonal oral contraceptives.[24][29][39] Although most reported side effects including nausea, headache, or mood swings will disappear as the therapy progresses or upon switching formulation, elevated blood pressure or blood clots in patients with cardiovascular conditions are documented side effects that requires medical attention if not termination of hormonal oral contraceptives.[24][29][39] It is because combined oral contraceptives uses have been found to be related to an increased risk of ischemic stroke or myocardial infarction, especially in combined oral contraceptives with >50 μg Oestrogen.[89] Besides, some ongoing studies giving evidence on the association between hormonal oral contraceptive use and escalated breast cancer risks cannot be neglected.[90][91][86][87]
According to WHO Medical Eligibility Criteria for Contraceptive Use 2015, Category 3 implies that the use of such contraception is usually not recommended, unless other more appropriate methods are neither available nor acceptable and with good resources of clinical judgment; Category 4 implies that the contraceptive method should not be used even with good resources of clinical judgment.[92] Both categories suggest that the contraceptive method should not be used with limited resources for clinical judgment.[92] The tables below summarise conditions of category 3 and 4 from World Health Organization Medical Eligibility Criteria for Contraceptive Use 2015.
Precautions and contraindications for combined oral contraceptives
[edit]| Condition | Category |
|---|---|
| Breastfeeding | |
| for < 6 weeks postpartum | 4 |
| for ≥ 6 weeks to < 6 months postpartum | 3 |
| Postpartum (non-breastfeeding) | |
| < 21 days postpartum without other risk factors for VTE | 3 |
| < 21 days postpartum with other risk factors for VTE | 4 |
| ≥ 21 days to 42 days postpartum with other risk factors for VTE | 3 |
| Smoking | |
| age ≥ 35 years and smoking < 15 cigarettes/day | 3 |
| age ≥ 35 years and smoking ≥ 15 cigarettes/day | 4 |
| Multiple risk factors for arterial cardiovascular disease | 3/4* |
| Hypertension | |
| history of hypertension, where blood pressure CANNOT be evaluated | 3 |
| adequately controlled hypertension, where blood pressure CAN be evaluated | 3 |
| elevated blood pressure levels (properly taken measurements)
with systolic 140–159 or diastolic 90–99 mm Hg |
3 |
| elevated blood pressure levels (properly taken measurements)
with systolic ≥ 160 or diastolic ≥ 100 mm Hg |
4 |
| elevated blood pressure levels (properly taken measurements) with Vascular disease | 4 |
| Deep vein thrombosis (DVT) / Pulmonary embolism (PE) | |
| with History of DVT/PE | 4 |
| with acute DVT/PE | 4 |
| with DVT/PE and established on anticoagulant therapy | 4 |
| with Major surgery with prolonged immobilization | 4 |
| Known thrombogenic mutations | 4 |
| Current and history of ischemic heart disease | 4 |
| Stroke (history of cerebrovascular accident) | 4 |
| Complicated valvular heart disease | 4 |
| Positive (or unknown) antiphospholipid antibodies Systemic Lupus Erythematous | 4 |
| Headache | |
| migraine without aura of age ≥ 35 years (for initiation of combined oral contraceptives) | 3 |
| migraine without aura of age < 35 years (for continuation of combined oral contraceptives) | 3 |
| migraine without aura of age ≥ 35 years (for continuation of combined oral contraceptives) | 4 |
| migraine with aura, at any age (for initiation and continuation of combined oral contraceptives) | 4 |
| Breast cancer | |
| current Breast cancer | 4 |
| past Breast Cancer and no evidence of current disease for 5 years | 3 |
| Nephropathy/retinopathy/neuropathy | 3/4* |
| Other vascular disease or diabetes of > 20 years' duration | 3/4* |
| Medically treated symptomatic gall bladder disease | 3 |
| Current symptomatic gall bladder disease | 3 |
| Past-combined oral contraceptive related history of Cholestasis | 3 |
| Acute or flare viral hepatitis (for initiation of combined oral contraceptives) | 3/4* |
| Severe cirrhosis (decompensated) | 4 |
| Liver tumors | |
| hepatocellular adenoma | 4 |
| malignant (hepatoma) | 4 |
| On anticonvulsant therapy | |
| with phenytoin, carbamazepine, barbiturates, primidone, topiramate, oxcarbazepine | 3 |
| with Lamotrigine | 3 |
| On antimicrobial therapy with Rifampicin or rifabutin therapy | |
*The category should be assessed according to the severity of the condition.
Hypercoagulability
[edit]Estrogen in high doses can increase risk of blood clots. All combined oral contraceptive pill users have a small increase in the risk of venous thromboembolism compared with non-users; this risk is greatest within the first year of combined oral contraceptive pill use.[93] Individuals with any pre-existing medical condition that also increases their risk for blood clots have a more significant increase in risk of thrombotic events with combined oral contraceptive pill use.[93] These conditions include but are not limited to high blood pressure, pre-existing cardiovascular disease (such as valvular heart disease or ischemic heart disease[94]), history of thromboembolism or pulmonary embolism, cerebrovascular accident, and a familial tendency to form blood clots (such as familial factor V Leiden).[95] There are conditions that, when associated with combined oral contraceptive pill use, increase risk of adverse effects other than thrombosis. For example, women with a history of migraine with aura have an increased risk of stroke when using combined oral contraceptive pills, and women who smoke over age 35 and use combined oral contraceptive pills are at higher risk of myocardial infarction.[88]
Pregnancy and postpartum
[edit]Women who are known to be pregnant should not take combined oral contraceptive pills. Those in the postpartum period who are breastfeeding are also advised not to start combined oral contraceptive pills until 4 weeks after birth due to increased risk of blood clots.[40] While studies have demonstrated conflicting results about the effects of combined oral contraceptive pills on lactation duration and milk volume, there exist concerns about the transient risk of combined oral contraceptive pills on breast milk production when breastfeeding is being established early postpartum.[96] Due to the stated risks and additional concerns on lactation, women who are breastfeeding are not advised to start combined oral contraceptive pills until at least six weeks postpartum, while women who are not breastfeeding and have no other risks factors for blood clots may start combined oral contraceptive pills after 21 days postpartum.[97][88]
Breast cancer
[edit]The World Health Organization (WHO) does not recommend the use of combined oral contraceptive pills in women with breast cancer.[41][98] Since combined oral contraceptive pills contain both estrogen and progestin, they are not recommended to be used in those with hormonally-sensitive cancers, including some types of breast cancer.[99][unreliable medical source?][100] Non-hormonal contraceptive methods, such as the Copper IUD or condoms,[101] should be the first-line contraceptive choice for these patients instead of combined oral contraceptive pills.[102][unreliable medical source?]
Other
[edit]Women with known or suspected endometrial cancer or unexplained uterine bleeding should also not take combined oral contraceptive pills to avoid health risks.[94] Combined oral contraceptive pills are also contraindicated for people with advanced diabetes, liver tumors, hepatic adenoma or severe cirrhosis of the liver.[41][95] Combined oral contraceptive pills are metabolized in the liver and thus liver disease can lead to reduced elimination of the medication. Additionally, severe hypercholesterolemia and hypertriglyceridemia are also contraindications, but the evidence showing that combined oral contraceptive pills lead to worse outcomes in this population is weak.[37][40] Obesity is not considered to be a contraindication to taking combined oral contraceptive pills.[40]
Side effects
[edit]It is generally accepted that the health risks of oral contraceptives are lower than those from pregnancy and birth,[103] and "the health benefits of any method of contraception are far greater than any risks from the method".[104] Some organizations have argued that comparing a contraceptive method to no method (pregnancy) is not relevant—instead, the comparison of safety should be among available methods of contraception.[105]
Common
[edit]Different sources note different incidence of side effects. The most common side effect is breakthrough bleeding. Combined oral contraceptive pills can improve conditions such as dysmenorrhea, premenstrual syndrome, and acne,[106] reduce symptoms of endometriosis and polycystic ovary syndrome, and decrease the risk of anemia.[107] Use of oral contraceptives also reduces lifetime risk of ovarian and endometrial cancer.[108][109][110]
Nausea, vomiting, headache, bloating, breast tenderness, swelling of the ankles/feet (fluid retention), or weight change may occur. Vaginal bleeding between periods (spotting) or missed/irregular periods may occur, especially during the first few months of use.[111]
Heart and blood vessels
[edit]Combined oral contraceptives are associated with an increased risk of venous thromboembolism, including deep vein thrombosis (DVT) and pulmonary embolism (PE).[112][113]
While lower doses of estrogen in combined oral contraceptive pills may have a lower risk of stroke and myocardial infarction compared to higher estrogen dose pills (50 μg/day), users of low estrogen dose combined oral contraceptive pills still have an increased risk compared to non-users.[114] These risks are greatest in women with additional risk factors, such as smoking (which increases risk substantially) and long-continued use of the pill, especially in women over 35 years of age.[115]
The overall absolute risk of venous thrombosis per 100,000 woman-years in current use of combined oral contraceptives is approximately 60, compared with 30 in non-users.[116] The risk of thromboembolism varies with different types of birth control pills; compared with combined oral contraceptives containing levonorgestrel (LNG), and with the same dose of estrogen and duration of use, the rate ratio of deep venous thrombosis for combined oral contraceptives with norethisterone is 0.98, with norgestimate 1.19, with desogestrel (DSG) 1.82, with gestodene 1.86, with drospirenone (DRSP) 1.64, and with cyproterone acetate 1.88.[116] In comparison, venous thromboembolism occurs in 100–200 per 100.000 pregnant women every year.[116]
One study showed more than a 600% increased risk of blood clots for women taking combined oral contraceptive pills with drospirenone compared with non-users, compared with 360% higher for women taking birth control pills containing levonorgestrel.[117] The US Food and Drug Administration (FDA) initiated studies evaluating the health of more than 800,000 women taking combined oral contraceptive pills and found that the risk of VTE was 93% higher for women who had been taking drospirenone combined oral contraceptive pills for 3 months or less and 290% higher for women taking drospirenone combined oral contraceptive pills for 7–12 months, compared with women taking other types of oral contraceptives.[118]
Based on these studies, in 2012, the FDA updated the label for drospirenone combined oral contraceptive pills to include a warning that contraceptives with drospirenone may have a higher risk of dangerous blood clots.[119]
A 2015 systematic review and meta-analysis found that combined birth control pills were associated with 7.6-fold higher risk of cerebral venous sinus thrombosis, a rare form of stroke in which blood clotting occurs in the cerebral venous sinuses.[120]
| Type | Route | Medications | Odds ratio (95% CI) |
|---|---|---|---|
| Menopausal hormone therapy | Oral | Estradiol alone ≤1 mg/day >1 mg/day |
1.27 (1.16–1.39)* 1.22 (1.09–1.37)* 1.35 (1.18–1.55)* |
| Conjugated estrogens alone ≤0.625 mg/day >0.625 mg/day |
1.49 (1.39–1.60)* 1.40 (1.28–1.53)* 1.71 (1.51–1.93)* | ||
| Estradiol/medroxyprogesterone acetate | 1.44 (1.09–1.89)* | ||
| Estradiol/dydrogesterone ≤1 mg/day E2 >1 mg/day E2 |
1.18 (0.98–1.42) 1.12 (0.90–1.40) 1.34 (0.94–1.90) | ||
| Estradiol/norethisterone ≤1 mg/day E2 >1 mg/day E2 |
1.68 (1.57–1.80)* 1.38 (1.23–1.56)* 1.84 (1.69–2.00)* | ||
| Estradiol/norgestrel or estradiol/drospirenone | 1.42 (1.00–2.03) | ||
| Conjugated estrogens/medroxyprogesterone acetate | 2.10 (1.92–2.31)* | ||
| Conjugated estrogens/norgestrel ≤0.625 mg/day CEEs >0.625 mg/day CEEs |
1.73 (1.57–1.91)* 1.53 (1.36–1.72)* 2.38 (1.99–2.85)* | ||
| Tibolone alone | 1.02 (0.90–1.15) | ||
| Raloxifene alone | 1.49 (1.24–1.79)* | ||
| Transdermal | Estradiol alone ≤50 μg/day >50 μg/day |
0.96 (0.88–1.04) 0.94 (0.85–1.03) 1.05 (0.88–1.24) | |
| Estradiol/progestogen | 0.88 (0.73–1.01) | ||
| Vaginal | Estradiol alone | 0.84 (0.73–0.97) | |
| Conjugated estrogens alone | 1.04 (0.76–1.43) | ||
| Combined birth control | Oral | Ethinylestradiol/norethisterone | 2.56 (2.15–3.06)* |
| Ethinylestradiol/levonorgestrel | 2.38 (2.18–2.59)* | ||
| Ethinylestradiol/norgestimate | 2.53 (2.17–2.96)* | ||
| Ethinylestradiol/desogestrel | 4.28 (3.66–5.01)* | ||
| Ethinylestradiol/gestodene | 3.64 (3.00–4.43)* | ||
| Ethinylestradiol/drospirenone | 4.12 (3.43–4.96)* | ||
| Ethinylestradiol/cyproterone acetate | 4.27 (3.57–5.11)* | ||
| Notes: (1) Nested case–control studies (2015, 2019) based on data from the QResearch and Clinical Practice Research Datalink (CPRD) databases. (2) Bioidentical progesterone was not included, but is known to be associated with no additional risk relative to estrogen alone. Footnotes: * = Statistically significant (p < 0.01). Sources: See template. | |||
Cancer
[edit]Decreased risk of ovarian, endometrial, and colorectal cancers
[edit]Usage of combined oral concetraption decreased the risk of ovarian cancer, endometrial cancer,[44] and colorectal cancer.[4][106][121] Two large cohort studies published in 2010 both found a significant reduction in adjusted relative risk of ovarian and endometrial cancer mortality in ever-users of OCs compared with never-users.[2][122] The use of oral contraceptives (birth control pills) for five years or more decreases the risk of ovarian cancer in later life by 50%.[121][123] Combined oral contraceptive use reduces the risk of ovarian cancer by 40% and the risk of endometrial cancer by 50% compared with never users. The risk reduction increases with duration of use, with an 80% reduction in risk for both ovarian and endometrial cancer with use for more than 10 years. The risk reduction for both ovarian and endometrial cancer persists for at least 20 years.[44]
Increased risk of Breast, cervical, and liver cancers
[edit]Combined oral concetraption is a IARC group 1 Carcinogen meaning there is sufficient evidence of carcinogenicity in humans.
An association between use of birth control pills and liver cancer has been suspected, but subsequent large population research has failed to confirm such an association.[124]
Increased risk of breast cancer was reported in women who take combined oral concetraption.[125][126] The relative risk of breast cancer in the current combined oral concetraption users was 1.24 (95% confidence interval [CI], 1.15–1.33), and this increased risk disappeared 10 years after discontinuation.[127] There was also a higher risk of premature deaths due to breast cancer in the population who used COCP (P < 0.0001) for longer duration.[128] 24 observational studies showed a higher risk of cervical cancer in women who use COCP, especially with an increased duration of COCP use.[129][126]
A 2013 meta-analysis concluded that every use of birth control pills is associated with a modest increase in the risk of breast cancer (relative risk 1.08) and a reduced risk of colorectal cancer (relative risk 0.86) and endometrial cancer (relative risk 0.57). Cervical cancer risk in those infected with HPV is increased.[130] A similar small increase in breast cancer risk was observed in other meta analyses.[131][132] A study of 1.8 million Danish women of reproductive age followed for 11 years found that the risk of breast cancer was 20% higher among those who currently or recently used hormonal contraceptives than among women who had never used hormonal contraceptives.[133] This risk increased with duration of use, with a 38% increase in risk after more than 10 years of use.[133]
Weight
[edit]A 2016 systematic review found low quality evidence that studies of combination hormonal contraceptives showed no large difference in weight when compared with placebo or no intervention groups.[134] The evidence was not strong enough to be certain that contraceptive methods do not cause some weight change, but no major effect was found.[134] This review also found "that women did not stop using the pill or patch because of weight change".[134]
Sexual function and risk aversion
[edit]Sexual desire
[edit]Some researchers question a causal link between combined oral contraceptive pill use and decreased libido;[135] a 2007 study of 1700 women found combined oral contraceptive pill users experienced no change in sexual satisfaction.[136] A 2005 laboratory study of genital arousal tested fourteen women before and after they began taking combined oral contraceptive pills. The study found that women experienced a significantly wider range of arousal responses after beginning pill use; decreases and increases in measures of arousal were equally common.[137][138]
In 2012, The Journal of Sexual Medicine published a review of research studying the effects of hormonal contraceptives on female sexual function that concluded that the sexual side effects of hormonal contraceptives are not well-studied and especially in regards to impacts on libido, with research establishing only mixed effects where only small percentages of women report experiencing an increase or decrease and majorities report being unaffected.[139] In 2013, The European Journal of Contraception & Reproductive Health Care published a review of 36 studies including 8,422 female subjects in total taking combined oral contraceptive pills that found that 5,358 subjects (or 63.6 percent) reported no change in libido, 1,826 subjects (or 21.7 percent) reported an increase, and 1,238 subjects (or 14.7 percent) reported a decrease.[140] In 2019, Neuroscience & Biobehavioral Reviews published a meta-analysis of 22 published and 4 unpublished studies (with 7,529 female subjects in total) that evaluated whether women expose themselves to greater health risks at different points in the menstrual cycle including by sexual activity with partners and found that subjects in the last third of the follicular phase and at ovulation (when levels of endogenous estradiol and luteinizing hormones are heightened) experienced increased sexual activity with partners as compared with the luteal phase and during menstruation.[141]
A 2006 study of 124 premenopausal women measured sex hormone-binding globulin (SHBG), including before and after discontinuation of the oral contraceptive pill. Women continuing use of oral contraceptives had SHBG levels four times higher than those who never used it, and levels remained elevated even in the group that had discontinued its use.[142][143] Theoretically, an increase in SHBG may be a physiologic response to increased hormone levels, but may decrease the free levels of other hormones, such as androgens, because of the unspecificity of its sex hormone binding. In 2020, The Lancet Diabetes & Endocrinology published a cross-sectional study of 588 premenopausal female subjects aged 18 to 39 years from the Australian states of Queensland, New South Wales, and Victoria with regular menstrual cycles whose SHBG levels were measured by immunoassay that found that after controlling for age, body mass index, cycle stage, smoking, parity, partner status, and psychoactive medication, SHBG was inversely correlated with sexual desire.[144]
Sexual attractiveness and function
[edit]Combined oral contraceptive pills may increase natural vaginal lubrication,[145] while some women experience decreased lubrication.[145][146]
In 2004, the Proceedings of the Royal Society B: Biological Sciences published a study where pairs of digital photographs of the faces of 48 women at Newcastle University and Charles University between the ages 19 and 33 who were not taking hormonal contraceptives during the study were photographed in the late follicular and early mid-luteal phases of their menstrual cycles and the photographs were then rated by 261 blinded subjects (130 male and 131 female) at their respective universities who compared the facial attractiveness of each photographed woman in their photograph pairs, and found that the subjects perceived the late follicular phase images of the photographed women as being more attractive than the luteal phase images by more than expected by random chance.[147]
In 2007, Evolution and Human Behavior published a study where 18 professional lap dancers recorded their menstrual cycles, work shifts, and tip earnings at gentlemen's clubs for 60 days that found by a mixed model analysis of 296 work shifts (or approximately 5,300 lap dances) that the 11 dancers with normal menstrual cycles earned US$335 per 5-hour shift during the late follicular phase and at ovulation, US$260 per shift during the luteal phase, and US$185 per shift during menstruation, while the 7 dancers using hormonal contraceptives showed no earnings peak during the late follicular phase and at ovulation.[148] In 2008, Evolution and Human Behavior published a study where the voices of 51 female students at the State University of New York at Albany were recorded with the women counting from 1 to 10 at four different points in their menstrual cycles were rated by blinded subjects who listened to the recordings to be more attractive at the points of the menstrual cycle with higher probabilities of conception, while the ratings of the voices of the women who were taking hormonal contraceptives showed no variation over the menstrual cycle in attractiveness.[149]
Risk-taking behaviour
[edit]In 1998, Evolution and Human Behavior published a study of 300 female undergraduate students at the State University of New York at Albany between the ages of 18 and 54 (with a mean age of 21.9 years) that surveyed the subjects engagement in 18 different behaviors over the 24 hours prior to filling out the study's questionnaire that varied in their risk of potential rape or sexual assault and the first day of their last menstruations, and found that subjects at ovulation showed statistically significant decreased engagement in behaviors that risked rape and sexual assault while subjects taking birth control pills showed no variation over their menstrual cycles in the same behaviors (suggesting a psychologically adaptive function of the hormonal fluctuations during the menstrual cycle in causing avoidance of behaviors that risk rape and sexual assault).[150][151] In 2003, Evolution and Human Behavior published a conceptual replication study of the 1998 survey that confirmed its findings.[152]
In 2006, a study presented at the annual conference of the Cognitive Science Society surveyed 176 female undergraduate students at Michigan State University (with a mean age of 19.9 years) in a decision-making experiment where the subjects chose between an option with a guaranteed outcome or an option involving risk and indicated the first day of their last menstruations, and found that the subjects risk aversion preferences varied over the menstrual cycle (with none of the subjects at ovulation preferring the risky option) and only subjects not taking hormonal contraceptives showed the menstrual cycle effect on risk aversion.[153] In the 2019 Neuroscience & Biobehavioral Reviews meta-analysis, the research reviewed also evaluated whether the 7,529 female subjects across the 26 studies showed greater risk recognition and avoidance of potentially threatening people and dangerous situations at different phases of the menstrual cycle and found that the subjects displayed better risk accuracy recognition during the late follicular phase and at ovulation as compared to the luteal phase.[141]
Depression
[edit]Low levels of serotonin, a neurotransmitter in the brain, have been linked to depression. High levels of estrogen, as in first-generation combined oral contraceptive pills, and progestin, as in some progestin-only contraceptives, have been shown to lower the brain serotonin levels by increasing the concentration of a brain enzyme that reduces serotonin.[citation needed]
Current medical reference textbooks on contraception[44] and major organizations such as the American ACOG,[154] the WHO,[88] and the United Kingdom's RCOG[155] agree that current evidence indicates low-dose combined oral contraceptives are unlikely to increase the risk of depression, and unlikely to worsen the condition in women that are depressed.
Hypertension
[edit]Bradykinin lowers blood pressure by causing blood vessel dilation. Certain enzymes are capable of breaking down bradykinin (Angiotensin Converting Enzyme, Aminopeptidase P). Progesterone can increase the levels of Aminopeptidase P (AP-P), thereby increasing the breakdown of bradykinin, which increases the risk of developing hypertension.[156]
Thyroid
[edit]Estrogen in oral contraceptives may increase thyroid binding globulin and decrease free T4. Thus, longer history of oral contraceptives use may be strongly associated with hypothyroidism, especially for more than 10 years. Also, a higher dose of thyroxine may be needed with oral contraceptives.[157]
Other effects
[edit]Other side effects associated with low-dose combined oral contraceptive pills are leukorrhea (increased vaginal secretions), reductions in menstrual flow, mastalgia (breast tenderness), and decrease in acne. Side effects associated with older high-dose combined oral contraceptive pills include nausea, vomiting, increases in blood pressure, and melasma (facial skin discoloration); these effects are not strongly associated with low-dose formulations.[medical citation needed]
Excess estrogen, such as from birth control pills, appears to increase cholesterol levels in bile and decrease gallbladder movement, which can lead to gallstones.[158] Progestins found in certain formulations of oral contraceptive pills can limit the effectiveness of weight training to increase muscle mass.[159] This effect is caused by the ability of some progestins to inhibit androgen receptors. One study claims that the pill may affect what male body odors a woman prefers, which may in turn influence her selection of partner.[160][161][162] Use of combined oral contraceptives is associated with a reduced risk of endometriosis, giving a relative risk of endometriosis of 0.63 during active use, yet with limited quality of evidence according to a systematic review.[163]
Combined oral contraception decreases total testosterone levels by approximately 0.5 nmol/L, free testosterone by approximately 60%, and increases the amount of sex hormone binding globulin (SHBG) by approximately 100 nmol/L. Contraceptives containing second generation progestins and/or estrogen doses of around 20 –25 mg EE were found to have less impact on SHBG concentrations.[164] Combined oral contraception may also reduce bone density.[165]
Drug interactions
[edit]Some drugs reduce the effect of the pill and can cause breakthrough bleeding, or increased chance of pregnancy. These include drugs such as rifampicin, barbiturates, phenytoin and carbamazepine. In addition cautions are given about broad spectrum antibiotics, such as ampicillin and doxycycline, which may cause problems "by impairing the bacterial flora responsible for recycling ethinylestradiol from the large bowel" (BNF 2003).[166][167][168][169]
The traditional medicinal herb St John's Wort has also been implicated due to its upregulation of the P450 system in the liver which could increase the metabolism of ethinyl estradiol and progestin components of some combined oral contraception.[170]
Accessibility
[edit]The availability of pharmaceutical products to the public is determined by the local governing body. In the US, the responsible organisation is the Food and Drug Administration (FDA). According to a press announcement in July 2023, a daily hormonal oral contraceptive was first made accessible to the public without a prescription.[171] Although this drug class was approved for prescription use as early as in 1973, it took an additional 50 years to de-escalate its legal status. Such allowance is made plausible thanks to the demonstration of its safe and effective use by the general public, not needing any guidance from healthcare professionals.[171] Ultimately, the governing body should act accordingly to applicants' evidence and update the local legislation.[171]
History
[edit]By the 1930s, scientists had isolated and determined the structure of the steroid hormones and found that high doses of androgens, estrogens or progesterone inhibited ovulation,[179][180][181][182] but obtaining these hormones, which were produced from animal extracts, from European pharmaceutical companies was extraordinarily expensive.[183]
In 1939, Russell Marker, a professor of organic chemistry at Pennsylvania State University, developed a method of synthesizing progesterone from plant steroid sapogenins, initially using sarsapogenin from sarsaparilla, which proved too expensive. After three years of extensive botanical research, he discovered a much better starting material, the saponin from inedible Mexican yams (Dioscorea mexicana and Dioscorea composita) found in the rain forests of Veracruz near Orizaba. The saponin could be converted in the lab to its aglycone moiety diosgenin. Unable to interest his research sponsor Parke-Davis in the commercial potential of synthesizing progesterone from Mexican yams, Marker left Penn State and in 1944 co-founded Syntex with two partners in Mexico City. When he left Syntex a year later the trade of the barbasco yam had started and the period of the heyday of the Mexican steroid industry had been started. Syntex broke the monopoly of European pharmaceutical companies on steroid hormones, reducing the price of progesterone almost 200-fold over the next eight years.[184][185][186]
Midway through the 20th century, the stage was set for the development of a hormonal contraceptive, but pharmaceutical companies, universities and governments showed no interest in pursuing research.[187]
Progesterone to prevent ovulation
[edit]Progesterone, given by injections, was first shown to inhibit ovulation in animals in 1937 by Makepeace and colleagues.[188]
In 1951, reproductive physiologist Gregory Pincus, a leader in hormone research and co-founder of the Worcester Foundation for Experimental Biology (WFEB) in Shrewsbury, Massachusetts, first met American birth control movement founder Margaret Sanger at a Manhattan dinner hosted by Abraham Stone, medical director and vice president of Planned Parenthood (PPFA), who helped Pincus obtain a small grant from PPFA to begin hormonal contraceptive research.[189][190][191] Research started in April 1951, with reproductive physiologist Min Chueh Chang repeating and extending the 1937 experiments of Makepeace et al. that was published in 1953 and showed that injections of progesterone suppressed ovulation in rabbits.[188] In October 1951, G. D. Searle & Company refused Pincus' request to fund his hormonal contraceptive research, but retained him as a consultant and continued to provide chemical compounds to evaluate.[183][192][193]
In March 1952, Sanger wrote a brief note mentioning Pincus' research to her longtime friend and supporter, suffragist and philanthropist Katharine Dexter McCormick, who visited the WFEB and its co-founder and old friend Hudson Hoagland in June 1952 to learn about contraceptive research there. Frustrated when research stalled from PPFA's lack of interest and meager funding, McCormick arranged a meeting at the WFEB in June 1953, with Sanger and Hoagland, where she first met Pincus who committed to dramatically expand and accelerate research with McCormick providing fifty times PPFA's previous funding.[192][194]
Pincus and McCormick enlisted Harvard clinical professor of gynecology John Rock, chief of gynecology at the Free Hospital for Women and an expert in the treatment of infertility, to lead clinical research with women. At a scientific conference in 1952, Pincus and Rock, who had known each other for many years, discovered they were using similar approaches to achieve opposite goals. In 1952, Rock induced a three-month anovulatory "pseudopregnancy" state in eighty of his infertility patients with continuous gradually increasing oral doses of an estrogen (5 to 30 mg/day diethylstilbestrol) and progesterone (50 to 300 mg/day), and within the following four months 15% of the women became pregnant.[192][195][196]
In 1953, at Pincus' suggestion, Rock induced a three-month anovulatory "pseudopregnancy" state in twenty-seven of his infertility patients with an oral 300 mg/day progesterone-only regimen for 20 days from cycle days 5–24 followed by pill-free days to produce withdrawal bleeding.[197] This produced the same 15% pregnancy rate during the following four months without the amenorrhea of the previous continuous estrogen and progesterone regimen.[197] But 20% of the women experienced breakthrough bleeding and in the first cycle ovulation was suppressed in only 85% of the women, indicating that even higher and more expensive oral doses of progesterone would be needed to initially consistently suppress ovulation.[197] Similarly, Ishikawa and colleagues found that ovulation inhibition occurred in only a "proportion" of cases with 300 mg/day oral progesterone.[198] Despite the incomplete inhibition of ovulation by oral progesterone, no pregnancies occurred in the two studies, although this could have simply been due to chance.[198][199] However, Ishikawa et al. reported that the cervical mucus in women taking oral progesterone became impenetrable to sperm, and this may have accounted for the absence of pregnancies.[198][199]
Progesterone was abandoned as an oral ovulation inhibitor following these clinical studies due to the high and expensive doses required, incomplete inhibition of ovulation, and the frequent incidence of breakthrough bleeding.[188][200] Instead, researchers would turn to much more potent synthetic progestogens for use in oral contraception in the future.[188][200]
Progestins to prevent ovulation
[edit]In October 1951, Chemist Luis Miramontes, working under the supervision of Carl Djerassi, and the direction of George Rosenkranz at Syntex in Mexico City, synthesized the first oral contraceptive, which was based on highly active progestin norethisterone. Frank B. Colton at Searle in Skokie, Illinois synthesized the orally highly active progestins noretynodrel (an isomer of norethisterone) in 1952 and norethandrolone in 1953.[183]
Pincus asked his contacts at pharmaceutical companies to send him chemical compounds with progestogenic activity. Chang screened nearly 200 chemical compounds in animals and found the three most promising were Syntex's norethisterone and Searle's noretynodrel and norethandrolone.[201]
In December 1954, Rock began the first studies of the ovulation-suppressing potential of 5–50 mg doses of the three oral progestins for three months (for 21 days per cycle—days 5–25 followed by pill-free days to produce withdrawal bleeding) in fifty of his patients with infertility in Brookline, Massachusetts. Norethisterone or noretynodrel 5 mg doses and all doses of norethandrolone suppressed ovulation but caused breakthrough bleeding, but 10 mg and higher doses of norethisterone or noretynodrel suppressed ovulation without breakthrough bleeding and led to a 14% pregnancy rate in the following five months. Pincus and Rock selected Searle's noretynodrel for the first contraceptive trials in women, citing its total lack of androgenicity versus Syntex's norethisterone very slight androgenicity in animal tests.[202][203]
Combined oral contraceptive
[edit]Noretynodrel (and norethisterone) were subsequently discovered to be contaminated with a small percentage of the estrogen mestranol (an intermediate in their synthesis), with the noretynodrel in Rock's 1954–5 study containing 4–7% mestranol. When further purifying noretynodrel to contain less than 1% mestranol led to breakthrough bleeding, it was decided to intentionally incorporate 2.2% mestranol, a percentage that was not associated with breakthrough bleeding, in the first contraceptive trials in women in 1954. The noretynodrel and mestranol combination was given the proprietary name Enovid.[203][204]
The first contraceptive trial of Enovid led by Celso-Ramón García and Edris Rice-Wray began in April 1956 in Río Piedras, Puerto Rico.[205][206][207] A second contraceptive trial of Enovid (and norethisterone) led by Edward T. Tyler began in June 1956 in Los Angeles.[186][208] In January 1957, Searle held a symposium reviewing gynecologic and contraceptive research on Enovid through 1956 and concluded Enovid's estrogen content could be reduced by 33% to lower the incidence of estrogenic gastrointestinal side effects without significantly increasing the incidence of breakthrough bleeding.[209]
While these large-scale trials contributed to the initial understanding of the pill formulation's clinical effects, the ethical implications of the trials generated significant controversy. Of note is the apparent lack of both autonomy and informed consent among participants in the Puerto Rican cohort prior to the trials. Many of these participants hailed from impoverished, working-class backgrounds.[10]
Public availability
[edit]As of 2013, less than a third of countries worldwide required a prescription for oral contraceptives.[210]
United States
[edit]
In June 1957, the Food and Drug Administration (FDA) approved Enovid 10 mg (9.85 mg noretynodrel and 150 μg mestranol) for menstrual disorders, based on data from its use by more than 600 women. Numerous additional contraceptive trials showed Enovid at 10, 5, and 2.5 mg doses to be highly effective. In July 1959, Searle filed a supplemental application to add contraception as an approved indication for 10, 5, and 2.5 mg doses of Enovid. The FDA refused to consider the application until Searle agreed to withdraw the lower dosage forms from the application. In May 1960, the FDA announced it would approve Enovid 10 mg for contraceptive use, and did so in June 1960. At that point, Enovid 10 mg had been in general use for three years and, by conservative estimate, at least half a million women had used it.[207][211][212]
Although FDA-approved for contraceptive use, Searle never marketed Enovid 10 mg as a contraceptive. Eight months later, in February 1961, the FDA approved Enovid 5 mg for contraceptive use. In July 1961, Searle finally began marketing Enovid 5 mg (5 mg noretynodrel and 75 μg mestranol) to physicians as a contraceptive.[211][213]
Although the FDA approved the first oral contraceptive in 1960, contraceptives were not available to married women in all states until Griswold v. Connecticut in 1965, and were not available to unmarried women in all states until Eisenstadt v. Baird in 1972.[187][213]
The first published case report of a blood clot and pulmonary embolism in a woman using Enavid (Enovid 10 mg in the US) at a dose of 20 mg/day did not appear until November 1961, four years after its approval, by which time it had been used by over one million women.[207][214][215] It would take almost a decade of epidemiological studies to conclusively establish an increased risk of venous thrombosis in oral contraceptive users and an increased risk of stroke and myocardial infarction in oral contraceptive users who smoke or have high blood pressure or other cardiovascular or cerebrovascular risk factors.[211] These risks of oral contraceptives were dramatized in the 1969 book The Doctors' Case Against the Pill by feminist journalist Barbara Seaman who helped arrange the 1970 Nelson Pill Hearings called by Senator Gaylord Nelson.[216] The hearings were conducted by senators who were all men and the witnesses in the first round of hearings were all men, leading Alice Wolfson and other feminists to protest the hearings and generate media attention.[213] Their work led to mandating the inclusion of patient package inserts with oral contraceptives to explain their possible side effects and risks to help facilitate informed consent.[217][218][219] Today's standard dose oral contraceptives contain an estrogen dose that is one third lower than the first marketed oral contraceptive and contain lower doses of different, more potent progestins in a variety of formulations.[44][211][213]
Beginning in 2015, certain states passed legislation allowing pharmacists to prescribe oral contraceptives. Such legislation was considered to address physician shortages and decrease barriers to birth control for women.[220] Pharmacists in Oregon, California, Colorado, Hawaii, Maryland, and New Mexico have authority to prescribe birth control after receiving specialized training and certification from their respective state Board of Pharmacy.[221][222] As of January 2024[update], pharmacists in 29 states can prescribe oral contraceptives.[223]
A progestin-based birth control pill (Opill) was approved by the FDA in 2023 and is available over the counter.[224] Estrogen-based pills still require prescriptions as of 2024.
Australia
[edit]The first oral contraceptive introduced outside the United States was Schering's Anovlar (norethisterone acetate 4 mg + ethinylestradiol 50 μg) in January 1961, in Australia.[225]
Germany
[edit]The first oral contraceptive introduced in Europe was Schering's Anovlar in June 1961, in West Germany.[225] The lower hormonal dose, still in use, was studied by the Belgian Gynaecologist Ferdinand Peeters.[226][227]
United Kingdom
[edit]Before the mid-1960s, the United Kingdom did not require pre-marketing approval of drugs. The British Family Planning Association (FPA) through its clinics was then the primary provider of family planning services in the UK and provided only contraceptives that were on its Approved List of Contraceptives (established in 1934). In 1957, Searle began marketing Enavid (Enovid 10 mg in the US) for menstrual disorders. Also in 1957, the FPA established a Council for the Investigation of Fertility Control (CIFC) to test and monitor oral contraceptives which began animal testing of oral contraceptives and in 1960 and 1961 began three large clinical trials in Birmingham, Slough, and London.[207][228]
In March 1960, the Birmingham FPA began trials of noretynodrel 2.5 mg + mestranol 50 μg, but a high pregnancy rate initially occurred when the pills accidentally contained only 36 μg of mestranol—the trials were continued with noretynodrel 5 mg + mestranol 75 μg (Conovid in the UK, Enovid 5 mg in the US).[229] In August 1960, the Slough FPA began trials of noretynodrel 2.5 mg + mestranol 100 μg (Conovid-E in the UK, Enovid-E in the US).[230] In May 1961, the London FPA began trials of Schering's Anovlar.[231]
In October 1961, at the recommendation of the Medical Advisory Council of its CIFC, the FPA added Searle's Conovid to its Approved List of Contraceptives.[232] In December 1961, Enoch Powell, then Minister of Health, announced that the oral contraceptive pill Conovid could be prescribed through the NHS at a subsidized price of 2s per month.[233][234] In 1962, Schering's Anovlar and Searle's Conovid-E were added to the FPA's Approved List of Contraceptives.[207][230][231]
France
[edit]In December 1967, the Neuwirth Law legalized contraception in France, including the pill.[235] The pill is the most popular form of contraception in France, especially among young women. It accounts for 60% of the birth control used in France. The abortion rate has remained stable since the introduction of the pill.[236]
Japan
[edit]In Japan, lobbying from the Japan Medical Association prevented the pill from being approved for general use for nearly 40 years. The higher dose "second generation" pill was approved for use in cases of gynecological problems, but not for birth control. Two main objections raised by the association were safety concerns over long-term use of the pill, and concerns that pill use would lead to decreased use of condoms and thereby potentially increase sexually transmitted infection (STI) rates.[237]
However, when the Ministry of Health and Welfare approved Viagra's use in Japan after only six months of the application's submission, while still claiming that the pill required more data before approval, women's groups cried foul.[238] The pill was subsequently approved for use in June 1999, when Japan became the last UN member country to do so.[239] However, the pill has not become popular in Japan.[240] According to estimates, only 1.3 percent of 28 million Japanese females of childbearing age use the pill, compared with 15.6 percent in the United States. The pill prescription guidelines the government has endorsed require pill users to visit a doctor every three months for pelvic examinations and undergo tests for sexually transmitted diseases and uterine cancer. In the United States and Europe, in contrast, an annual or bi-annual clinic visit is standard for pill users. However, beginning as far back as 2007, many Japanese OBGYNs have required only a yearly visit for pill users, with multiple checks a year recommended only for those who are older or at increased risk of side effects.[241] As of 2004, condoms accounted for 80% of birth control use in Japan, and this may explain Japan's comparatively low rates of AIDS.[241]
Society and culture
[edit]
In countries such as Ireland where contraceptives were illegal or otherwise not commonly available, the baby boom lasted longer.[242]
The pill was approved by the US FDA in the early 1960s; its use spread rapidly in the late part of that decade, generating an enormous social impact. Time magazine placed the pill on its cover in April 1967.[243][244] In the first place, it was more effective than most previous reversible methods of birth control, giving women unprecedented control over their fertility.[245] Its use was separate from intercourse, requiring no special preparations at the time of sexual activity that might interfere with spontaneity or sensation, and the choice to take the pill was a private one. This combination of factors served to make the pill immensely popular within a few years of its introduction.[184][213]
Claudia Goldin, among others, argue that this new contraceptive technology was a key player in forming women's modern economic role, in that it prolonged the age at which women first married allowing them to invest in education and other forms of human capital as well as generally become more career-oriented. Soon after the birth control pill was legalized, there was a sharp increase in college attendance and graduation rates for women.[246] From an economic point of view, the birth control pill reduced the cost of staying in school. The ability to control fertility without sacrificing sexual relationships allowed women to make long term educational and career plans.[247]
Because the pill was so effective, and soon so widespread, it also heightened the debate about the moral and health consequences of pre-marital sex and promiscuity. Never before had sexual activity been so divorced from reproduction. For a couple using the pill, intercourse became purely an expression of love, or a means of physical pleasure, or both; but it was no longer a means of reproduction. While this was true of previous contraceptives, their relatively high failure rates and their less widespread use failed to emphasize this distinction as clearly as did the pill. The spread of oral contraceptive use thus led many religious figures and institutions to debate the proper role of sexuality and its relationship to procreation. The Roman Catholic Church in particular, after studying the phenomenon of oral contraceptives, re-emphasized the stated teaching on birth control in the 1968 papal encyclical Humanae vitae. The encyclical reiterated the established Catholic teaching that artificial contraception distorts the nature and purpose of sex.[248] On the other side Anglican and other Protestant churches, such as the Protestant Church in Germany (EKD), accepted the combined oral contraceptive pill.[249]
The United States Senate began hearings on the pill in 1970 and where different viewpoints were heard from medical professionals. Dr. Michael Newton, President of the College of Obstetricians and Gynecologists said:
The evidence is not yet clear that these still do in fact cause cancer or related to it. The FDA Advisory Committee made comments about this, that if there wasn't enough evidence to indicate whether or not these pills were related to the development of cancer, and I think that's still thin; you have to be cautious about them, but I don't think there is clear evidence, either one way or the other, that they do or don't cause cancer.[250]
Another physician, Dr. Roy Hertz of the Population Council, said that anyone who takes this should know of "our knowledge and ignorance in these matters" and that all women should be made aware of this so they can decide to take the pill or not.[250]
The Secretary of Health, Education, and Welfare at the time, Robert Finch, announced the federal government had accepted a compromise warning statement which would accompany all sales of birth control pills.[250]
Result on popular culture
[edit]The introduction of the birth control pill in 1960 allowed more women to find employment opportunities and further their education. As a result of women getting more jobs and an education, their husbands had to start taking over household tasks like cooking.[251] Wanting to stop the change that was occurring in terms of gender norms in an American household, many films, television shows, and other popular culture items portrayed what an ideal American family should be. Below are listed some examples:
Poem
[edit]- The Pill Versus the Springhill Mine Disaster is the title poem of a 1968 collection by Richard Brautigan.[252]
Music
[edit]- Singer Loretta Lynn commented on how women no longer had to choose between a relationship and a career in her 1974 album with a song entitled "The Pill", which told the story of a married woman's use of the drug to liberate herself from her traditional role as wife and mother.[253]
Environmental impact
[edit]A woman using combined oral contraceptive pills excretes in her urine and feces natural estrogens, estrone (E1) and estradiol (E2), and synthetic estrogen ethinylestradiol (EE2).[254] These hormones can pass through water treatment plants and into rivers.[255] Other forms of contraception, such as the contraceptive patch, use the same synthetic estrogen (EE2) that is found in combined oral contraceptive pills, and can add to the hormonal concentration in the water when flushed down the toilet.[256] This excretion is shown to play a role in causing endocrine disruption, which affects the sexual development and reproduction of wild fish populations in segments of streams contaminated by treated sewage effluents.[254][257] A study done in British rivers supported the hypothesis that the incidence and the severity of intersex wild fish populations were significantly correlated with the concentrations of the E1, E2, and EE2 in the rivers.[254]
A review of activated sludge plant performance found estrogen removal rates varied considerably but averaged 78% for estrone, 91% for estradiol, and 76% for ethinylestradiol (estriol effluent concentrations are between those of estrone and estradiol, but estriol is a much less potent endocrine disruptor to fish).[258]
Several studies have suggested that reducing human population growth through increased access to contraception, including birth control pills, can be an effective strategy for climate change mitigation as well as adaptation.[259][260] According to Thomas Wire, contraception is the 'greenest technology' because of its cost-effectiveness in combating global warming — each $7 spent on contraceptives would reduce global carbon emissions by 1 tonne over four decades, while achieving the same result with low-carbon technologies would require $32.[261]
See also
[edit]References
[edit]- ^ a b Trussell J (2011). "Contraceptive efficacy". In Hatcher RA, Trussell J, Nelson A, Cates W, Kowal D, Policar M (eds.). Contraceptive technology (20th revised ed.). New York: Ardent Media. pp. 779–863. ISBN 978-1-59708-004-0. ISSN 0091-9721. OCLC 781956734. Table 26–1 = Table 3–2 Percentage of women experiencing an unintended pregnancy during the first year of typical use and the first year of perfect use of contraception, and the percentage continuing use at the end of the first year. United States. Archived 15 February 2017 at the Wayback Machine
- ^ a b Hannaford PC, Iversen L, Macfarlane TV, Elliott AM, Angus V, Lee AJ (March 2010). "Mortality among contraceptive pill users: cohort evidence from Royal College of General Practitioners' Oral Contraception Study". BMJ. 340: c927. doi:10.1136/bmj.c927. PMC 2837145. PMID 20223876.
- ^ "Oral Contraceptives and Cancer Risk". National Cancer Institute. 22 February 2018. Archived from the original on 27 May 2020. Retrieved 10 May 2020.
- ^ a b IARC working group (2007). "Combined Estrogen-Progestogen Contraceptives" (PDF). IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. 91. Archived (PDF) from the original on 3 August 2016. Retrieved 14 September 2010.
- ^ Collaborative Group on Hormonal Factors in Breast Cancer (June 1996). "Breast cancer and hormonal contraceptives: collaborative reanalysis of individual data on 53 297 women with breast cancer and 100 239 women without breast cancer from 54 epidemiological studies". Lancet. 347 (9017): 1713–27. doi:10.1016/S0140-6736(96)90806-5. PMID 8656904. S2CID 36136756.
- ^ Kemmeren JM, Tanis BC, van den Bosch MA, Bollen EL, Helmerhorst FM, van der Graaf Y, et al. (May 2002). "Risk of Arterial Thrombosis in Relation to Oral Contraceptives (RATIO) study: oral contraceptives and the risk of ischemic stroke". Stroke. 33 (5): 1202–8. doi:10.1161/01.STR.0000015345.61324.3F. PMID 11988591.
- ^ Baillargeon JP, McClish DK, Essah PA, Nestler JE (July 2005). "Association between the current use of low-dose oral contraceptives and cardiovascular arterial disease: a meta-analysis". The Journal of Clinical Endocrinology and Metabolism. 90 (7): 3863–70. doi:10.1210/jc.2004-1958. PMID 15814774.
- ^ "Birth Control Pills - Birth Control Pill - The Pill". Archived from the original on 5 August 2011. Retrieved 3 April 2009.
- ^ a b c d e Teal S, Edelman A (December 2021). "Contraception Selection, Effectiveness, and Adverse Effects: A Review". JAMA. 326 (24): 2507–2518. doi:10.1001/jama.2021.21392. PMID 34962522. S2CID 245557522.
- ^ a b "Guinea pigs or pioneers? How Puerto Rican women were used to test the birth control pill". The Washington Post. ISSN 0190-8286. Archived from the original on 8 November 2022. Retrieved 14 September 2022.
- ^ "Birth Control Pill (for Teens) - Nemours KidsHealth". kidshealth.org. Archived from the original on 21 September 2022. Retrieved 21 September 2022.
- ^ Tyrer L (1999). "Introduction of the pill and its impact". Contraception. 59 (1): 11S – 16S. doi:10.1016/s0010-7824(98)00131-0. PMID 10342090.
- ^ Contraceptive use by method 2019: data booklet. [New York, NY]: United Nations. Department of Economic and Social Affairs. Population Division. 2019. ISBN 978-92-1-148329-1. OCLC 1135665739.
- ^ Christin-Maitre S (February 2013). "History of oral contraceptive drugs and their use worldwide". Best Practice & Research. Clinical Endocrinology & Metabolism. 27 (1): 3–12. doi:10.1016/j.beem.2012.11.004. PMID 23384741.
- ^ "Products - Data Briefs - Number 327 - December 2018". U.S. Centers for Disease Control and Prevention (CDC). 11 July 2022. Archived from the original on 13 September 2022. Retrieved 14 September 2022.
- ^ Sech LA, Mishell DR (November 2015). "Oral steroid contraception". Women's Health. 11 (6): 743–748. doi:10.2217/whe.15.82. PMID 26673988. S2CID 6433771.
- ^ "Current Contraceptive Status Among Women Aged 15–49: United States, 2015–2017". U.S. Centers for Disease Control and Prevention (CDC). 7 June 2019. Archived from the original on 13 September 2022. Retrieved 2 August 2019.
- ^ UN Population Division (2006). World Contraceptive Use 2005 (PDF). New York: United Nations. ISBN 978-92-1-151418-6. Archived (PDF) from the original on 26 April 2018. Retrieved 28 June 2017. women aged 15–49 married or in consensual union
- ^ Delvin D (15 June 2016). "Contraception – the contraceptive pill: How many women take it in the UK?". Netdoctor. Archived from the original on 4 January 2011. Retrieved 25 December 2010.
- ^ Taylor T, Keyse L, Bryant A (2006). Contraception and Sexual Health, 2005/06 (PDF). London: Office for National Statistics. ISBN 978-1-85774-638-9. Archived from the original (PDF) on 9 January 2007. British women aged 16–49: 24% use the pill as of 2016[update] (17% use Combined pill, 5% use Minipill, 2% don't know type)
- ^ Yoshida H, Sakamoto H, Leslie A, Takahashi O, Tsuboi S, Kitamura K (June 2016). "Contraception in Japan: Current trends". Contraception. 93 (6): 475–477. doi:10.1016/j.contraception.2016.02.006. PMID 26872717.
- ^ World Health Organization (2023). The selection and use of essential medicines 2023: web annex A: World Health Organization model list of essential medicines: 23rd list (2023). Geneva: World Health Organization. hdl:10665/371090. WHO/MHP/HPS/EML/2023.02.
- ^ Harris G (3 May 2010). "The Pill Started More Than One Revolution". The New York Times. Archived from the original on 27 September 2015. Retrieved 21 September 2015.
- ^ a b c d e f g h i j k l m n o Cooper DB, Patel P, Mahdy H (2024), "Oral Contraceptive Pills", StatPearls, Treasure Island (FL): StatPearls Publishing, PMID 28613632, retrieved 28 February 2024
- ^ Edwards M, Can AS (2024), "Progestins", StatPearls, Treasure Island (FL): StatPearls Publishing, PMID 33085358, retrieved 28 February 2024
- ^ a b c "How to Use Birth Control Pills". Planned Parenthood. Archived from the original on 6 December 2017. Retrieved 29 November 2017.
- ^ a b c d e "Combined hormonal contraceptives | European Medicines Agency". www.ema.europa.eu. 12 July 2013. Retrieved 28 February 2024.
- ^ a b c d e f g h i j Thiyagarajan DK, Basit H, Jeanmonod R (2024), "Physiology, Menstrual Cycle", StatPearls, Treasure Island (FL): StatPearls Publishing, PMID 29763196, retrieved 28 February 2024
- ^ a b c d e f g h i j k l "Estrogen and Progestin (Oral Contraceptives): MedlinePlus Drug Information". medlineplus.gov. Retrieved 28 February 2024.
- ^ a b c d e f Hatcher RA, Trussell J, Nelson A, Cates W, Kowal D, Policar M, eds. (2011). "Combined oral contraceptives (COCs)". Contraceptive technology (20th revised ed.). New York: Ardent Media. pp. 249–341. ISBN 978-1-59708-004-0. ISSN 0091-9721. OCLC 781956734. pp. 257–258:
Mechanism of action
Combined oral contraceptive pills prevent fertilization and, therefore, qualify as contraceptives. There is no significant evidence that they work after fertilization. The progestins in all combined oral contraceptives provide most of the contraceptive effect by suppressing ovulation and thickening cervical mucus, although the estrogens also make a small contribution to ovulation suppression. Cycle control is enhanced by the estrogen.
Because combined oral contraceptives so effectively suppress ovulation and block ascent of sperm into the upper genital tract, the potential impact on endometrial receptivity to implantation is almost academic. When the two primary mechanisms fail, the fact that pregnancy occurs despite the endometrial changes demonstrates that those endometrial changes do not significantly contribute to the pill's mechanism of action. - ^ a b c Speroff L, Darney PD (2011). "Oral contraception". A clinical guide for contraception (5th ed.). Philadelphia: Lippincott Williams & Wilkins. pp. 19–152. ISBN 978-1-60831-610-6.
- ^ a b c Levin ER, Hammes SR (2011). "Estrogens and progestins". In Goodman LS, Brunton LL, Chabner BA, Knollmann BC (eds.). Goodman & Gilman's pharmacological basis of therapeutics (12th ed.). New York: McGraw-Hill Medical. pp. 1163–1194. ISBN 978-0-07-162442-8.
- ^ Glasier A (2010). "Contraception". In Jameson JL, De Groot LJ (eds.). Endocrinology (6th ed.). Philadelphia: Saunders Elsevier. pp. 2417–2427. ISBN 978-1-4160-5583-9.
- ^ Barbieri RL (2014), Rosenwaks Z, Wassarman PM (eds.), "The Endocrinology of the Menstrual Cycle", Human Fertility: Methods and Protocols, Methods in Molecular Biology, vol. 1154, New York, NY: Springer, pp. 145–169, doi:10.1007/978-1-4939-0659-8_7, ISBN 978-1-4939-0659-8, PMID 24782009
- ^ a b c d e Nelson AL, Cwiak C (2011). "Combined oral contraceptives". In Hatcher RA, Trussell J, Nelson A, Cates W, Kowal D, Policar M (eds.). Contraceptive technology (20th revised ed.). New York: Ardent Media. pp. 253–254. ISBN 978-1-59708-004-0. ISSN 0091-9721. OCLC 781956734.
Ten different progestins have been used in the combined oral contraceptives that have been sold in the United States. Several different classification systems for the progestins exist, but the one most commonly used system recapitulates the history of the pill in the United States by categorizing the progestins into the so-called "generations of progestins". The first three generations of progestins are derived from 19-nortestosterone. The fourth generation is drospirenone. Newer progestins are hybrids.
First-generation progestins. First-generation progestins include noretynodrel, norethisterone, norethisterone acetate, and etynodiol diacetate... These compounds have the lowest potency and relatively short half-lives. The short half-life did not matter in the early, high-dose pills but as doses of progestin were decreased in the more modern pills, problems with unscheduled spotting and bleeding became more common.
Second-generation progestins. To solve the problem of unscheduled bleeding and spotting, the second generation progestins (norgestrol and levonorgestrel) were designed to be significantly more potent and to have longer half-lives than norethisterone-related progestins ... The second-generation progestins have been associated with more androgen-related side-effects such as adverse effect on lipids, oily skin, acne, and facial hair growth.
Third-generation progestins. Third-generation progestins (desogestrel, norgestimate and elsewhere, gestodene) were introduced to maintain the potent progestational activity of second-generation progestins, but to reduce androgeneic side effects. Reduction in androgen impacts allows a fuller expression of the pill's estrogen impacts. This has some clinical benefits... On the other hand, concern arose that the increased expression of estrogen might increase the risk of venous thromboembolism (VTE). This concern introduced a pill scare in Europe until international studies were completed and correctly interpreted.
Fourth-generation progestins. Drospirenone is an analogue of spironolactone, a potassium-sparing diuretic used to treat hypertension. Drospirenone possesses anti-mineralocorticoid and anti-androgenic properties. These properties have led to new contraceptive applications, such as treatment of premenstrual dysphoric disorder and acne... In the wake of concerns around possible increased VTE risk with less androgenic third-generation formulations, those issues were anticipated with drospirenone. They were clearly answered by large international studies.
Next-generation progestins. Progestins have been developed with properties that are shared with different generations of progestins. They have more profound, diverse, and discrete effects on the endometrium than prior progestins. This class would include dienogest (United States) and nomegestrol (Europe). - ^ a b c d e Speroff L, Darney PD (2011). "Oral contraception". A clinical guide for contraception (5th ed.). Philadelphia: Lippincott Williams & Wilkins. p. 40. ISBN 978-1-60831-610-6.
- ^ a b c d e f Callahan TL, Caughey AB (2013). Blueprints obstetrics & gynecology (6th ed.). Baltimore, MD: Lippincott Williams & Wilkins. ISBN 978-1-4511-1702-8. OCLC 800907400.
- ^ "Birth Control Pills All Guides". October 2014. Archived from the original on 31 May 2023. Retrieved 20 October 2018.
- ^ a b c d e f g h "Combined pill". nhs.uk. 21 December 2017. Retrieved 28 February 2024.
- ^ a b c d e World Health Organization (2016). Selected practice recommendations for contraceptive use (Third ed.). Geneva: World Health Organization. p. 150. hdl:10665/252267. ISBN 978-92-4-156540-0. OCLC 985676200.
- ^ a b c d e Curtis KM, Tepper NK, Jatlaoui TC, Berry-Bibee E, Horton LG, Zapata LB, et al. (July 2016). "U.S. Medical Eligibility Criteria for Contraceptive Use, 2016" (PDF). MMWR. Recommendations and Reports. 65 (3): 1–103. doi:10.15585/mmwr.rr6503a1. ISSN 1057-5987. PMID 27467196. Archived (PDF) from the original on 16 October 2020. Retrieved 11 February 2024.
- ^ Trussell J (April 2009). "Understanding contraceptive failure". Best Practice & Research. Clinical Obstetrics & Gynaecology. Contraception and Sexual Health. 23 (2): 199–209. doi:10.1016/j.bpobgyn.2008.11.008. PMC 3638203. PMID 19223239.
- ^ Trussell J (May 2011). "Contraceptive failure in the United States". Contraception. 83 (5): 397–404. doi:10.1016/j.contraception.2011.01.021. PMC 3638209. PMID 21477680.
- ^ a b c d e f g h Speroff L, Darney PD (2005). "Oral Contraception". A Clinical Guide for Contraception (4th ed.). Philadelphia: Lippincott Williams & Wilkins. pp. 21–138. ISBN 978-0-7817-6488-9.
- ^ FFPRHC (2007). "Clinical Guidance: First Prescription of Combined Oral Contraception" (PDF). Archived from the original (PDF) on 4 July 2007. Retrieved 26 June 2007.
- ^ a b Edelman A, Micks E, Gallo MF, Jensen JT, Grimes DA (July 2014). "Continuous or extended cycle vs. cyclic use of combined hormonal contraceptives for contraception". The Cochrane Database of Systematic Reviews. 2014 (7) CD004695. doi:10.1002/14651858.CD004695.pub3. PMC 6837850. PMID 25072731.
- ^ Curtis KM, Jatlaoui TC, Tepper NK, Zapata LB, Horton LG, Jamieson DJ, et al. (July 2016). "U.S. Selected Practice Recommendations for Contraceptive Use, 2016". MMWR. Recommendations and Reports. 65 (4): 1–66. doi:10.15585/mmwr.rr6504a1. PMID 27467319.
- ^ "What are sugar birth control pills?". www.plannedparenthood.org. Archived from the original on 18 April 2025. Retrieved 21 June 2025.
- ^ Gladwell M (10 March 2000). "John Rock's Error". The New Yorker. Archived from the original on 11 May 2013. Retrieved 4 February 2009.
- ^ Mayo Clinic staff. "Birth control pill FAQ: Benefits, risks and choices". Mayo Clinic. Archived from the original on 26 December 2012. Retrieved 1 February 2013.
- ^ "US Patent:Oral contraceptive:Patent 6451778 Issued on September 17, 2002 Estimated Expiration Date: July 2, 2017". PatentStorm LLC. Archived from the original on 13 June 2011. Retrieved 19 November 2010.
- ^ Hercberg S, Preziosi P, Galan P (April 2001). "Iron deficiency in Europe". Public Health Nutrition. 4 (2B): 537–545. doi:10.1079/phn2001139. PMID 11683548.
- ^ Viswanathan M, Treiman KA, Kish-Doto J, Middleton JC, Coker-Schwimmer EJ, Nicholson WK (January 2017). "Folic Acid Supplementation for the Prevention of Neural Tube Defects: An Updated Evidence Report and Systematic Review for the US Preventive Services Task Force". JAMA. 317 (2): 190–203. doi:10.1001/jama.2016.19193. PMID 28097361.
- ^ Lassi ZS, Bhutta ZA (April 2012). "Clinical utility of folate-containing oral contraceptives". International Journal of Women's Health. 4: 185–190. doi:10.2147/IJWH.S18611. PMC 3346209. PMID 22570577.
- ^ "FDA Approves Seasonal Oral Contraceptive". U.S. Food and Drug Administration (FDA). 25 September 2003. Archived from the original on 7 October 2006. Retrieved 9 November 2006.
- ^ CYWH Staff (18 October 2011). "Medical Uses of the Birth Control Pill". Archived from the original on 5 February 2013. Retrieved 1 February 2013.
- ^ "Information for Consumers (Drugs) - Find Information about a Drug". U.S. Food and Drug Administration (FDA). Archived from the original on 14 November 2017. Retrieved 13 December 2017.
- ^ "Patient education: Polycystic ovary syndrome (PCOS) (Beyond the Basics)". UpToDate. Archived from the original on 15 September 2022. Retrieved 15 September 2022.
- ^ Dumesic DA, Lobo RA (August 2013). "Cancer risk and PCOS". Steroids. 78 (8): 782–785. doi:10.1016/j.steroids.2013.04.004. ISSN 1878-5867. PMID 23624028. S2CID 10185317.
- ^ "Polycystic Ovary Syndrome (PCOS)". American College of Obstetricians and Gynecologists. Archived from the original on 15 September 2022. Retrieved 15 September 2022.
- ^ Barakat RR, Park RC, Grigsby PW, et al. Corpus: Epithelial Tumors. In: Principles and Practice of Gynecologic Oncology, 2nd, Hoskins WH, Perez CA, Young RC (Eds), Lippincott-Raven Publishers, Philadelphia 1997. p.859
- ^ Hardiman P, Pillay OC, Atiomo W (May 2003). "Polycystic ovary syndrome and endometrial carcinoma". Lancet. 361 (9371): 1810–2. doi:10.1016/s0140-6736(03)13409-5. PMID 12781553. S2CID 27453081.
- ^ "Can birth control pills cure PCOS". American College of Obstetricians and Gynecologists. Archived from the original on 20 September 2022. Retrieved 18 September 2022.
- ^ Huber J, Walch K (January 2006). "Treating acne with oral contraceptives: use of lower doses". Contraception. 73 (1): 23–9. doi:10.1016/j.contraception.2005.07.010. PMID 16371290.
- ^ "Hormonal Contraceptives and Acne: A Retrospective Analysis of 2147 Patients". JDDonline - Journal of Drugs in Dermatology. Archived from the original on 19 May 2022. Retrieved 15 September 2022.
- ^ Chang L. "Birth Control of Acne". WebMD, LLC. Archived from the original on 26 January 2013. Retrieved 1 February 2013.
- ^ "DailyMed - ORTHO TRI CYCLEN- norgestimate and ethinyl estradiol ORTHO CYCLEN- norgestimate and ethinyl estradiol". dailymed.nlm.nih.gov. Archived from the original on 14 December 2017. Retrieved 13 December 2017.
- ^ "Beyaz Package Insert" (PDF). U.S. Food and Drug Administration (FDA). Archived from the original (PDF) on 15 April 2023. Retrieved 6 August 2019.
- ^ "Patient education: Hirsutism (excess hair growth in females) (Beyond the Basics)". UpToDate. Archived from the original on 20 September 2022. Retrieved 18 September 2022.
- ^ "Hirsutism: What It Is, In Women, Causes, PCOS & Treatment". Cleveland Clinic. Archived from the original on 19 September 2022. Retrieved 18 September 2022.
- ^ a b Arowojolu A, Gallo M, Grimes D, Garner S (19 July 2004), The Cochrane Collaboration (ed.), "Combined oral contraceptive pills for treatment of acne", Cochrane Database of Systematic Reviews (3) CD004425, Chichester, UK: John Wiley & Sons, Ltd, doi:10.1002/14651858.cd004425.pub2, PMID 15266533, retrieved 28 February 2024
- ^ Frances E Casey MD (January 2023). "Contraception and its impact on acne". GYN Journal. Vol 68 No 01. 68 (1).
- ^ "ACOG Endometriosis FAQ". Archived from the original on 1 February 2020. Retrieved 3 March 2019.
- ^ Zorbas KA, Economopoulos KP, Vlahos NF (July 2015). "Continuous versus cyclic oral contraceptives for the treatment of endometriosis: a systematic review". Archives of Gynecology and Obstetrics. 292 (1): 37–43. doi:10.1007/s00404-015-3641-1. PMID 25644508. S2CID 23340983.
- ^ "Heavy Menstrual Bleeding". American College of Obstetricians and Gynecologists. Archived from the original on 20 September 2022. Retrieved 18 September 2022.
- ^ Apgar BS, Kaufman AH, George-Nwogu U, Kittendorf A (15 June 2007). "Treatment of Menorrhagia". American Family Physician. 75 (12): 1813–1819. PMID 17619523. Archived from the original on 6 October 2022. Retrieved 15 September 2022.
- ^ "Patient education: Heavy or prolonged menstrual bleeding (menorrhagia) (Beyond the Basics)". UpToDate. Archived from the original on 15 September 2022. Retrieved 15 September 2022.
- ^ "Noncontraceptive Benefits of Birth Control Pills". American Society for Reproductive Medicine (ASRM). Archived from the original on 13 September 2022. Retrieved 15 September 2022.
- ^ a b American Medical Society for Sports Medicine (24 April 2014), "Five Things Physicians and Patients Should Question", Choosing Wisely: an initiative of the ABIM Foundation, American Medical Society for Sports Medicine, archived from the original on 29 July 2014, retrieved 29 July 2014
- ^ a b c "Hormonal contraception for menstrual suppression". www.uptodate.com. Retrieved 28 February 2024.
- ^ Irvine GA, Campbell-Brown MB, Lumsden MA, Heikkilä A, Walker JJ, Cameron IT (June 1998). "Randomised comparative trial of the levonorgestrel intrauterine system and norethisterone for treatment of idiopathic menorrhagia". British Journal of Obstetrics and Gynaecology. 105 (6): 592–598. doi:10.1111/j.1471-0528.1998.tb10172.x. ISSN 0306-5456. PMID 9647148.
- ^ a b c d e Jacobson JC, Likis FE, Murphy PA (2012). "Extended and continuous combined contraceptive regimens for menstrual suppression". Journal of Midwifery & Women's Health. 57 (6): 585–592. doi:10.1111/j.1542-2011.2012.00250.x. ISSN 1542-2011. PMID 23217068.
- ^ a b Sulak PJ, Scow RD, Preece C, Riggs MW, Kuehl TJ (February 2000). "Hormone withdrawal symptoms in oral contraceptive users". Obstetrics and Gynecology. 95 (2): 261–266. doi:10.1016/s0029-7844(99)00524-4. ISSN 0029-7844. PMID 10674591.
- ^ a b Loder EW, Buse DC, Golub JR (March 2005). "Headache and Combination Estrogen-Progestin Oral Contraceptives: Integrating Evidence, Guidelines, and Clinical Practice". Headache: The Journal of Head and Face Pain. 45 (3): 224–231. doi:10.1111/j.1526-4610.2005.05049.x. ISSN 0017-8748. PMID 15836597.
- ^ a b Silberstein SD (September 1999). "Menstrual Migraine". Journal of Women's Health & Gender-Based Medicine. 8 (7): 919–931. doi:10.1089/jwh.1.1999.8.919. ISSN 1524-6094. PMID 10534294.
- ^ a b Huber D, Seitz S, Kast K, Emons G, Ortmann O (April 2020). "Use of oral contraceptives in BRCA mutation carriers and risk for ovarian and breast cancer: a systematic review". Archives of Gynecology and Obstetrics. 301 (4): 875–884. doi:10.1007/s00404-020-05458-w. ISSN 1432-0711. PMC 8494665. PMID 32140806.
- ^ a b van Bommel MH, IntHout J, Veldmate G, Kets CM, de Hullu JA, van Altena AM, et al. (1 March 2023). "Contraceptives and cancer risks in BRCA1/2 pathogenic variant carriers: a systematic review and meta-analysis". Human Reproduction Update. 29 (2): 197–217. doi:10.1093/humupd/dmac038. ISSN 1460-2369. PMC 9976973. PMID 36383189.
- ^ a b c d World Health Organization (2015). Medical eligibility criteria for contraceptive use (Fifth ed.). Geneva, Switzerland: World Health Organization. hdl:10665/181468. ISBN 978-92-4-154915-8. OCLC 932048744. Archived from the original on 11 February 2024. Retrieved 11 February 2024.
- ^ Roach RE, Helmerhorst FM, Lijfering WM, Stijnen T, Algra A, Dekkers OM (27 August 2015). "Combined oral contraceptives: the risk of myocardial infarction and ischemic stroke". The Cochrane Database of Systematic Reviews. 2015 (8) CD011054. doi:10.1002/14651858.CD011054.pub2. ISSN 1469-493X. PMC 6494192. PMID 26310586.
- ^ Fitzpatrick D, Pirie K, Reeves G, Green J, Beral V (March 2023). "Combined and progestagen-only hormonal contraceptives and breast cancer risk: A UK nested case-control study and meta-analysis". PLOS Medicine. 20 (3) e1004188. doi:10.1371/journal.pmed.1004188. ISSN 1549-1676. PMC 10030023. PMID 36943819.
- ^ Anastasiou E, McCarthy KJ, Gollub EL, Ralph L, van de Wijgert JH, Jones HE (March 2022). "The relationship between hormonal contraception and cervical dysplasia/cancer controlling for human papillomavirus infection: A systematic review". Contraception. 107: 1–9. doi:10.1016/j.contraception.2021.10.018. ISSN 1879-0518. PMC 8837691. PMID 34752778.
- ^ a b "Medical eligibility criteria for contraceptive use". World Health Organization (WHO). Retrieved 28 February 2024.
- ^ a b Black A, Guilbert E, Costescu D, Dunn S, Fisher W, Kives S, et al. (April 2017). "No. 329-Canadian Contraception Consensus Part 4 of 4 Chapter 9: Combined Hormonal Contraception". Journal of Obstetrics and Gynaecology Canada. 39 (4): 229–268.e5. doi:10.1016/j.jogc.2016.10.005. PMID 28413042.
- ^ a b Cooper DB, Patel P, Mahdy H (2019). "Oral Contraceptive Pills". StatPearls. StatPearls Publishing. PMID 28613632. Archived from the original on 3 May 2019. Retrieved 5 August 2019.
- ^ a b "Can Any Woman Take Birth Control Pills?". WebMD. Archived from the original on 3 May 2016. Retrieved 8 May 2016.
- ^ Lopez LM, Grey TW, Stuebe AM, Chen M, Truitt ST, Gallo MF, et al. (Cochrane Fertility Regulation Group) (March 2015). "Combined hormonal versus nonhormonal versus progestin-only contraception in lactation". The Cochrane Database of Systematic Reviews. 2015 (3) CD003988. doi:10.1002/14651858.CD003988.pub2. PMC 10644229. PMID 25793657.
- ^ "Classifications for Combined Hormonal Contraceptives". U.S. Centers for Disease Control and Prevention (CDC). 9 April 2020. Archived from the original on 27 November 2020. Retrieved 7 December 2020.
- ^ Tepper NK, Curtis KM, Cox S, Whiteman MK (April 2020). "Update to U.S. Medical Eligibility Criteria for Contraceptive Use, 2016: Updated Recommendations for the Use of Contraception Among Women at High Risk for HIV Infection" (PDF). MMWR. Morbidity and Mortality Weekly Report. 69 (14): 405–410. doi:10.15585/mmwr.mm6914a3. PMC 7147901. PMID 32271729. Archived (PDF) from the original on 9 June 2023. Retrieved 11 February 2024.
- ^ "Is There a Link Between Birth Control Pills and Higher Breast Cancer Risk?". www.breastcancer.org. Archived from the original on 20 September 2022. Retrieved 18 September 2022.
- ^ Pernambuco-Holsten C (25 September 2018). "Birth Control and Cancer Risk: 6 Things You Should Know". Memorial Sloan Kettering Cancer Center. Archived from the original on 20 September 2022. Retrieved 18 September 2022.
- ^ "What are the best birth control options that aren't hormonal?". Planned Parenthood. Archived from the original on 20 September 2022. Retrieved 18 September 2022.
- ^ "Do Hormonal Contraceptives Increase Breast Cancer Risk?". www.breastcancer.org. Archived from the original on 20 September 2022. Retrieved 18 September 2022.
- ^ Crooks RL, Baur K (2005). Our Sexuality. Belmont, CA: Thomson Wadsworth. ISBN 978-0-534-65176-3.[page needed]
- ^ "Appendix 10: Myths about contraception". Decision-Making Tool for Family Planning Clients and Providers. WHO. 2005. Archived from the original on 7 January 2007. Retrieved 14 September 2022.
- ^ Holck S. "Contraceptive Safety". Special Challenges in Third World Women's Health. 1989 Annual Meeting of the American Public Health Association. Archived from the original on 8 November 2017. Retrieved 7 October 2006.
- ^ a b Huber JC, Bentz EK, Ott J, Tempfer CB (September 2008). "Non-contraceptive benefits of oral contraceptives". Expert Opinion on Pharmacotherapy. 9 (13): 2317–2325. doi:10.1517/14656566.9.13.2317. PMID 18710356. S2CID 73326364.
- ^ Nelson RJ (2005). An introduction to behavioral endocrinology (3rd ed.). Sunderland, Mass: Sinauer Associates. ISBN 978-0-87893-617-5.[page needed]
- ^ Vo C, Carney ME (December 2007). "Ovarian cancer hormonal and environmental risk effect". Obstetrics and Gynecology Clinics of North America. 34 (4): 687–700, viii. doi:10.1016/j.ogc.2007.09.008. PMID 18061864.
- ^ Bandera CA (June 2005). "Advances in the understanding of risk factors for ovarian cancer". The Journal of Reproductive Medicine. 50 (6): 399–406. PMID 16050564.
- ^ Pragout D, Laurence V, Baffet H, Raccah-Tebeka B, Rousset-Jablonski C (December 2018). "Contraception et cancer. RPC Contraception CNGOF" [Contraception and cancer: CNGOF Contraception Guidelines]. Gynécologie Obstétrique Fertilité & Sénologie. 46 (12): 834–844. doi:10.1016/j.gofs.2018.10.010. PMID 30385358. S2CID 196536513.
- ^ "Apri oral: Uses, Side Effects, Interactions, Pictures, Warnings & Dosing". Archived from the original on 13 December 2016. Retrieved 2 December 2016.
- ^ Blanco-Molina A, Monreal M (February 2010). "Venous thromboembolism in women taking hormonal contraceptives". Expert Review of Cardiovascular Therapy. 8 (2): 211–5. doi:10.1586/erc.09.175. PMID 20136607. S2CID 41309800.
- ^ de Bastos M, Stegeman BH, Rosendaal FR, Van Hylckama Vlieg A, Helmerhorst FM, Stijnen T, et al. (3 March 2014). Cochrane Fertility Regulation Group (ed.). "Combined oral contraceptives: venous thrombosis". Cochrane Database of Systematic Reviews. 2014 (3) CD010813. doi:10.1002/14651858.CD010813.pub2. PMC 10637279. PMID 24590565.
- ^ Roach RE, Helmerhorst FM, Lijfering WM, Stijnen T, Algra A, Dekkers OM (August 2015). "Combined oral contraceptives: the risk of myocardial infarction and ischemic stroke". The Cochrane Database of Systematic Reviews. 8 (8) CD011054. doi:10.1002/14651858.CD011054.pub2. PMC 6494192. PMID 26310586.
- ^ Rang HP, Dale MM, Ritter JM, Flower RJ, Henderson G (2012). "The reproductive system". Rang and Dale's pharmacology (7th ed.). Edinburgh: Elsevier/Churchill Livingstone. p. 426. ISBN 978-0-7020-3471-8.
- ^ a b c ESHRE Capri Workshop Group (2013). "Venous thromboembolism in women: a specific reproductive health risk". Human Reproduction Update. 19 (5): 471–82. doi:10.1093/humupd/dmt028. PMID 23825156.
- ^ Lidegaard Ø, Milsom I, Geirsson RT, Skjeldestad FE (July 2012). "Hormonal contraception and venous thromboembolism". Acta Obstetricia et Gynecologica Scandinavica. 91 (7): 769–78. doi:10.1111/j.1600-0412.2012.01444.x. PMID 22568831. S2CID 2691199.
- ^ Dunn N (April 2011). "The risk of deep venous thrombosis with oral contraceptives containing drospirenone". BMJ. 342 d2519. doi:10.1136/bmj.d2519. PMID 21511807. S2CID 42721801.
- ^ "Yasmin- drospirenone and ethinyl estradiol kit". DailyMed. 19 May 2023. Retrieved 28 December 2024.
- ^ Amoozegar F, Ronksley PE, Sauve R, Menon BK (2015). "Hormonal contraceptives and cerebral venous thrombosis risk: a systematic review and meta-analysis". Front Neurol. 6: 7. doi:10.3389/fneur.2015.00007. PMC 4313700. PMID 25699010.
- ^ a b Bast RC, Brewer M, Zou C, Hernandez MA, Daley M, Ozols R, et al. (2007). "Prevention and Early Detection of Ovarian Cancer: Mission Impossible?". Cancer Prevention. Recent Results in Cancer Research. Vol. 174. pp. 91–100. doi:10.1007/978-3-540-37696-5_9. ISBN 978-3-540-37695-8. PMID 17302189.
- ^ Vessey M, Yeates D, Flynn S (September 2010). "Factors affecting mortality in a large cohort study with special reference to oral contraceptive use". Contraception. 82 (3): 221–9. doi:10.1016/j.contraception.2010.04.006. PMID 20705149.
- ^ Nappi RE, Pellegrinelli A, Campolo F, Lanzo G, Santamaria V, Suragna A, et al. (2 January 2015). "Effects of combined hormonal contraception on health and wellbeing: Women's knowledge in northern Italy". The European Journal of Contraception & Reproductive Health Care. 20 (1): 36–46. doi:10.3109/13625187.2014.961598. ISSN 1362-5187. PMID 25317952. S2CID 26048792.
- ^ Watling CZ, Sweetland S, Wojt A, Butera G, Graubard BI, Floud S, et al. (July 2025). "Oral contraceptive use and risk of liver cancer: a population-based study, systematic review, and meta-analysis". Lancet Oncol. 26 (8): 1031–1042. doi:10.1016/S1470-2045(25)00222-0. PMC 12303860. PMID 40617239.
- ^ Collaborative Group on Hormonal Factors in Breast Cancer (22 June 1996). "Breast cancer and hormonal contraceptives: collaborative reanalysis of individual data on 53 297 women with breast cancer and 100 239 women without breast cancer from 54 epidemiological studies". The Lancet. 347 (9017): 1713–1727. doi:10.1016/s0140-6736(96)90806-5. ISSN 0140-6736. PMID 8656904.
- ^ a b Shah D, Patil M, On behalf of the National PCOS Working Group (2018). "Consensus statement on the use of oral contraceptive pills in polycystic ovarian syndrome women in India". Journal of Human Reproductive Sciences. 11 (2): 96–118. doi:10.4103/jhrs.JHRS_72_18. ISSN 0974-1208. PMC 6094524. PMID 30158805.
- ^ Shah D, Patil M, On behalf of the National PCOS Working Group (2018). "Consensus statement on the use of oral contraceptive pills in polycystic ovarian syndrome women in India". Journal of Human Reproductive Sciences. 11 (2): 96–118. doi:10.4103/jhrs.JHRS_72_18. ISSN 0974-1208. PMC 6094524. PMID 30158805.
- ^ Charlton BM, Rich-Edwards JW, Colditz GA, Missmer SA, Rosner BA, Hankinson SE, et al. (31 October 2014). "Oral contraceptive use and mortality after 36 years of follow-up in the Nurses' Health Study: prospective cohort study". BMJ. 349 g6356. doi:10.1136/bmj.g6356. ISSN 1756-1833. PMC 4216099. PMID 25361731.
- ^ International Collaboration of Epidemiological Studies of Cervical Cancer (10 November 2007). "Cervical cancer and hormonal contraceptives: collaborative reanalysis of individual data for 16 573 women with cervical cancer and 35 509 women without cervical cancer from 24 epidemiological studies". The Lancet. 370 (9599): 1609–1621. doi:10.1016/S0140-6736(07)61684-5. ISSN 0140-6736. PMID 17993361.
- ^ Gierisch JM, Coeytaux RR, Urrutia RP, Havrilesky LJ, Moorman PG, Lowery WJ, et al. (November 2013). "Oral contraceptive use and risk of breast, cervical, colorectal, and endometrial cancers: a systematic review". Cancer Epidemiology, Biomarkers & Prevention. 22 (11): 1931–43. doi:10.1158/1055-9965.EPI-13-0298. PMID 24014598.
- ^ Anothaisintawee T, Wiratkapun C, Lerdsitthichai P, Kasamesup V, Wongwaisayawan S, Srinakarin J, et al. (September 2013). "Risk factors of breast cancer: a systematic review and meta-analysis". Asia-Pacific Journal of Public Health. 25 (5): 368–87. doi:10.1177/1010539513488795. PMID 23709491. S2CID 206616972.
- ^ Zhu H, Lei X, Feng J, Wang Y (December 2012). "Oral contraceptive use and risk of breast cancer: a meta-analysis of prospective cohort studies". The European Journal of Contraception & Reproductive Health Care. 17 (6): 402–14. doi:10.3109/13625187.2012.715357. PMID 23061743. S2CID 33708638.
- ^ a b Mørch LS, Skovlund CW, Hannaford PC, Iversen L, Fielding S, Lidegaard Ø (7 December 2017). "Contemporary Hormonal Contraception and the Risk of Breast Cancer". The New England Journal of Medicine. 377 (23): 2228–2239. doi:10.1056/NEJMoa1700732. hdl:2164/15157. PMID 29211679. S2CID 4498610.
- ^ a b c Lopez LM, Ramesh S, Chen M, Edelman A, Otterness C, Trussell J, et al. (28 August 2016). "Progestin-only contraceptives: effects on weight". The Cochrane Database of Systematic Reviews. 2016 (8) CD008815. doi:10.1002/14651858.CD008815.pub4. ISSN 1469-493X. PMC 5034734. PMID 27567593.
- ^ Weir GC, DeGroot LJ, Grossman A, Marshall JF, Melmed S, Potts JT (2006). Endocrinology (5th ed.). St. Louis, Mo: Elsevier Saunders. p. 2999. ISBN 978-0-7216-0376-6.[page needed]
- ^ Westhoff CL, Heartwell S, Edwards S, Zieman M, Stuart G, Cwiak C, et al. (April 2007). "Oral contraceptive discontinuation: do side effects matter?". American Journal of Obstetrics and Gynecology. 196 (4): 412.e1–6, discussion 412.e6–7. doi:10.1016/j.ajog.2006.12.015. PMC 1903378. PMID 17403440.
- ^ Seal BN, Brotto LA, Gorzalka BB (August 2005). "Oral contraceptive use and female genital arousal: methodological considerations". Journal of Sex Research. 42 (3): 249–58. doi:10.1080/00224490509552279. PMID 19817038. S2CID 10402534.
- ^ Higgins JA, Davis AR (July 2014). "Contraceptive sex acceptability: a commentary, synopsis and agenda for future research". Contraception. 90 (1): 4–10. doi:10.1016/j.contraception.2014.02.029. PMC 4247241. PMID 24792147.
- ^ Burrows LJ, Basha M, Goldstein AT (2012). "The Effects of Hormonal Contraceptives on Female Sexuality: A Review". The Journal of Sexual Medicine. 9 (9). Elsevier: 2213–2223. doi:10.1111/j.1743-6109.2012.02848.x. PMID 22788250.
- ^ Pastor Z, Holla K, Chmel R (2013). "The influence of combined oral contraceptives on female sexual desire: a systematic review". The European Journal of Contraception & Reproductive Health Care. 18 (1). Taylor & Francis: 27–43. doi:10.3109/13625187.2012.728643. PMID 23320933. S2CID 34748865.
- ^ a b Boudesseul J, Gildersleeve KA, Haselton MG, Bègue L (2019). "Do women expose themselves to more health-related risks in certain phases of the menstrual cycle? A meta-analytic review". Neuroscience & Biobehavioral Reviews. 107: 505–524. doi:10.1016/j.neubiorev.2019.08.016. PMID 31513819. Archived from the original on 6 May 2023. Retrieved 27 April 2023.
- ^ Panzer C, Wise S, Fantini G, Kang D, Munarriz R, Guay A, et al. (January 2006). "Impact of oral contraceptives on sex hormone-binding globulin and androgen levels: a retrospective study in women with sexual dysfunction". The Journal of Sexual Medicine. 3 (1): 104–13. doi:10.1111/j.1743-6109.2005.00198.x. PMID 16409223.
Description of the study results in Medical News Today: "Birth Control Pill Could Cause Long-Term Problems With Testosterone, New Research Indicates". 4 January 2006. Archived from the original on 24 April 2011. Retrieved 9 April 2011. - ^ Panzer C, Wise S, Fantini G, Kang D, Munarriz R, Guay A, et al. (January 2006). "Impact of oral contraceptives on sex hormone-binding globulin and androgen levels: a retrospective study in women with sexual dysfunction". The Journal of Sexual Medicine. 3 (1): 104–13. doi:10.1111/j.1743-6109.2005.00198.x. PMID 16409223.
- ^ Zheng J, Islam RM, Skiba MA, Bell RJ, Davis SR (2020). "Associations between androgens and sexual function in premenopausal women: a cross-sectional study". The Lancet Diabetes & Endocrinology. 8 (8). Elsevier: 693–702. doi:10.1016/S2213-8587(20)30239-4. PMID 32707117. S2CID 225473332.
- ^ a b Hatcher RD, Nelson AL (2004). "Combined Hormonal Contraceptive Methods". In Hatcher RD (ed.). Contraceptive technology (18th ed.). New York: Ardent Media, Inc. pp. 403, 432, 434. ISBN 978-0-9664902-5-1.
- ^ Speroff L (2005). A clinical guide for contraception (4th ed.). Hagerstown, MD: Lippincott Williams & Wilkins. p. 72. ISBN 978-0-7817-6488-9.
- ^ Roberts SC, Havlicek J, Flegr J, Hruskova M, Little AC, Jones BC, et al. (2004). "Female facial attractiveness increases during the fertile phase of the menstrual cycle". Proceedings of the Royal Society B: Biological Sciences. 271 (Supplemental 5). Royal Society: S270 – S272. doi:10.1098/rsbl.2004.0174. JSTOR 4142824. PMC 1810066. PMID 15503991.
- ^ Miller GF, Tybur JM, Jordan BD (2007). "Ovulatory cycle effects on tip earnings by lap dancers: economic evidence for human estrus?" (PDF). Evolution and Human Behavior. 28 (6): 375–381. CiteSeerX 10.1.1.154.8176. doi:10.1016/j.evolhumbehav.2007.06.002. Archived (PDF) from the original on 13 June 2023. Retrieved 16 May 2023.
- ^ Pipitone RN, Gallup GG (2008). "Women's voice attractiveness varies across the menstrual cycle". Evolution and Human Behavior. 29 (4). Elsevier: 268–274. Bibcode:2008EHumB..29..268P. doi:10.1016/j.evolhumbehav.2008.02.001.
- ^ Chavanne TJ, Gallup GG (1998). "Variation in Risk Taking Behavior Among Female College Students as a Function of the Menstrual Cycle". Evolution and Human Behavior. 19 (1). Elsevier: 27–32. Bibcode:1998EHumB..19...27C. doi:10.1016/S1090-5138(98)00016-6.
- ^ Buss DM (2016) [1994]. The Evolution of Desire: Strategies of Human Mating (3rd ed.). New York: Basic Books. pp. 260–262. ISBN 978-0-465-09776-0.
- ^ Bröder A, Hohmann N (2003). "Variations in risk taking behavior over the menstrual cycle: An improved replication". Evolution and Human Behavior. 24 (6). Elsevier: 391–398. Bibcode:2003EHumB..24..391B. doi:10.1016/S1090-5138(03)00055-2.
- ^ Burns BD (2006). "Cognitive Neuroendocrinology: Risk Preference Changes Across the Menstrual Cycle". Proceedings of the Annual Meeting of the Cognitive Science Society. 28: 125–130. ISSN 1069-7977. Archived from the original on 27 April 2023. Retrieved 27 April 2023.
- ^ ACOG Committee on Practice Bulletins-Gynecology (2006). "ACOG Practice Bulletin No. 73: Use of Hormonal Contraception in Women with Coexisting Medical Conditions". Obstetrics & Gynecology. 107 (6): 1453–72. doi:10.1097/00006250-200606000-00055. PMID 16738183.
- ^ FFPRHC (2006). "The UK Medical Eligibility Criteria for Contraceptive Use (2005/2006)" (PDF). Archived from the original (PDF) on 19 June 2007. Retrieved 31 March 2007.
- ^ Cilia La Corte AL, Carter AM, Turner AJ, Grant PJ, Hooper NM (December 2008). "The bradykinin-degrading aminopeptidase P is increased in women taking the oral contraceptive pill". Journal of the Renin-Angiotensin-Aldosterone System. 9 (4): 221–5. doi:10.1177/1470320308096405. PMID 19126663. S2CID 206729914.
- ^ Qiu Y, Hu Y (23 June 2021). "Birth control pills and risk of hypothyroidism: a cross-sectional study of the National Health and Nutrition Examination Survey, 2007–2012". BMJ Open. 11 (6) e046607. doi:10.1136/bmjopen-2020-046607. PMC 8230965. PMID 34162647.
- ^ "Gallstones". NDDIC. July 2007. Archived from the original on 11 August 2010. Retrieved 13 August 2010.
- ^ Raloff J (23 April 2009). "Birth control pills can limit muscle-training gains". Science News. Archived from the original on 11 June 2012. Retrieved 22 October 2018.
- ^ "Love woes can be blamed on contraceptive pill: research – ABC News (Australian Broadcasting Corporation)". ABC News. Abc.net.au. 14 August 2008. Archived from the original on 22 July 2010. Retrieved 20 March 2010.
- ^ Kollndorfer K, Ohrenberger I, Schöpf V (2016). "Contraceptive Use Affects Overall Olfactory Performance: Investigation of Estradiol Dosage and Duration of Intake". PLOS ONE. 11 (12) e0167520. Bibcode:2016PLoSO..1167520K. doi:10.1371/journal.pone.0167520. PMC 5176159. PMID 28002464.
- ^ Roberts SC, Gosling LM, Carter V, Petrie M (December 2008). "MHC-correlated odour preferences in humans and the use of oral contraceptives". Proceedings. Biological Sciences. 275 (1652): 2715–22. doi:10.1098/rspb.2008.0825. PMC 2605820. PMID 18700206.
- ^ Vercellini P, Eskenazi B, Consonni D, Somigliana E, Parazzini F, Abbiati A, et al. (2010). "Oral contraceptives and risk of endometriosis: a systematic review and meta-analysis". Human Reproduction Update. 17 (2): 159–70. doi:10.1093/humupd/dmq042. PMID 20833638.
- ^ Zimmerman Y, Eijkemans MJ, Coelingh Bennink HJ, Blankenstein MA, Fauser BC (2013). "The effect of combined oral contraception on testosterone levels in healthy women: a systematic review and meta-analysis". Human Reproduction Update. 20 (1): 76–105. doi:10.1093/humupd/dmt038. PMC 3845679. PMID 24082040.
- ^ Scholes D, Hubbard RA, Ichikawa LE, LaCroix AZ, Spangler L, Beasley JM, et al. (September 2011). "Oral contraceptive use and bone density change in adolescent and young adult women: a prospective study of age, hormone dose, and discontinuation". The Journal of Clinical Endocrinology and Metabolism. 96 (9): E1380–7. doi:10.1210/jc.2010-3027. PMC 3167673. PMID 21752879.
- ^ The effects of broad-spectrum antibiotics on Combined contraceptive pills is not found on systematic interaction metanalysis (Archer, 2002), although "individual patients do show large decreases in the plasma concentrations of ethinylestradiol when they take certain other antibiotics" (Dickinson, 2001). "experts on this topic still recommend informing oral contraceptive users of the potential for a rare interaction" (DeRossi, 2002) and this remains current (2006) UK Family Planning Association advice Archived 8 February 2007 at the Wayback Machine.
- ^ Archer JS, Archer DF (June 2002). "Oral contraceptive efficacy and antibiotic interaction: a myth debunked". Journal of the American Academy of Dermatology. 46 (6): 917–23. doi:10.1067/mjd.2002.120448. PMID 12063491.
- ^ Dickinson BD, Altman RD, Nielsen NH, Sterling ML (November 2001). "Drug interactions between oral contraceptives and antibiotics". Obstetrics and Gynecology. 98 (5 Pt 1): 853–60. doi:10.1016/S0029-7844(01)01532-0. PMID 11704183. S2CID 41354899.
- ^ DeRossi SS, Hersh EV (October 2002). "Antibiotics and oral contraceptives". Dental Clinics of North America. 46 (4): 653–64. CiteSeerX 10.1.1.620.9933. doi:10.1016/S0011-8532(02)00017-4. PMID 12436822.
- ^ Berry-Bibee EN, Kim MJ, Tepper NK, Riley HE, Curtis KM (December 2016). "Co-administration of St. John's wort and hormonal contraceptives: a systematic review". Contraception. 94 (6): 668–677. doi:10.1016/j.contraception.2016.07.010. PMC 11283811. PMID 27444983.
- ^ a b c "FDA Approves First Nonprescription Daily Oral Contraceptive". U.S. Food and Drug Administration (FDA) (Press release). July 2023. Retrieved 6 April 2024.
- ^ Marks L (2001). Sexual Chemistry: A History of the Contraceptive Pill. Yale University Press. pp. 73–75, 77–78. ISBN 978-0-300-08943-1. Archived from the original on 11 January 2023. Retrieved 27 September 2020.
- ^ Gelijns A (1991). Innovation in Clinical Practice: The Dynamics of Medical Technology Development. National Academies. pp. 167–. NAP:13513. Archived from the original on 13 January 2023. Retrieved 27 September 2020.
- ^ Blum RW (22 October 2013). Adolescent Health Care: Clinical Issues. Elsevier Science. pp. 216–. ISBN 978-1-4832-7738-7.
- ^ Tone A, Watkins ES (8 January 2007). Medicating Modern America: Prescription Drugs in History. NYU Press. pp. 117–119. ISBN 978-0-8147-8301-6.
- ^ McGuire JL (2000). Pharmaceuticals, 4 Volume Set. Wiley. p. 1580,1599. ISBN 978-3-527-29874-7.
- ^ Rao B (1 January 1998). Clinical Gynecology (4th ed.). Orient Blackswan. pp. 163–. ISBN 978-81-250-1622-9.
- ^ Labhart A (6 December 2012). Clinical Endocrinology: Theory and Practice. Springer Science & Business Media. pp. 571–. ISBN 978-3-642-96158-8.
- ^ Goldzieher JW, Rudel HW (October 1974). "How the oral contraceptives came to be developed". JAMA. 230 (3): 421–5. doi:10.1001/jama.230.3.421. PMID 4606623.
- ^ Goldzieher JW (May 1982). "Estrogens in oral contraceptives: historical perspectives". The Johns Hopkins Medical Journal. 150 (5): 165–9. PMID 7043034.
- ^ Perone N (Spring 1993). "The history of steroidal contraceptive development: the progestins". Perspectives in Biology and Medicine. 36 (3): 347–62. doi:10.1353/pbm.1993.0054. PMID 8506121. S2CID 46312750.
- ^ Goldzieher JW (Spring 1993). "The history of steroidal contraceptive development: the estrogens". Perspectives in Biology and Medicine. 36 (3): 363–8. doi:10.1353/pbm.1993.0066. PMID 8506122. S2CID 28975213.
- ^ a b c Maisel AQ (1965). The Hormone Quest. New York: Random House. OCLC 543168.
- ^ a b Asbell B (1995). The Pill: A Biography of the Drug That Changed the World. New York: Random House. ISBN 978-0-679-43555-6.
- ^ Lehmann PA, Bolivar A, Quintero R (March 1973). "Russell E. Marker. Pioneer of the Mexican steroid industry". Journal of Chemical Education. 50 (3): 195–9. Bibcode:1973JChEd..50..195L. doi:10.1021/ed050p195. PMID 4569922.
- ^ a b Vaughan P (1970). The Pill on Trial. New York: Coward-McCann. OCLC 97780.
- ^ a b Tone A (2001). Devices & Desires: A History of Contraceptives in America. New York: Hill and Wang. ISBN 978-0-8090-3817-6.
- ^ a b c d Pincus G, Bialy G (1964). Drugs Used in Control of Reproduction. Advances in Pharmacology. Vol. 3. Academic Press. pp. 285–313. doi:10.1016/S1054-3589(08)61115-1. ISBN 978-0-12-032903-8. PMID 14232795.
The original observation of Makepeace et al. (1937) that progesterone inhibited ovulation in the rabbit was substantiated by Pincus and Chang (1953). In women, 300 mg of progesterone per day taken orally resulted in ovulation inhibition in 80% of cases (Pincus, 1956). The high dosage and frequent incidence of breakthrough bleeding limited the practical application of the method. Subsequently, the utilization of potent 19-norsteroids, which could be given orally, opened the field to practical oral contraception.
{{cite book}}: ISBN / Date incompatibility (help) - ^ Nance K (16 October 2014). "Jonathan Eig on 'The Birth of the Pill'". Chicago Tribune. Archived from the original on 19 April 2019. Retrieved 19 October 2014.
- ^ "The Birth of the Pill". W. W. Norton & Company. Archived from the original on 11 October 2014. Retrieved 19 October 2014.
- ^ Manning K (17 October 2014). "Book review: 'The Birth of the Pill,' and the reinvention of sex, by Jonathan Eig". The Washington Post. Archived from the original on 9 December 2022. Retrieved 25 August 2017.
- ^ a b c Reed J (1978). From Private Vice to Public Virtue: The Birth Control Movement and American Society Since 1830. New York: Basic Books. ISBN 978-0-465-02582-4.
- ^ Speroff L (2009). A Good Man: Gregory Goodwin Pincus: The Man, His Story, The Birth Control Pill. Portland, Oregon: Arnica. ISBN 978-0-9801942-9-6.
- ^ Fields A (2003). Katharine Dexter McCormick: Pioneer for Women's Rights. Westport, Conn.: Prager. ISBN 978-0-275-98004-7.
- ^ McLaughlin L (1982). The Pill, John Rock, and the Church: The Biography of a Revolution. Boston: Little, Brown. ISBN 978-0-316-56095-5.
- ^ Rock J, Garcia CR, Pincus G (1957). "Synthetic progestins in the normal human menstrual cycle". Recent Progress in Hormone Research. 13: 323–39, discussion 339–46. PMID 13477811.
- ^ a b c Pincus G (December 1958). "The hormonal control of ovulation and early development". Postgraduate Medicine. 24 (6): 654–60. doi:10.1080/00325481.1958.11692305. PMID 13614060.
- ^ a b c Pincus G (1959). Progestational Agents and the Control of Fertility. Vitamins & Hormones. Vol. 17. Academic Press. pp. 307–324. doi:10.1016/S0083-6729(08)60274-5. ISBN 978-0-12-709817-3. ISSN 0083-6729.
Ishikawa et al. (1957) employing the same regime of progesterone administration also observed suppression of ovulation in a proportion of the cases taken to laparotomy. Although sexual intercourse was practised freely by the subjects of our experiments and those of Ishikawa el al., no pregnancies occurred. Since ovulation presumably took place in a proportion of cycles, the lack of any pregnancies may be due to chance, but Ishikawa et al. (1957) have presented data indicating that in women receiving oral progesterone the cervical mucus becomes impenetrable to sperm.
{{cite book}}: ISBN / Date incompatibility (help) - ^ a b Diczfalusy E (December 1965). "Probable mode of action of oral contraceptives". BMJ. 2 (5475): 1394–9. doi:10.1136/bmj.2.5475.1394. PMC 1847181. PMID 5848673.
At the Fifth International Conference on Planned Parenthood in Tokyo, Pincus (1955) reported an ovulation inhibition by progesterone or norethynodrel1 taken orally by women. This report indicated the beginning of a new era in the history of contraception. ... That the cervical mucus might be one of the principal sites of action was suggested by the first studies of Pincus (1956, 1959) and of Ishikawa et al. (1957). These investigators found that no pregnancies occurred in women treated orally with large doses of progesterone, though ovulation was inhibited only in some 70% of the cases studied. ... The mechanism of protection in this method—and probably in that of Pincus (1956) and of Ishikawa et al. (1957)—must involve an effect on the cervical mucus and/or endometrium and Fallopian tubes.
- ^ a b Ramírez de Arellano AB, Seipp C (10 October 2017). Colonialism, Catholicism, and Contraception: A History of Birth Control in Puerto Rico. University of North Carolina Press. pp. 107–. ISBN 978-1-4696-4001-3. Archived from the original on 15 July 2023. Retrieved 2 May 2019.
Still, neither of the two researchers was completely satisfied with the results. Progesterone tended to cause "premature menses", or breakthrough bleeding, in approximately 20 percent of the cycles, an occurrence that disturbed the patients and worried Rock.17 In addition, Pincus was concerned about the failure to inhibit ovulation in all the cases. Only large doses of orally administered progesterone could insure the suppression of ovulation, and these doses were expensive. The mass use of this regimen as a birth control method was thus seriously imperiled.
- ^ Chang MC (September 1978). "Development of the oral contraceptives". American Journal of Obstetrics and Gynecology. 132 (2): 217–9. doi:10.1016/0002-9378(78)90928-6. PMID 356615.
- ^ Garcia CR, Pincus G, Rock J (November 1956). "Effects of certain 19-nor steroids on the normal human menstrual cycle". Science. 124 (3227): 891–3. Bibcode:1956Sci...124..891R. doi:10.1126/science.124.3227.891. PMID 13380401.
- ^ a b Rock J, García CR (1957). "Observed effects of 19-nor steroids on ovulation and menstruation". Proceedings of a Symposium on 19-Nor Progestational Steroids. Chicago: Searle Research Laboratories. pp. 14–31. OCLC 935295.
- ^ Pincus G, Rock J, Garcia CR, Ricewray E, Paniagua M, Rodriguez I (June 1958). "Fertility control with oral medication". American Journal of Obstetrics and Gynecology. 75 (6): 1333–46. doi:10.1016/0002-9378(58)90722-1. PMID 13545267.
- ^ García CR (December 2004). "Development of the pill". Annals of the New York Academy of Sciences. 1038 (1): 223–6. Bibcode:2004NYASA1038..223G. doi:10.1196/annals.1315.031. PMID 15838117. S2CID 25550745.
- ^ Strauss JF, Mastroianni L (January 2005). "In memoriam: Celso-Ramon Garcia, M.D. (1922-2004), reproductive medicine visionary". Journal of Experimental & Clinical Assisted Reproduction. 2 (1) 2. doi:10.1186/1743-1050-2-2. PMC 548289. PMID 15673473.
- ^ a b c d e Junod SW, Marks L (April 2002). "Women's trials: the approval of the first oral contraceptive pill in the United States and Great Britain". Journal of the History of Medicine and Allied Sciences. 57 (2): 117–60. doi:10.1093/jhmas/57.2.117. PMID 11995593. S2CID 36533080.
- ^ Tyler ET, Olson HJ (April 1959). "Fertility promoting and inhibiting effects of new steroid hormonal substances". Journal of the American Medical Association. 169 (16): 1843–54. doi:10.1001/jama.1959.03000330015003. PMID 13640942.
- ^ Winter IC (1957). "Summary". Proceedings of a Symposium on 19-Nor Progestational Steroids. Chicago: Searle Research Laboratories. pp. 120–122. OCLC 935295.
- ^ Grindlay K, Burns B, Grossman D (July 2013). "Prescription requirements and over-the-counter access to oral contraceptives: a global review". Contraception. 88 (1): 91–96. doi:10.1016/j.contraception.2012.11.021. ISSN 1879-0518. PMID 23352799.
- ^ a b c d Marks L (2001). Sexual Chemistry: A History of the Contraceptive Pill. New Haven: Yale University Press. ISBN 978-0-300-08943-1.
- ^ Winter IC (May 1970). "Industrial pressure and the population problem--the FDA and the pill". JAMA. 212 (6): 1067–8. doi:10.1001/jama.212.6.1067. PMID 5467404.
- ^ a b c d e Watkins ES (1998). On the Pill: A Social History of Oral Contraceptives, 1950–1970. Baltimore: Johns Hopkins University Press. ISBN 978-0-8018-5876-5.
- ^ Winter IC (March 1965). "The incidence of thromboembolism in Enovid users". Metabolism. 14 (Supplement): SUPPL:422–8. doi:10.1016/0026-0495(65)90029-6. PMID 14261427.
- ^ Jordan WM, Anand JK (18 November 1961). "Pulmonary embolism". Lancet. 278 (7212): 1146–1147. doi:10.1016/S0140-6736(61)91061-3.
- ^ Seaman B (1969). The Doctors' Case Against the Pill. New York: P. H. Wyden. ISBN 978-0-385-14575-6.
- ^ US Food and Drug Administration (11 June 1970). "Statement of policy concerning oral contraceptive labeling directed to users". Federal Register. 35 (113): 9001–9003.
- ^ US Food and Drug Administration (31 January 1978). "Oral contraceptives; requirement for labeling directed to the patient". Federal Register. 43 (21): 4313–4334.
- ^ US Food and Drug Administration (25 May 1989). "Oral contraceptives; patient package insert requirement". Federal Register. 54 (100): 22585–22588.
- ^ "Prescriptive Authority for Pharmacists: Oral Contraceptives". Pharmacy Times. November 2018 Cough, Cold, & Flu. 84 (11). 19 November 2018. Archived from the original on 2 August 2019. Retrieved 2 August 2019.
- ^ "Pharmacist Prescribing for Hormonal Contraceptive Medications". NASPA. Archived from the original on 2 August 2019. Retrieved 2 August 2019.
- ^ "Pharmacists Authorized to Prescribe Birth Control in More States". NASPA. 4 May 2017. Archived from the original on 2 August 2019. Retrieved 2 August 2019.
- ^ "Doctor: Why I'm cheering about pharmacists in my state prescribing birth control pills". CNN. 30 January 2024. Archived from the original on 8 February 2024. Retrieved 11 February 2024.
- ^ Commissioner Oo (9 August 2024). "FDA Approves First Nonprescription Daily Oral Contraceptive". FDA. Archived from the original on 13 July 2023. Retrieved 25 September 2024.
- ^ a b "History of Schering AG". Archived from the original on 15 April 2008. Retrieved 6 December 2007.
{{cite web}}: CS1 maint: bot: original URL status unknown (link) - ^ Van den Broeck K (5 March 2010). "Gynaecoloog Ferdinand Peeters: De vergeten stiefvader van de pil" [Gynecologist Ferdinand Peeters: The forgotten stepfather of the pil]. Knack (in Dutch). pp. 6–13. Extra #4. Archived from the original on 14 September 2022. Retrieved 14 September 2022.
- ^ Hope A (24 May 2010). "The little pill that could". Flanders Today. Archived from the original on 1 January 2021. Retrieved 28 December 2014.
- ^ Mears E (November 1961). "Clinical trials of oral contraceptives". British Medical Journal. 2 (5261): 1179–83. doi:10.1136/bmj.2.5261.1179. PMC 1970272. PMID 14471934.
- ^ Eckstein P, Waterhouse JA, Bond GM, Mills WG, Sandilands DM, Shotton DM (November 1961). "The Birmingham oral contraceptive trial". British Medical Journal. 2 (5261): 1172–9. doi:10.1136/bmj.2.5261.1172. PMC 1970253. PMID 13889122.
- ^ a b Pullen D (October 1962). ""Conovid-E" as an oral contraceptive". British Medical Journal. 2 (5311): 1016–9. doi:10.1136/bmj.2.5311.1016. PMC 1926317. PMID 13972503.
- ^ a b Mears E, Grant EC (July 1962). ""Anovlar" as an oral contraceptive". British Medical Journal. 2 (5297): 75–9. doi:10.1136/bmj.2.5297.75. PMC 1925289. PMID 14471933.
- ^ "Annotations". British Medical Journal. 2 (5258): 1007–9. October 1961. doi:10.1136/bmj.2.3490.1009. PMC 1970146. PMID 20789252.
"Medical News". BMJ. 2 (5258): 1032–1034. 1961. doi:10.1136/bmj.2.5258.1032. S2CID 51696624. - ^ Chàvez L, Takahashi A, Yoshimoto T, Su CC, Sugawara T, Fujii Y (March 1990). "Morphological changes in normal canine basilar arteries after transluminal angioplasty". Neurological Research. 12 (1): 12–6. doi:10.1136/bmj.2.5266.1584. PMID 1970619. S2CID 8849008.
- ^ "Subsidizing birth control". Time. Vol. 78, no. 24. 15 December 1961. p. 55. Archived from the original on 5 February 2008.
- ^ Dourlen Rollier AV (October 1972). "[Contraception: yes, but...]". Fertilité, Orthogénie. 4 (4): 185–8. PMID 12306278.
- ^ "The Aids Generation: the pill takes priority?". Science Actualities. 2000. Archived from the original on 1 December 2006. Retrieved 7 September 2006.
- ^ "Djerassi on birth control in Japan – abortion 'yes,' pill 'no'" (Press release). Stanford University News Service. 14 February 1996. Archived from the original on 6 January 2007. Retrieved 23 August 2006.
- ^ Wudunn S (27 April 1999). "Japan's Tale of Two Pills: Viagra and Birth Control". The New York Times. Archived from the original on 19 April 2023. Retrieved 15 February 2017.
- ^ Efron S (3 June 1999). "Japan OKs Birth Control Pill After Decades of Delay". Los Angeles Times. Archived from the original on 31 August 2022. Retrieved 31 August 2022.
- ^ Efron S (3 June 1999). "Japan OKs Birth Control Pill After Decades of Delay". Los Angeles Times. Archived from the original on 10 August 2014. Retrieved 15 January 2015.
- ^ a b Hayashi A (20 August 2004). "Japanese Women Shun The Pill". CBS News. Archived from the original on 29 June 2006. Retrieved 12 June 2006.
- ^ Coleman DA (1992). "The Demographic Transition in Ireland in International Context" (PDF). Proceedings of the British Academy. 79: 65.
- ^ "The Pill". Time. 7 April 1967. Archived from the original on 19 February 2005. Retrieved 16 June 2020.
- ^ Westhoff C (15 December 2015). "How Obamacare Explains the Rising Popularity of IUDs". Public Health Now at Columbia University. Archived from the original on 23 December 2021. Retrieved 17 June 2020.
- ^ "The Birth Control Pill: A History" (PDF). Archived from the original (PDF) on 9 December 2021. Retrieved 20 October 2018.
- ^ Goldin C, Katz LF (2002). "The Power of the Pill: Oral Contraceptives and Women's Career and Marriage Decisions" (PDF). Journal of Political Economy. 110 (4): 730–770. CiteSeerX 10.1.1.473.6514. doi:10.1086/340778. S2CID 221286686. Archived (PDF) from the original on 29 May 2023. Retrieved 1 November 2017.
- ^ "The Pill". Equality Archive. 1 March 2017. Archived from the original on 23 December 2021. Retrieved 9 March 2017.
- ^ Weigel G (2002). The Courage to Be Catholic: Crisis, Reform, and the Renewal of the Church. Basic Books.
- ^ "Pillenverbot bleibt Streitfrage zwischen den Konfessionen". Focus Online. Archived from the original on 6 July 2021. Retrieved 22 March 2016.
- ^ a b c "1970 Year in Review". UPI. Archived from the original on 23 May 2009. Retrieved 8 April 2009.
- ^ Winnick C (1968). "The Beige Epoch: Depolarization of Sex Roles in America". The Annals of the American Academy of Political and Social Science. 376: 18–24. doi:10.1177/000271626837600103. JSTOR 1037799. S2CID 145158114.
- ^ Brautigan R (2007). "The Pill versus The Springhill Mine Disaster". Brautigan. doi:10.7273/nvgh-ca61. Archived from the original on 14 January 2009. Retrieved 1 December 2017.
- ^ "The pill and the marriage revolution". The Clayman Institute for Gender Research. Archived from the original on 12 December 2017. Retrieved 1 December 2017.
- ^ a b c Williams RJ, Johnson AC, Smith JJ, Kanda R (May 2003). "Steroid estrogens profiles along river stretches arising from sewage treatment works discharges". Environmental Science & Technology. 37 (9): 1744–50. Bibcode:2003EnST...37.1744W. doi:10.1021/es0202107. PMID 12775044.
- ^ "Not Quite Worry-Free". Environment. 45 (1): 6–7. January–February 2003. doi:10.1080/00139150309604545. S2CID 218496430.
- ^ Batt S (Spring 2005). "Pouring Drugs Down the Drain" (PDF). Herizons. 18 (4): 12–3. Archived from the original (PDF) on 15 March 2012. Retrieved 4 December 2011.
- ^ Zeilinger J, Steger-Hartmann T, Maser E, Goller S, Vonk R, Länge R (December 2009). "Effects of synthetic gestagens on fish reproduction". Environmental Toxicology and Chemistry. 28 (12): 2663–70. Bibcode:2009EnvTC..28.2663Z. doi:10.1897/08-485.1. PMID 19469587.
- ^ Johnson AC, Williams RJ, Simpson P, Kanda R (May 2007). "What difference might sewage treatment performance make to endocrine disruption in rivers?". Environmental Pollution. 147 (1): 194–202. Bibcode:2007EPoll.147..194J. doi:10.1016/j.envpol.2006.08.032. PMID 17030080.
- ^ Potts M, Marsh L (February 2010). "THE POPULATION FACTOR: How does it relate to climate change?" (PDF). OurPlanet.com. Archived from the original (PDF) on 4 March 2016. Retrieved 22 June 2015.
- ^ Cafaro P. "Alternative Climate Wedges – Population Wedge". Philip Cafaro. Archived from the original on 22 June 2015. Retrieved 22 June 2015.
- ^ Wire T (10 September 2009). "Contraception is 'greenest' technology". London School of Economics. Archived from the original on 22 June 2015. Retrieved 22 June 2015.
Further reading
[edit]- Black A, Guilbert E, Costescu D, Dunn S, Fisher W, Kives S, et al. (April 2017). "No. 329-Canadian Contraception Consensus Part 4 of 4 Chapter 9: Combined Hormonal Contraception". Journal of Obstetrics and Gynaecology Canada. 39 (4): 229–268.e5. doi:10.1016/j.jogc.2016.10.005. PMID 28413042.
External links
[edit]- The Birth Control Pill—CBC Digital Archives
- The Birth of the Pill—slide show by Life magazine
Combined oral contraceptive pill
View on GrokipediaBiological and Physiological Basis
The Natural Menstrual Cycle
The natural menstrual cycle encompasses recurring hormonal and physiological changes in the female reproductive system, typically spanning an average of 28 days from the first day of one menstruation to the first day of the next, though cycles ranging from 21 to 35 days are considered normal in adults.[8] This process is orchestrated by the hypothalamic-pituitary-ovarian axis, where the hypothalamus secretes gonadotropin-releasing hormone (GnRH) in a pulsatile manner to stimulate the anterior pituitary gland to release follicle-stimulating hormone (FSH) and luteinizing hormone (LH).[8] These gonadotropins regulate ovarian follicle development and ovulation, while ovarian hormones—primarily estradiol (a form of estrogen) and progesterone—feedback to modulate pituitary secretion and prepare the endometrium for potential implantation.[9] The cycle divides into the follicular phase, ovulation, and luteal phase, with menstruation marking the onset if pregnancy does not occur.[8] In the early follicular phase, low hormone levels following menstruation trigger elevated FSH, which promotes the recruitment and growth of multiple antral follicles in the ovaries; estradiol production rises as follicles mature, exerting negative feedback to suppress further FSH secretion and allowing a dominant follicle to emerge.[9] Estradiol levels peak just prior to ovulation, shifting to positive feedback that induces a mid-cycle surge in LH (and to a lesser extent FSH), culminating in ovulation approximately 36 hours later, typically around day 14 in a 28-day cycle.[8] Post-ovulation, the dominant follicle transforms into the corpus luteum, which secretes progesterone to maintain endometrial secretory changes conducive to blastocyst implantation, alongside sustained estradiol production.[9] If fertilization and implantation fail, the corpus luteum involutes after about 14 days due to the absence of human chorionic gonadotropin (hCG), causing progesterone and estradiol levels to decline sharply; this withdrawal triggers endometrial breakdown and menstruation, lasting 3 to 7 days and shedding the functional layer of the endometrium.[8] The luteal phase duration remains relatively fixed at 12 to 14 days, while follicular phase variability accounts for most inter-individual and intra-individual cycle length differences, with greater irregularity observed in adolescents (cycles 21–45 days) and perimenopausal women.[8] Empirical tracking data confirm that cycle lengths outside 24–38 days in reproductive-age women often signal underlying ovulatory dysfunction, though natural variation persists even in eumenorrheic cycles.[10]Hormonal Mechanisms of Ovulation and Implantation
The hormonal mechanisms governing ovulation and implantation are orchestrated by the hypothalamic-pituitary-ovarian (HPO) axis, involving gonadotropin-releasing hormone (GnRH) from the hypothalamus, which stimulates the anterior pituitary to secrete follicle-stimulating hormone (FSH) and luteinizing hormone (LH).[9] FSH initiates follicular development in the ovaries during the follicular phase of the menstrual cycle, promoting granulosa cell proliferation and estradiol production by dominant follicles.[11] Rising estradiol levels exert positive feedback on the pituitary, culminating in an LH surge approximately 36 hours before ovulation.[11] The LH surge triggers final oocyte maturation, resumption of meiosis in the oocyte, and follicular rupture, releasing the mature oocyte into the fallopian tube.[11] This process involves increased intrafollicular proteolytic enzymes that weaken the ovarian wall, allowing expulsion of the oocyte-cumulus complex.[11] Post-ovulation, the ruptured follicle transforms into the corpus luteum under LH influence, which secretes progesterone to maintain the luteal phase.[9] Progesterone induces secretory changes in the endometrium, transforming it from proliferative to receptive for potential implantation.[12] It promotes endometrial gland development, stromal cell decidualization, and vascular remodeling, creating a nutrient-rich environment for blastocyst attachment.[13] Estrogen, in synergy with progesterone, modulates receptivity by downregulating barriers like mucin 1 (MUC1) on epithelial cells, enabling blastocyst adhesion typically 6-10 days post-ovulation.[14] Without fertilization, corpus luteum regression leads to progesterone withdrawal, triggering menstruation and cycle reset.[9]Mechanism of Action
Inhibition of Ovulation
The combined oral contraceptive (COC) pill inhibits ovulation primarily by suppressing the release of gonadotropins, follicle-stimulating hormone (FSH) and luteinizing hormone (LH), through negative feedback on the hypothalamic-pituitary-ovarian axis.[1] The synthetic estrogen component mimics elevated estradiol levels, inhibiting gonadotropin-releasing hormone (GnRH) pulsatility from the hypothalamus and directly suppressing LH secretion from the anterior pituitary.[15] Concurrently, the progestin component further diminishes FSH secretion, limiting follicular recruitment and growth in the ovaries, while also blunting the mid-cycle LH surge essential for final oocyte maturation and rupture of the dominant follicle.[1] This dual hormonal action sustains a pseudoluteal state, preventing the estrogen-positive feedback loop that triggers ovulation in natural cycles.[15] Follicular development is arrested early due to persistently low FSH levels, with ovarian follicles rarely exceeding 10-13 mm in diameter during COC use, insufficient for ovulation.[16] The progestin enhances this by reducing ovarian responsiveness to any residual FSH and inhibiting LH synthesis at the pituitary level.[15] Estrogen doses as low as 20-35 μg ethinylestradiol, combined with progestins like levonorgestrel or desogestrel, achieve reliable suppression, with ovulation rates below 2% in compliant users across multiple formulations.[17] Breakthrough ovulation is rare but can occur with missed pills or drug interactions that lower hormone levels, though backup mechanisms like altered cervical mucus often prevent fertilization.[1] Clinical evidence from ultrasonography and hormonal assays confirms ovulation inhibition in over 97% of cycles with standard COCs taken correctly for at least 7 days.[17] A literature review of 23 studies found desogestrel-containing COCs and traditional progestin-only pills comparably effective, with no ovulation in most monitored cycles under controlled conditions.[17] Even extended or continuous regimens maintain suppression, though slight follicular activity may increase with lower-dose estrogens, without compromising overall efficacy.[18] These findings underscore ovulation inhibition as the dominant contraceptive mechanism, supported by direct measurement of absent LH peaks and immature follicles.[16]Effects on Cervical Mucus and Endometrium
The progestin component in combined oral contraceptive pills (COCPs) primarily induces changes in cervical mucus by increasing its viscosity and altering its composition, resulting in a thick, scanty, and opaque secretion that forms a barrier impermeable to sperm penetration into the upper genital tract.[19][20] This effect mimics the postovulatory progesterone-dominated phase of the natural cycle but is sustained due to exogenous progestin, thereby inhibiting sperm capacitation and transport as a secondary contraceptive mechanism beyond ovulation suppression.[21] Clinical evaluations, such as Insler scoring, demonstrate rapid mucus thickening within hours of progestin administration, with scores remaining low during active pill phases in both standard 21/7 and extended 24/4 regimens.[22][23] Regarding the endometrium, COCPs promote glandular atrophy, stromal decidualization, and overall thinning of the lining under progestin influence, which counteracts estrogen-driven proliferation and diminishes vascularity and receptivity to blastocyst implantation. This thinning frequently results in minimal or absent withdrawal bleeding during hormone-free intervals, as there is little endometrial tissue to shed.[24][25] Endometrial biopsies after 3–13 cycles of low-dose COCPs show atrophic changes in 41–100% of users, depending on duration, with mean thicknesses reduced to approximately 8–9 mm on cycle day 10 in long-term users compared to non-users.[26][27] This thinning persists during continuous regimens, rendering the endometrium inactive and reducing hyperplasia risk, though recovery may require weeks post-discontinuation.[28] These alterations collectively contribute to contraceptive efficacy by creating an inhospitable environment for fertilized ova, supported by histological and ultrasonographic evidence from controlled studies.[2]Additional Physiological Impacts
The progestin component of combined oral contraceptives reduces fallopian tube motility and peristalsis, slowing the transport of gametes and thereby impeding fertilization even if sperm penetrate the cervical barrier.[29][30] This effect stems from progestin mimicking luteal-phase suppression of oviduct contractility, with studies documenting decreased tubal propulsion in users compared to non-users.[7] Beyond primary ovulation suppression, combined oral contraceptives inhibit follicular maturation, leading to persistent small, non-functional follicles that produce minimal estradiol and are unlikely to yield fertilizable ova during breakthrough cycles, which occur in approximately 2-5% of cycles depending on formulation adherence.[31][28] Endogenous gonadotropin levels remain low, curtailing ovarian steroidogenesis and maintaining hypoestrogenic states outside the pill-free interval.[1] In rare escape ovulations, the continuous progestin exposure sustains endometrial atrophy and dyssynchrony, creating a hostile environment for implantation; evidence from animal models and human ectopic pregnancy data supports potential postfertilization interference, though human incidence is low due to dominant pre-ovulatory mechanisms.[32][33] Systemically, ethinylestradiol induces hepatic synthesis of sex hormone-binding globulin (SHBG), elevating levels 2- to 4-fold within weeks, which sequesters free testosterone and androgens, reducing their bioavailability and contributing to physiological shifts like decreased hirsutism in hyperandrogenic states but potential impacts on muscle and libido.[34][35] Progestins variably influence insulin sensitivity, with some formulations increasing resistance via androgenic effects, while others improve it through anti-androgenic action.[36][37] These changes reflect dose-dependent interactions with metabolic pathways, independent of contraceptive efficacy.Formulations and Administration
Estrogen and Progestin Components
The estrogen component in combined oral contraceptive pills (COCs) consists primarily of ethinylestradiol (EE), a synthetic derivative of estradiol with enhanced oral bioavailability due to ethinylation at carbon 17, rendering it resistant to first-pass hepatic metabolism.[38] Most formulations deliver 20 to 35 mcg of EE per active tablet, a reduction from earlier 50 mcg doses that correlated with higher risks of thromboembolism and other estrogen-related adverse effects.[29] [1] This low-dose EE primarily stabilizes the endometrial lining and enhances progestin-mediated ovulation suppression, though progestins alone can inhibit ovulation at sufficient doses.[39] Newer COC variants incorporate bioidentical or modified natural estrogens, such as estradiol valerate (converted to estradiol in vivo) or estetrol (E4), a fetal estrogen with selective receptor affinity that may reduce clotting factor activation compared to EE.[29] [40] For instance, estradiol-based pills like those containing 1 to 3 mg estradiol valerate paired with dienogest aim to mimic physiologic estrogen levels more closely, potentially lowering venous thromboembolism incidence, though long-term data remain limited.[1] Mestranol, an EE prodrug used in early formulations, has largely been phased out due to requiring higher doses for equivalent activity.[38] Progestins in COCs are synthetic analogues of progesterone, classified into generations based on chemical structure and pharmacologic profile, primarily derived from 19-nortestosterone or spironolactone scaffolds.[41] First-generation progestins, such as norethindrone (0.35–1 mg doses), exhibit moderate androgenic activity, contributing to potential acne or hirsutism in susceptible users.[1] Second-generation agents like levonorgestrel (0.1–0.15 mg) offer higher progestogenic potency with residual androgenicity, effective for ovulation blockade via strong luteinizing hormone suppression.[41] Third-generation progestins, including desogestrel (0.15 mg, prodrug to etonogestrel), norgestimate (0.18–0.25 mg), and gestodene (0.075 mg), feature reduced androgen binding to receptors, minimizing metabolic side effects like dyslipidemia while maintaining contraceptive reliability.[1] Fourth-generation progestins, such as drospirenone (3 mg, structurally akin to spironolactone) and dienogest (2–3 mg), provide anti-androgenic effects beneficial for acne and premenstrual dysphoric disorder, alongside anti-mineralocorticoid activity that counters estrogen-induced fluid retention.[41] [42] Progestin selection influences non-contraceptive benefits, with no established differences in ovulation inhibition or overall efficacy across types when combined with adequate estrogen.[41][43]| Generation | Key Examples (Typical Dose) | Pharmacologic Notes |
|---|---|---|
| First | Norethindrone (0.35–1 mg) | Androgenic; derived from 19-nortestosterone[1] |
| Second | Levonorgestrel (0.1–0.15 mg) | High progestogenic potency; androgenic[41] |
| Third | Desogestrel (0.15 mg), Norgestimate (0.18–0.25 mg) | Lower androgenicity; improved lipid profiles[1] |
| Fourth | Drospirenone (3 mg), Dienogest (2–3 mg) | Anti-androgenic, anti-mineralocorticoid[41] |
Standard and Extended Regimens
The standard regimen for combined oral contraceptive pills (COCs) consists of 21 consecutive days of active pills containing fixed doses of estrogen and progestin, followed by a 7-day hormone-free interval (HFI) of placebo pills or no pills, during which withdrawal bleeding typically occurs.[44] This 28-day cyclic schedule, introduced in the 1960s and refined with lower hormone doses by the 1980s, aligns with the approximate length of the natural menstrual cycle to facilitate user adherence and endometrial shedding.[45] Absence of withdrawal bleeding during the HFI is often normal and common, as the hormones suppress ovulation and thin the uterine lining, leaving little or no endometrium to shed; this is particularly prevalent with low-dose pills, long-term use, or continuous cycling approaches.[46] However, it can indicate pregnancy if adherence is imperfect, such as missed or late doses, or impaired absorption from vomiting or diarrhea, warranting a pregnancy test. Less common etiologies include switching to a new formulation, interacting medications, excessive exercise, low body weight, or conditions like thyroid dysfunction or polycystic ovary syndrome (PCOS). Provided pills are taken correctly without accompanying symptoms, absent bleeding typically poses no concern, though persistent absence merits medical consultation. Variations include 24 active days followed by 4 HFIs, often with low-dose estrogen in the latter to minimize unscheduled bleeding, as seen in formulations approved since the early 2000s.[29] Pills must be taken daily at the same time for optimal efficacy, with backup contraception recommended if doses are missed during the active phase.[46] Extended regimens extend the active pill phase beyond 21 or 24 days to reduce withdrawal bleed frequency, such as 84 active days followed by a 7-day HFI, yielding approximately four periods annually, or fully continuous use without scheduled HFIs.[44] Formulations like those with 84 days of 20 μg ethinyl estradiol and 100 μg levonorgestrel, approved by the FDA in 2003, support this approach while maintaining low cumulative hormone exposure.[47] These regimens, increasingly prescribed since the 1990s for conditions involving heavy or painful bleeding, involve skipping placebo phases across multiple packs or using continuous dosing, with initial breakthrough bleeding often resolving after 3–6 months.[48] Contraceptive efficacy in extended use matches standard cyclic regimens (Pearl Index approximately 0.3 with perfect use), with no significant differences in overall safety profiles reported in comparative studies up to 2024.[49][50] Providers may tailor regimens by omitting HFIs in standard packs, though dedicated extended-cycle products minimize dosing errors.[51]Recent Formulation Advances
Advances in combined oral contraceptive (COC) formulations since the early 2000s have primarily focused on reducing hormone doses to minimize adverse effects while preserving efficacy, introducing novel progestins with improved side-effect profiles, and developing extended or continuous regimens to decrease withdrawal bleeding frequency.[52] Contemporary low-dose COCs typically contain 20 to 35 micrograms (mcg) of ethinyl estradiol (EE), a significant reduction from earlier high-dose formulations exceeding 50 mcg, which has lowered risks such as venous thromboembolism without compromising ovulation suppression.[29] These ultra-low-dose options, including those with 10 to 15 mcg EE paired with potent progestins like levonorgestrel, maintain contraceptive effectiveness rates above 99% with perfect use but may increase unscheduled bleeding in some users.[53] New progestins have addressed androgenic side effects common in older generations, such as acne and hirsutism. Drospirenone (DRSP), introduced in COCs like Yasmin in 2001, exhibits anti-mineralocorticoid and anti-androgenic properties akin to spironolactone, reducing bloating and improving premenstrual symptoms, though it carries a slightly elevated thrombotic risk compared to levonorgestrel-based pills.[54] Dienogest (DNG), combined with EE in formulations approved in the 2000s, offers strong progestogenic activity with minimal androgenicity or estrogenicity, benefiting conditions like endometriosis while maintaining endometrial transformation for contraception.[55] More recently, estetrol (E4), a natural estrogen derived from fetal metabolism, has been incorporated into COCs with DRSP (e.g., Nextstellis, approved by the FDA in 2021), demonstrating reduced impacts on coagulation, lipids, and carbohydrate metabolism relative to EE-based pills in clinical trials.[39] Extended-cycle and continuous regimens represent a shift from the traditional 21/7 (active/hormone-free days) model, extending active pill intake to 84 or more days followed by shortened or no hormone-free intervals, thereby suppressing menstruation to four or fewer times annually.[44] Seasonique, approved in 2006, combines 84 days of 30 mcg EE/0.15 mg levonorgestrel with 7 days of 10 mcg EE (no progestin), mitigating breakthrough bleeding and hypoestrogenic symptoms better than placebo intervals.[47] These regimens, supported by evidence of sustained follicular suppression, improve quality of life for users with dysmenorrhea or heavy bleeding but require monitoring for cumulative estrogen exposure.[48] Phasic formulations, such as biphasic or triphasic pills varying hormone doses within cycles, further optimize bleeding patterns and tolerability, with triphasic norgestimate/EE showing reduced cycle control issues in comparative studies.[56]Efficacy and Clinical Use
Contraceptive Effectiveness Rates
The combined oral contraceptive pill (COCP) demonstrates high contraceptive efficacy under ideal conditions. With perfect use—involving daily ingestion at the same time without omissions, delays, or confounding factors such as vomiting or drug interactions—the first-year failure rate is approximately 0.3%, meaning 0.3 pregnancies occur per 100 woman-years of exposure.[57][1] This rate aligns with the Pearl Index calculations from clinical trials, where adherence is closely monitored, yielding values typically between 0.1% and 1.0% depending on the specific estrogen-progestin formulation tested.[58]00049-7/fulltext) In typical use, which incorporates real-world inconsistencies like missed doses (reported in up to 50% of users over a year), incorrect timing, or interactions with antibiotics and anticonvulsants, the first-year failure rate increases to 7-9%.[1][57][59] These estimates derive from large-scale demographic surveys adjusting for user demographics, with higher failure rates observed among younger women (e.g., 9% for ages 15-19) and lower among older or higher-income groups due to better adherence.[57]| Use Type | First-Year Failure Rate | Effectiveness Rate | Key Factors Influencing Rate |
|---|---|---|---|
| Perfect Use | 0.3% | >99% | Strict daily adherence, no interactions[57] |
| Typical Use | 7-9% | 91-93% | Missed pills, timing errors, user demographics[1][59] |
Factors Affecting Efficacy
The efficacy of combined oral contraceptive pills (COCs) depends on consistent daily intake, with perfect use yielding a failure rate of 0.3 pregnancies per 100 woman-years, while typical use, incorporating real-world inconsistencies, results in a rate of 7% to 9%.[20][1] This disparity arises primarily from user-dependent factors that disrupt steady hormone levels necessary to inhibit ovulation and alter cervical mucus and endometrial receptivity. Adherence represents the dominant influence on efficacy, as missed pills, delayed dosing, or irregular timing can lead to follicular development and ovulation. Non-compliance accounts for most unintended pregnancies among COC users, with surveys showing that up to 50% of young women forget doses periodically, elevating first-year failure rates to 3% to 8% under typical conditions. Interventions like reminders or extended-cycle regimens can mitigate this, but lapses beyond 24 hours typically require backup contraception for 7 days to restore efficacy.[61][62][63] Pharmacokinetic interactions with concurrent medications significantly impair efficacy by accelerating hepatic metabolism of ethinylestradiol and progestins via cytochrome P450 induction. Rifampin, certain anticonvulsants (e.g., phenytoin, carbamazepine, phenobarbital), and some antiretroviral protease inhibitors reduce hormone bioavailability, prompting guidelines to recommend alternative or supplemental contraception during co-administration and for 28 days post-discontinuation. While broad-spectrum antibiotics like amoxicillin show no consistent impact in pharmacokinetic studies, enzyme-inducing agents pose a clear risk, with failure rates potentially doubling in affected users.[64][65][66] Gastrointestinal events compromise absorption if occurring soon after dosing; vomiting within 2 to 3 hours warrants repeating the pill, while severe diarrhea exceeding 24 hours mimics missed doses by reducing enterohepatic recirculation of hormones, necessitating backup methods until recovery and for 7 subsequent days. Chronic conditions like inflammatory bowel disease may chronically elevate failure risk through similar mechanisms.[67][68][69] Body mass index (BMI) has a debated but minimal net effect on efficacy per meta-analyses of large cohorts; higher-quality prospective studies report no substantial increase in failure rates for BMI ≥30 kg/m² versus <25 kg/m², attributing early concerns to confounding factors like adherence rather than pharmacokinetics. Pharmacodynamic modeling confirms adequate ovulation suppression across weight ranges with standard formulations, though ultra-low-dose variants may warrant caution in obesity due to marginally lower plasma levels.62524-4/fulltext)00272-5/fulltext)00603-4/fulltext) Formulation-specific elements, including estrogen dose and progestin type, indirectly affect efficacy through tolerability; lower-dose COCs (e.g., ≤20 μg ethinylestradiol) demand stricter timing to maintain suppression, while user errors in initiation (e.g., not starting on day 1 of menses) transiently heighten risk.[70][1]Non-Contraceptive Medical Applications
Combined oral contraceptives (COCs) are employed to manage various gynecological conditions by suppressing ovulation, stabilizing endometrial proliferation, and modulating hormone levels, thereby alleviating symptoms without addressing underlying pathologies.[71] In polycystic ovary syndrome (PCOS), COCs serve as first-line therapy alongside lifestyle interventions, improving menstrual regularity (achieving 100% cycle normalization versus 0% with no treatment, per low-certainty evidence from randomized trials) and reducing hyperandrogenism manifestations such as hirsutism and acne through increased sex hormone-binding globulin and decreased free testosterone.[72][30] For hyperandrogenic disorders, COCs effectively diminish acne severity and hirsutism in women with PCOS, with formulations containing anti-androgenic progestins (e.g., cyproterone acetate or drospirenone) showing superior outcomes in reducing sebum production and pilosebaceous activity compared to standard types.[73][74] Menstrual irregularities, including heavy bleeding (menorrhagia) and painful periods (dysmenorrhea), are ameliorated by COCs via reduced menstrual blood loss (up to 40-50% decrease) and prostaglandin-mediated uterine contractility, with evidence from systematic reviews indicating consistent symptom relief across diverse populations.[71][4] In endometriosis, COCs mitigate pelvic pain and dysmenorrhea by inducing endometrial atrophy and decreasing lesion growth, though they do not eradicate ectopic tissue; observational data and guidelines endorse their use for symptom control, particularly in extended regimens to minimize cyclic bleeding.[75][76] Premenstrual syndrome (PMS) and associated migraines benefit from COC-induced cycle regulation, with suppression of ovulatory hormone fluctuations reducing symptom intensity in affected individuals.[4] Approximately 40-60% of COC prescriptions in Europe target such gynecological disturbances rather than contraception alone, underscoring their therapeutic prevalence.[77] Despite these applications, COCs provide symptomatic management rather than curative effects, necessitating ongoing monitoring for metabolic impacts in conditions like PCOS.[78]Evidence-Based Health Benefits
Reductions in Ovarian, Endometrial, and Colorectal Cancers
Use of combined oral contraceptives (COCs) has been consistently associated with a reduced incidence of ovarian cancer, with the magnitude of risk reduction increasing with duration of use. A 2023 meta-analysis of epidemiological studies reported up to a 50% decrease in ovarian cancer incidence among users after 10 or more years of exposure.[79] Similarly, a 2024 systematic review found that hormonal contraceptive users, particularly those using COCs, experienced a 36% lower risk (relative risk [RR] 0.64, 95% confidence interval [CI] 0.60–0.68).[80] This protective effect persists long-term, remaining evident up to 30 years after discontinuation, attributed mechanistically to the suppression of ovulation and resultant fewer ovulatory cycles that may promote ovarian epithelial damage.[81] Long-term use also mitigates risk across diverse lifestyle and genetic factors, including in BRCA1/2 mutation carriers.[82][83] For endometrial cancer, COCs exert a dose-dependent protective effect, with longer durations yielding greater reductions. A 2025 meta-analysis indicated that use for 10 or more years lowered odds by approximately 69% (odds ratio [OR] 0.31, 95% CI 0.13–0.70), building on prior evidence of a 50% risk decrease for ever-users in large cohort and case-control studies.[84][85] Every additional 5 years of use correlates with further risk attenuation, as shown in a 2015 pooled analysis of 36 studies, where the effect stems from progestin-induced endometrial atrophy that counters estrogen-driven proliferation.[86] Overall long-term use reduces risk by at least 30–34%, with benefits enduring for decades post-cessation.[6][82] Evidence for colorectal cancer risk reduction is also supportive but slightly less pronounced than for gynecological sites. Meta-analyses of cohort studies estimate a 15–20% lower risk among ever-users (OR 0.84, 95% CI 0.72–0.97), with some large prospective data like the Nurses' Health Study confirming a 19% overall reduction.[6][87][88] This association holds across multiple epidemiologic designs, potentially linked to progestins' modulation of bile acid metabolism and anti-inflammatory effects in the colon, though the effect size appears independent of duration and may wane more rapidly after stopping compared to ovarian or endometrial protection.[89]Treatment of Conditions like PCOS, Acne, and Endometriosis
Combined oral contraceptive pills (COCPs) are frequently prescribed as a first-line treatment for polycystic ovary syndrome (PCOS), particularly to regulate menstrual cycles and mitigate hyperandrogenism symptoms such as hirsutism and acne, alongside lifestyle interventions.[30] By suppressing gonadotropin-releasing hormone (GnRH) pulsatility, COCPs reduce ovarian androgen production and increase sex hormone-binding globulin (SHBG) levels, thereby lowering free testosterone concentrations by approximately 30-50% after 6-12 months of use.[90] Randomized controlled trials indicate that COCPs improve menstrual regularity in 70-90% of PCOS patients, with formulations containing cyproterone acetate (CPA) demonstrating superior efficacy in reducing hirsutism scores compared to other progestins, as measured by the Ferriman-Gallwey scale.00339-5/fulltext) Continuous or extended-cycle regimens may further protect the endometrium from unopposed estrogen exposure, reducing hyperplasia risk in anovulatory PCOS.[91] For acne vulgaris, COCPs are approved by regulatory bodies like the FDA for moderate cases in women, with specific formulations including Yaz, Ortho Tri-Cyclen, and Estrostep approved for this indication due to their ability to regulate hormones and reduce breakouts. Evidence from systematic reviews shows significant reductions in inflammatory and noninflammatory lesion counts versus placebo, achieving up to 55% overall lesion reduction after 6 months.[92] This effect stems from decreased androgen-mediated sebum production and anti-inflammatory properties of specific progestins, such as drospirenone (DRSP), where ethinyl estradiol 20 μg/DRSP 3 mg regimens outperform placebo in pooled analyses of phase III trials involving over 2,000 participants.[93] Guidelines from dermatological societies endorse COCPs as adjunctive therapy when topical treatments fail, particularly for hormonal acne linked to menstrual cycles, though efficacy varies by formulation, with norgestimate and DRSP showing consistent benefits in reducing total lesions by 40-60%.[94][95] In endometriosis management, COCPs alleviate symptoms like dysmenorrhea and pelvic pain by inducing endometrial atrophy and suppressing ovulation, with meta-analyses confirming comparable efficacy to progestogens in reducing pain scores by 20-40% over 6-24 months.[96] Continuous dosing minimizes menstrual flow and retrograde menstruation, potentially limiting lesion progression, as supported by trials where estradiol-based or estetrol-containing COCPs outperformed ethinyl estradiol variants in pain relief for endometriosis-associated dysmenorrhea.[97] Long-term studies, including those post-surgery, report sustained dyspareunia and chronic pain reduction similar to long-acting progestogens, with adherence rates favoring COCPs due to fewer irregular bleeding episodes in some regimens.[98] However, COCPs do not eradicate ectopic tissue and are less effective for advanced disease requiring surgical intervention.[99]Other Gynecological Benefits
Combined oral contraceptive pills (COCPs) effectively reduce symptoms of primary dysmenorrhea through ovulation suppression and decreased endometrial tissue growth, which lowers prostaglandin production responsible for uterine contractions. A 2023 Cochrane systematic review of 13 randomized controlled trials involving over 2,000 women found that COCPs significantly alleviate dysmenorrhea pain compared to placebo, with a number needed to treat of approximately 4 for moderate to severe cases; however, they increase risks of irregular bleeding (risk ratio 1.67) and possibly headache and nausea.[100] Observational and clinical data further support consistent benefits, particularly with low-dose formulations, though efficacy may vary by individual pain severity and pill type. COCPs also manage heavy menstrual bleeding (menorrhagia) by inducing endometrial atrophy, resulting in reduced menstrual blood loss. A 2019 Cochrane review of five randomized trials with 704 women demonstrated moderate-quality evidence that COCPs over six months achieve amenorrhea or spotting in up to 77% of users with unacceptable heavy bleeding, compared to 12% on placebo or no treatment; pictorial blood loss assessments showed mean reductions of 40-60 mL per cycle.[101] This benefit is attributed to stable hormone levels preventing excessive endometrial buildup, with extended-cycle regimens enhancing outcomes by minimizing bleeding episodes.[102] Beyond these, COCPs regulate irregular menstrual cycles by stabilizing hormone fluctuations, promoting predictable withdrawal bleeding and shorter cycle lengths. Clinical guidelines note their use in adolescents and adults with oligomenorrhea or polymenorrhea, where they normalize cycles within 3-6 months by overriding endogenous ovarian activity.[103] For premenstrual syndrome (PMS), formulations containing drospirenone yield superior relief of physical symptoms like bloating and breast tenderness versus standard COCPs, per a 2023 meta-analysis of trials showing improved daily functioning in women with moderate to severe PMS.[104]Health Risks and Adverse Effects
Cardiovascular and Thrombotic Risks
Combined oral contraceptive pills (COCPs) are associated with an increased risk of venous thromboembolism (VTE), including deep vein thrombosis and pulmonary embolism, with a relative risk of approximately 3 to 5 compared to non-users.[105] [106] The absolute risk remains low, typically 7 to 10 events per 10,000 woman-years among users versus 4 to 5 per 10,000 in non-pregnant, non-postpartum non-users, though this varies by formulation and individual factors. For example, drospirenone-containing COCPs are associated with roughly 8–12 VTE events per 10,000 woman-years, while levonorgestrel-containing COCPs have 5–7 per 10,000 (versus 1–2 per 10,000 in non-users).[106] Third-generation progestins (e.g., desogestrel, gestodene) and drospirenone-containing COCPs carry a higher VTE risk than second-generation ones (e.g., levonorgestrel), with relative risks 50% to 80% elevated in meta-analyses of randomized and observational data.[106] [107] Inherited thrombophilias amplify this risk substantially; for instance, women with factor V Leiden or prothrombin G20210A mutations using COCPs face relative risks up to 30-fold higher for VTE compared to non-users without thrombophilia, with absolute incidences reaching 4.3 to 4.6% in severe cases versus near-zero in mild ones among relatives.[108] [109] Acquired risks, such as obesity (BMI >30 kg/m²), smoking, and age over 35, further elevate VTE incidence multiplicatively, with combined effects potentially tripling baseline user risks.[110] [111] COCPs also confer arterial thrombotic risks, including ischemic stroke and myocardial infarction (MI), with relative risks of 1.5 to 2.8 for MI and similar for stroke among users versus non-users, after adjusting for confounders like hypertension and smoking.[112] [113] These risks escalate with age, smoking (up to 10-fold in heavy smokers over 35), hypertension, diabetes, and migraine with aura, though absolute incidences remain rare in young, healthy users (e.g., <1 MI per 10,000 woman-years).[114] [115] Recent Danish cohort data indicate slightly higher ischemic stroke and MI risks with certain contemporary formulations, including progestin-only options, but no significant association with cardiovascular mortality overall.[116] [117] Blood pressure elevations occur in some users, with meta-analyses showing modest mean increases (3-5 mmHg systolic) linked to estrogen dose, though progression to clinical hypertension is not consistently elevated across studies.[118] [119] Risks generally attenuate post-discontinuation, returning to baseline within months, but persist longer in those with predisposing factors.[111] Guidelines recommend screening for modifiable risks and preferring lower-risk formulations (e.g., levonorgestrel) in vulnerable populations to minimize events.[106][120]Oncogenic Risks: Breast, Cervical, and Liver Cancers
Use of combined oral contraceptives (COCs) is associated with a modest increase in breast cancer risk, particularly among current or recent users. A 2023 meta-analysis of hormonal contraception found an odds ratio (OR) of 1.33 (95% CI: 1.19-1.49) for breast cancer in ever-users compared to never-users.[121] Current or recent use of estrogen-progestagen COCs elevates risk by approximately 20-30%, though this elevation is not consistently observed across all studies and tends to diminish or disappear about 10 years after discontinuation.[122][123] The absolute increase in risk remains small, with estimates suggesting an additional 1-2 cases per 10,000 women per year among current users aged 16-49.[122] For cervical cancer, prolonged COC use correlates with elevated risk, potentially independent of human papillomavirus (HPV) infection, though confounding factors like sexual behavior and screening practices complicate attribution. The National Cancer Institute reports a duration-dependent effect: less than 5 years of use yields a 10% risk increase, rising to 60% for 10 or more years.[6] A 2023 review confirmed a relative risk (RR) of 1.40 (95% CI: 1.28-1.53) for recent or current users.[124] Population studies indicate higher incidence among those using COCs for over 5 years, with risk declining after cessation but persisting for long-term users.[125] Liver cancer risk from COCs primarily involves rare benign hepatocellular adenomas (HCAs), which occur in approximately 3-4 per 100,000 users and carry a small potential for malignant transformation to hepatocellular carcinoma. HCAs are strongly linked to estrogen-containing COCs, especially high-dose formulations used historically, with prevalence rising in reproductive-age women on long-term therapy.[126] A 2025 meta-analysis reported a slightly increased liver cancer risk per 5 years of COC use, though absolute incidence remains low at under 1 per 100,000 women annually.[127] Discontinuation often leads to regression of HCAs, reducing malignancy risk, which is estimated at 4-10% for adenomas over 5 cm.[128] Modern low-dose COCs appear to confer lower adenoma risk than earlier formulations.[129]Mental Health and Neurological Effects
Observational studies have linked combined oral contraceptive (COC) use to elevated risks of depressive symptoms, particularly among adolescents and young women. A systematic review of studies comparing COC users to non-users reported consistent increases in depression, anxiety, and eating disorder symptoms, with factors like younger age and prior side effects amplifying vulnerability.[130] Adolescent COC users specifically exhibit heightened depressive symptoms compared to non-users, though overall population-level associations remain mixed due to confounding variables such as self-selection and underlying mental health predispositions.[131] COC use has been associated with increased suicidal behavior in large cohort analyses. A Danish registry study of over 1 million women found hormonal contraceptive use doubled the rate of suicide attempts and tripled completed suicides, independent of prior psychiatric diagnoses in some subgroups.[132] A meta-analysis confirmed a positive association between hormonal contraceptives and suicide consumption, suggesting heightened risk in susceptible populations prior to initiation.[133] However, analyses excluding women with pre-existing psychiatric illness indicate no elevated suicide risk from combined formulations, pointing to mediation by underlying vulnerabilities rather than universal causation.[134][135] Short-term experimental studies demonstrate acute mood disruptions, with COC users showing a 12.67% increase in negative affect and 7.42% rise in anxiety during stress tasks compared to controls.[136] Broader reviews note conflicting general-population results—ranging from slight risk elevations (RR 1.13 for depression) to protective effects in postpartum or comorbid cases—but emphasize observational biases and the need for causal inference via randomized designs, which remain limited.[137][138] Neurologically, COCs induce alterations in brain structure, function, and connectivity. Neuroimaging reveals changes in regional volumes, with macrostructural increases and decreases across cortical and subcortical areas, alongside shifts in functional connectivity during emotional processing.[139] Functional MRI studies show COC users exhibit modified dynamic brain states and reduced reward-related activation, correlating with reported mood declines.[140] These effects stem from synthetic estrogens and progestins mimicking or suppressing endogenous hormones, influencing neuroendocrine pathways and stress responses, though long-term implications for cognition and resilience require further longitudinal data.[141][142]Metabolic and Other Side Effects
Combined oral contraceptives (COCs) influence lipid metabolism, typically increasing triglycerides and high-density lipoprotein cholesterol (HDL-C), with variable effects on low-density lipoprotein cholesterol (LDL-C) depending on the progestin component.[143] [144] Most formulations elevate triglycerides due to estrogen's stimulation of hepatic production, alongside rises in HDL-C from progestins, though third-generation progestins may attenuate LDL-C increases compared to earlier types.[145] [146] These shifts generally lack clinical significance for cardiovascular risk in healthy users but warrant monitoring in those with dyslipidemia.[147] Regarding carbohydrate metabolism, COCs exert minor effects on insulin sensitivity, fasting glucose, and HbA1c in normoglycemic women, with meta-analyses indicating no substantial impairment of glycemic control.[148] [143] However, in subgroups such as women with polycystic ovary syndrome (PCOS) or obesity, usage can worsen glucose tolerance, potentially amplifying insulin resistance via progestin-mediated androgen suppression and estrogen-induced hepatic glucose output.[149] Acute post-ingestion glucose excursions may also rise, though long-term diabetes risk remains unestablished.[150] Weight gain is not consistently linked to COC use; systematic reviews find no clinically meaningful increase in body mass index (BMI) beyond placebo levels, attributing perceived gains to fluid retention or behavioral factors rather than direct metabolic causation.[147] [143] [151] COCs may modestly elevate blood pressure, with average systolic increases of 4-5 mmHg observed in some users, particularly smokers or those with baseline hypertension, though most remain normotensive.[118] Non-metabolic side effects include transient nausea, headaches, and breast tenderness, affecting up to 10-20% of initiators but diminishing within 3 months as adaptation occurs.[45] [152] These arise from estrogen's vascular and gastrointestinal effects or progestin's mammary stimulation, often resolvable by dose adjustment or formulation switch.[153] Long-term use associates with heightened gallbladder disease risk, including cholelithiasis and cholecystitis, via estrogen-promoted cholesterol supersaturation in bile; relative risks approximate 1.3-2.0 for ever-users versus never-users, escalating with duration beyond 5 years.[154] On bone health, low-dose COCs in adolescents may reduce bone mineral density (BMD) accrual by 1-2% annually compared to non-users, potentially due to suppressed ovarian estrogen peaks, though effects reverse post-discontinuation and appear neutral or protective in adults.[155] [156] High-quality evidence remains limited, emphasizing individualized assessment for young users.[157]Contraindications, Precautions, and Interactions
Absolute and Relative Contraindications
Absolute contraindications for combined oral contraceptive pills (COCs) encompass medical conditions classified as category 4 in the U.S. Medical Eligibility Criteria (USMEC) for combined hormonal contraceptives, indicating an unacceptable health risk if used. These conditions primarily involve heightened risks of thromboembolism, cardiovascular events, or hormone-sensitive malignancies, where the estrogen component exacerbates underlying pathologies. Guidelines emphasize that COCs should not be initiated or continued in such cases, with alternative contraception recommended.[65] Key absolute contraindications include:- Current or history of breast cancer, due to potential promotion of hormone-sensitive tumor growth or recurrence.[65][1]
- Known or suspected pregnancy, as exogenous hormones offer no benefit and may pose fetal risks.[65]
- Current venous thromboembolism (VTE), deep vein thrombosis (DVT), or pulmonary embolism (PE), or history thereof without ongoing anticoagulation therapy, owing to estrogen's prothrombotic effects elevating recurrence risk.[65][1]
- Acute myocardial infarction, current or history of ischemic heart disease, or stroke, reflecting amplified arterial thrombotic hazards.[65]
- Thrombogenic mutations (e.g., factor V Leiden, prothrombin mutation, protein C/S or antithrombin deficiencies), which compound VTE propensity.[65][1]
- Benign hepatocellular adenoma or malignant liver tumors, with risk of hemorrhage or progression from estrogen influence.[65][1]
- Severe (decompensated) cirrhosis or acute liver disease, impairing metabolism and increasing thrombotic complications.[65]
- Uncontrolled hypertension (systolic ≥160 mm Hg or diastolic ≥100 mm Hg), associated with cerebrovascular and cardiac event escalation.[65][1]
- Migraine with aura, particularly in those aged ≥35 years, due to stroke risk augmentation.[65][1]
- Major surgery with prolonged immobilization (≥1 week), heightening postoperative VTE incidence.[65]
- Postpartum period <21 days (breastfeeding or non-breastfeeding women), driven by elevated baseline VTE susceptibility.[65]
- Complicated valvular heart disease or peripartum cardiomyopathy within 6 months postpartum with impaired function, predisposing to thrombosis.[65]
- Hypertension adequately controlled or with systolic 140–159 mm Hg or diastolic 90–99 mm Hg, necessitating blood pressure surveillance.[65][1]
- Smoking in women aged ≥35 years (<15 cigarettes/day), with dose-dependent cardiovascular hazard.[65][1]
- Multiple risk factors for atherosclerotic cardiovascular disease (e.g., age ≥40 years, diabetes, obesity), warranting risk-benefit assessment.[65]
- History of DVT/PE on anticoagulation therapy, where ongoing treatment may offset but not eliminate recurrence potential.[65]
- Diabetes mellitus with vascular complications (e.g., nephropathy, retinopathy) or duration >20 years, due to microvascular and macrovascular interplay.[65][1]
- Postpartum 21–42 days with additional VTE risk factors (e.g., cesarean delivery, obesity), balancing puerperal thrombosis against contraceptive needs.[65]
- Breast cancer history without evidence of disease for ≥5 years, with lingering recurrence concerns.[65]
- Current or treated symptomatic gallbladder disease or past COC-related cholestasis, risking hepatic or biliary exacerbation.[65]
- Certain drug interactions (e.g., rifampin, some anticonvulsants like phenytoin), reducing efficacy and requiring backups.[65]

