Recent from talks
Nothing was collected or created yet.
Pinus halepensis
View on Wikipedia
| Pinus halepensis | |
|---|---|

| |
| Pinus halepensis in Sounion Natural Park, Greece | |
| Scientific classification | |
| Kingdom: | Plantae |
| Clade: | Tracheophytes |
| Clade: | Gymnospermae |
| Division: | Pinophyta |
| Class: | Pinopsida |
| Order: | Pinales |
| Family: | Pinaceae |
| Genus: | Pinus |
| Subgenus: | P. subg. Pinus |
| Section: | P. sect. Pinus |
| Subsection: | Pinus subsect. Pinaster |
| Species: | P. halepensis
|
| Binomial name | |
| Pinus halepensis | |
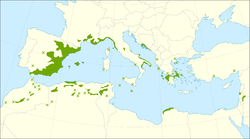
| |
| Distribution map | |
Pinus halepensis, commonly known as the Aleppo pine, also known as the Jerusalem pine,[2] is a pine native to the Mediterranean region. It was officially named by the botanist Philip Miller in his 1768 book The Gardener's Dictionary; he probably never went to Aleppo but mentions seeing large specimens at Goodwood in the garden of the Duke of Richmond, which were transplanted (perhaps sent by Alexander Russell from Syria) in 1739.[3]
Description
[edit]Pinus halepensis is a small to medium-sized tree, 15–25 metres (49–82 feet) tall, with a trunk diameter up to 60 centimetres (24 inches), exceptionally up to 1 m (3 ft 3 in). The bark is orange-red, thick, and deeply fissured at the base of the trunk, and thin and flaky in the upper crown. The leaves ('needles') are very slender, 6–12 cm (2+1⁄4–4+3⁄4 in) long, distinctly yellowish green, and produced in pairs (rarely a few in threes). The cones are narrow conic, 5–12 cm (2–4+3⁄4 in) long and 2–3 cm (3⁄4–1+1⁄4 in) broad at the base when closed, green at first, ripening glossy red-brown when 24 months old. They open slowly over the next few years, a process quickened if they are exposed to heat such as in forest fires. The cones open 5–8 cm (2–3+1⁄4 in) wide to allow the seeds to disperse. The seeds are 5–6 millimetres (3⁄16–1⁄4 in) long, with a 20 mm (13⁄16 in) wing, and are wind-dispersed.[4][5][6]
Related species
[edit]The Aleppo pine is closely related to the Turkish pine, Canary Island pine, and maritime pine, which all share many of its characteristics. Some authors include the Turkish pine as a subspecies of the Aleppo pine, as Pinus halepensis subsp. brutia (Ten.) Holmboe,[7] but it is usually regarded as a distinct species.[4][5][6][8] It is a relatively nonvariable species, in that its morphological characteristics stay constant over the entire range.[4]
Distribution and habitat
[edit]The native range of Pinus halepensis extends from Morocco, Algeria, Tunisia, and Spain north to southern France, Malta, Italy, Croatia, Montenegro, and Albania, and east to Greece. It has been introduced into many parts of the world, including Portugal. There is an outlying population (from which it was first described) in Syria, Lebanon, southern Turkey, Jordan, Israel and Palestine.
The species is generally found at low altitudes, mostly from sea level to 200 m (660 ft), but can grow above 1,000 m (3,300 ft) in southern and eastern Spain, well over 1,200 m (3,900 ft) on Crete, and up to 1,700 m (5,600 ft) in the south, in Morocco, Algeria and Tunisia.[4][5] The tree is able to quickly colonize open and disturbed areas. It is classed as an invasive species in South Africa.[9] It can grow on all substrates and almost in all bioclimates in the Mediterranean.[10]
Pinus halepensis is a diagnostic species of the vegetation class Pinetea halepensis.[11]
-
Cones
-
Pinus halepensis, Afhir Forest in Tlemcen.
-
Foliage
-
A grove of Aleppo pines in Pinet
-
Pinus halepensis forest at the island of Mljet
-
Bark and trunk
-
Plate from Lambert's Description of the Genus Pinus
-
Cone of pinus halepensis in Hebron
-
A dead Aleppo pine in front of the Étang de Thau
Uses
[edit]The resin of the Aleppo pine is used to flavor the Greek wine retsina.
From the pine nuts of the Aleppo pine is made a pudding called asidet zgougou in the Tunisian dialect; it is served in bowls, covered with cream, and topped with almonds and small candies.
The Maltese dessert prinjolata is also prepared using these pine nuts, both in its filling as well as a topping.
Aleppo pine are used for bonsai.
Forestry
[edit]In its native area, P. halepensis is widely planted for its fine timber, making it one of the most important forestry trees in Algeria and Morocco.[6]
In Israel, natural patches of Aleppo pine forests can be found in the Carmel and Galilee regions.[12] The Aleppo pine, along with Pinus brutia, has been planted extensively by the Jewish National Fund. It proved very successful in Yatir Forest in the northern Negev (on the edge of the desert), where foresters had not expected it to survive. Many Aleppo pine forests exist today in Israel and are used for recreational purposes. Although it is a local species, some argue that the historical replacement of natural oak maquis shrubland and garrigue with tall stands of pine has created "ecological deserts" and has significantly changed the species assemblage of these regions.[13] The species produces timber which is valued for its hardness, density and unproblematic seasoning. Seasoned timber is inclined to tear out with planing, but this can be avoided by using sharp blades or adjusting the sharpening angle of tools.[14]
The Aleppo pine is considered an invasive species though useful in South Africa; in South Australia, a control program is in place on Eyre Peninsula.
Landscape
[edit]Pinus halepensis is a popular ornamental tree, extensively planted in gardens, parks, and private and agency landscapes in hot dry areas such as Southern California and the Karoo in South Africa, where the Aleppo pine's considerable heat and drought tolerance, fast growth, and aesthetic qualities are highly valued.[citation needed]
In culture
[edit]Paul Cézanne had an Aleppo pine in his garden at Aix-en-Provence; this tree was the inspiration and model for his painting The Big Trees. As of 2005, the tree is still growing in Cézanne's garden.[15]
The Aleppo pine is associated with ANZAC Day and the ANZACs in Australia due to its use by soldiers in the Battle of Lone Pine during the Gallipoli campaign. It is often planted at war memorials.[citation needed]
References
[edit]- ^ Farjon, A. (2013). "Pinus halepensis". IUCN Red List of Threatened Species. 2013 e.T42366A2975569. doi:10.2305/IUCN.UK.2013-1.RLTS.T42366A2975569.en. Retrieved 19 November 2021.
- ^ Aisner, R.; Terkel, J. (1992-08-01). "Ontogeny of pine cone opening behaviour in the black rat, Rattus rattus". Animal Behaviour. 44: 327–336. doi:10.1016/0003-3472(92)90038-B. ISSN 0003-3472. S2CID 53148456.
- ^ Miller, Philip (1768). The Gardener's Dictionary. Vol. 3. biodiversitylibrary.org.
- ^ a b c d Farjon, A. (2005). Pines. Drawings and Descriptions of the genus Pinus. Brill, Leiden. ISBN 90-04-13916-8.
- ^ a b c Rushforth, K. (1999). Trees of Britain and Europe. Collins ISBN 0-00-220013-9.
- ^ a b c Nahal, I. (1962). Le Pin d'Alep (Pinus halepensis Miller). Étude taxonomique, phytogéographique, écologique et sylvicole. Annales de l'École National des Eaux et Forêts (Nancy) 19: 1–207.
- ^ Christensen, K. I. (1997). Gymnospermae. Pp. 1–17 in Strid, A., & Tan, K., eds., Flora Hellenica 1. Königstein.
- ^ Richardson, D. M., ed. (1998). Ecology and Biogeography of Pinus. Cambridge University Press ISBN 0-521-55176-5.
- ^ "Aleppo pine – Invasive Species South Africa". invasives.org.za. Retrieved 2024-05-19.
- ^ Facy, B.; Semerci, H. & Vendramin, G.G. (2003). "Aleppo and Brutia pines - Pinus halepensis/Pinus brutia" (PDF). EUFORGEN Technical Guidelines for Genetic Conservation and Use. Archived from the original (PDF) on 2018-09-30. Retrieved 2016-10-24.
- ^ Bonari, Gianmaria; Fernández-González, Federico; Çoban, Süleyman; Monteiro-Henriques, Tiago; Bergmeier, Erwin; Didukh, Yakiv P.; Xystrakis, Fotios; Angiolini, Claudia; Chytrý, Kryštof; Acosta, Alicia T.R.; Agrillo, Emiliano (January 2021). Ewald, Jörg (ed.). "Classification of the Mediterranean lowland to submontane pine forest vegetation". Applied Vegetation Science. 24 (1) e12544. Bibcode:2021AppVS..24E2544B. doi:10.1111/avsc.12544. hdl:10400.5/21923. ISSN 1402-2001. S2CID 228839165.
- ^ "Development Site: Forestry - Aleppo pine". Newman Information Center for Desert Research and Development, desert.bgu.ac.il. 2 October 2006. Archived from the original on 2 October 2006. Retrieved 19 May 2024 – via web.archive.org.
- ^ F.T. Maestre, J. Cortina . "Are Pinus halepensis plantations useful as a restoration tool in semiarid Mediterranean areas?" Forest Ecology and Management, 2004 (Elsevier).
- ^ Reducing Tear Out when Wood Planingwww.evenfallstudios.com Archived 2018-09-29 at the Wayback Machine
- ^ Cézanne, P. "Visions". In Architectural Digest, December 2005: 117.
External links
[edit]- Gymnosperm Database: Pinus halepensis
- Pinus halepensis—distribution map, genetic conservation units and related resources. European Forest Genetic Resources Programme (EUFORGEN)
Pinus halepensis
View on GrokipediaTaxonomy
Etymology
The genus name Pinus originates from the Latin term for pine trees, a word used in ancient Roman and Greek texts to describe resinous conifers valued for their timber and pitch.[5] The specific epithet halepensis is derived from "Halep," the Arabic name for Aleppo, a city in Syria, indicating the species' association with that region where early European botanists encountered it.[2] This binomial name was formally established by the Scottish botanist Philip Miller in the eighth edition of his The Gardener's Dictionary (1768), marking the first valid description of the species under the Linnaean system of binomial nomenclature, which had been introduced by Carl Linnaeus in 1753 to systematically classify plants, including those from the Mediterranean flora.[6] Miller's work reflected the growing 18th-century European effort to document and name exotic trees introduced to gardens from the Levant and North Africa. In common usage, Pinus halepensis is widely known as Aleppo pine in English, reflecting its namesake origin, and also as Jerusalem pine due to its prevalence in the region around the city.[7] Regional variants include pin d'Alep in French and الصنوبر الحلبي (ṣanawbar al-ḥalabī) in Arabic, emphasizing its cultural significance in Mediterranean landscapes.[2]Classification and related species
Pinus halepensis belongs to the kingdom Plantae, phylum Tracheophyta, class Pinopsida, order Pinales, family Pinaceae, genus Pinus, subgenus Pinus, section Pinus, and subsection Pinaster.[2] This classification places it among the hard pines (diploxylon pines) characterized by two vascular bundles in their needles.[2] Phylogenetic analyses using chloroplast genes such as rbcL and matK have positioned P. halepensis within a distinct Mediterranean clade of subgenus Pinus, highlighting its evolutionary ties to other Eurasian pines adapted to similar environments.[8] More recent phylogenomic studies based on transcriptome data from over 1,600 genes confirm this placement, estimating the divergence of the Mediterranean pine clade (subsection Pinaster) in the Miocene, approximately 20-15 million years ago.[9] The closest relative of P. halepensis is Pinus brutia (Turkish pine), with which it forms a sister species pair sharing a recent common ancestor; genetic analyses of chloroplast microsatellites reveal distinct but overlapping haplotypes, while morphological differences include longer needles (10-18 cm vs. 5-12 cm) and similar-sized cones (both 6-12 cm long) in P. brutia.[2] [10] [11] Other close relatives include Pinus pinea (stone pine) and Pinus pinaster (maritime pine), both in the broader Mediterranean pine group; P. pinea differs genetically through unique seed cone adaptations and morphologically by its rounded crown and edible seeds, whereas P. pinaster exhibits greater cold tolerance and thicker bark, supported by differences in nuclear and chloroplast DNA sequences.[9] [2] No subspecies or varieties of P. halepensis are widely recognized in current taxonomic revisions; proposed infraspecific taxa, such as subsp. ceciliae, have been reduced to synonymy based on morphological and genetic evidence showing insufficient differentiation.[1]Description
Morphology
Pinus halepensis is an evergreen conifer that attains a mature height of 15–25 m, with a trunk diameter reaching up to 1.5 m. The trunk is typically single and straight in younger trees but may become sinuous or divided with age. Older specimens feature an irregular, often umbrella-shaped crown formed by slender, spreading branches that can become pendulous, contributing to a rounded or flat-topped appearance.[12] The bark is thick, deeply fissured, and scaly, exhibiting purple-brown color, which provides significant fire resistance by protecting the underlying cambium from lethal temperatures during wildfires.[12][13] Needles occur in pairs (fascicles of two), are 6–12 cm long and 1–1.2 mm wide, stiff, slightly twisted, grayish-green, and persist for 2–3 years; they have finely toothed margins and stomatal lines on all faces.[12][7] Seed cones are ovoid to conical, measuring 6–12 cm in length and 4–7 cm in width, red- to purple-brown, and borne singly or in clusters of 2–6 on short stalks; they are moderately to highly serotinous, remaining closed on the tree for years until heat from fire or intense sun exposure causes the scales to open, enabling effective seed dispersal in post-disturbance environments.[12][14] The seeds themselves are 5–6 mm long with a light brown body and a 2.5 cm wing that aids wind dispersal; research indicates high viability, with germination rates typically ranging from 87–90% under optimal conditions and seeds retaining full viability for at least three years in canopy storage.[12][15][16]Reproduction and growth
Pinus halepensis primarily reproduces sexually through wind-pollinated cones, with male cones producing pollen during spring from March to April. Female conelets initiate development in the late growing season of the previous year and receive pollen the following spring, but fertilization occurs in the subsequent year due to delayed embryo development. The resulting cones take approximately three years to mature, exhibiting serotiny by remaining tightly closed and retaining seeds for extended periods, often decades, until triggered open by heat from wildfires.[17][18][19] Seeds ripen by late spring, with dispersal commencing in June to July, though the serotinous nature ensures most release is fire-dependent for effective regeneration in natural settings. Germination requires breaking seed coat dormancy through heat scarification, typically from fire exposure at 90–110°C for 1–5 minutes, which enhances viability without damaging embryos. Optimal germination occurs at soil temperatures of 20–25°C in well-drained, mineral-rich substrates, achieving rates above 70% under favorable light and moisture conditions.[17][20][21] Trees enter the reproductive phase early, often at 3–6 years of age under favorable conditions, with full stand maturity reached by 12–20 years. Initial post-germination growth is slow, with annual height increments of 8–15 cm in the first 5–7 years, accelerating to 0.5–1 m per year as saplings mature, particularly between 20 and 60 years when radial and height growth peak. Lifespan typically ranges from 150 to 200 years, though individuals in optimal habitats may exceed 300 years.[17][22][23] Asexual reproduction is limited in natural populations, with occasional root suckering observed in disturbed or post-fire soils, but it plays a minor role compared to seed-based regeneration. In cultivation, vegetative propagation via semi-hardwood cuttings from mature trees achieves rooting success up to 50% when treated with auxins like indole-3-butyric acid, enabling clonal production of selected genotypes.[24] The juvenile phase features primary needles that are decurrent and solitary, differing structurally from the paired secondary needles of adults; juvenile needles are narrower, possess lower dry mass per unit area (1.6–2.4 times less), and exhibit higher photosynthetic capacity to support rapid early establishment. This heteroblastic transition occurs within the first few years, aligning with the shift to reproductive maturity.[25][26]Distribution and habitat
Native range
Pinus halepensis is native to the Mediterranean Basin, with its core distribution encompassing southeastern Spain, southern France, Italy, Greece, Turkey, and the Levant region including Syria, Israel, and Lebanon, as well as North Africa in Algeria, Tunisia, and Morocco.[27][28] Pollen records and anthracological evidence indicate that the species has been present in the region since the Upper Pleistocene, with significant post-glacial expansion occurring around 10,000 years ago during the early Holocene, marking its establishment in current coastal and inland habitats.[29][30] The tree typically occupies altitudinal ranges from sea level to 1,000 meters, favoring coastal zones and inland hills where it forms open woodlands.[31][32] It thrives in Mediterranean climates characterized by hot, dry summers and mild, wet winters, with annual precipitation varying between 250 and 800 mm, enabling its adaptation to semi-arid conditions across its native extent.[33][14]Introduced ranges and invasiveness
Pinus halepensis has been introduced to several regions outside its native Mediterranean range, primarily during the 19th and early 20th centuries, for purposes such as soil stabilization, timber production, and ornamental landscaping.[14] In Australia, the species was first planted in the 1800s for erosion control and windbreaks, particularly in South Australia, where it has since become widely naturalized and invasive in semi-arid and coastal habitats similar to its native environment.[34] Similarly, introductions to California in the United States occurred in the 1800s for ornamental and forestry uses, leading to its establishment in the southwestern states, with notable invasiveness on islands like Catalina.[14] In the Southern Hemisphere, the tree was brought to Chile, South Africa, and New Zealand, where it thrives in Mediterranean-like climates; for instance, in South Africa, plantings began in the mid-19th century for timber and amenity purposes, while in New Zealand, arrivals date to the mid-19th century, often as contaminants or deliberate imports.[35][36][37] The invasiveness of P. halepensis stems from its prolific seed production, fire-adapted regeneration, and ability to form dense monospecific stands that outcompete native vegetation. As of 2025, recent records confirm its establishment on Santa Cruz Island, California, and studies highlight changes in fuel traits in invaded high-altitude grasslands in Argentina, increasing wildfire risks.[38][39] In Australia and California, it invades grasslands and shrublands, altering soil nutrient cycles and reducing biodiversity by shading out understory plants and preventing native seedling establishment.[40][14] These stands also increase wildfire fuel loads, exacerbating fire intensity and frequency in invaded areas, which further favors its spread post-disturbance.[14] In South Africa and New Zealand, similar patterns occur, with the species listed as an environmental weed due to its displacement of indigenous flora in fynbos and coastal ecosystems.[35][36] Control efforts typically involve mechanical removal, such as cutting and herbicide application, often targeting young seedlings to prevent re-establishment from seed banks.[40][34] Recent studies indicate that P. halepensis is expanding its introduced ranges, driven by climate change, which extends suitable semi-arid habitats.[37] Ecological niche modeling predicts further invasion potential in central Chile, eastern Argentina, and Uruguay by 2050, as warming temperatures and altered precipitation patterns align with the species' tolerances.[37] In Australia and South Africa, ongoing spread is linked to increased drought resilience, heightening risks to biodiversity hotspots.[14] These projections underscore the need for proactive management to mitigate ecological impacts in vulnerable regions.[37]Ecology
Habitat preferences
Pinus halepensis thrives in a variety of soil types, particularly poor, rocky, and calcareous substrates such as rendzina soils that drain rapidly.[41] It tolerates a wide pH range from mildly acidic to strongly alkaline, typically between 6 and 8, and exhibits resilience to low fertility and salinity conditions.[42][31] Its drought resistance is enhanced by a deep taproot system that facilitates access to subsurface water, allowing survival in arid environments.[43] The species is highly xerophytic, capable of enduring annual rainfall as low as 250-300 mm, though optimal growth occurs with 350-700 mm.[44][45] Adaptations for water conservation include stomatal regulation and a narrow hydraulic safety margin, which enable efficient resource use during prolonged dry periods.[46] Pinus halepensis requires full sun exposure and cannot tolerate shade, reflecting its adaptation to open, sunny Mediterranean landscapes.[42] It withstands temperatures from -12°C to over 40°C, but young seedlings are particularly sensitive to frost.[14] In its native Mediterranean range, Pinus halepensis forms open woodlands often associated with oak species such as Quercus ilex and contributes to maquis shrublands.[14][47] It plays a key successional role in post-fire ecosystems, rapidly colonizing disturbed areas to stabilize soils and facilitate understory recovery.[48]Ecological interactions and threats
Pinus halepensis forms ectomycorrhizal associations with various fungi, which significantly enhance nutrient uptake, particularly of phosphorus and nitrogen, in nutrient-limited Mediterranean soils. These symbiotic relationships improve seedling growth and stress tolerance, as demonstrated in studies where inoculation with ectomycorrhizal fungi like Scleroderma species increased biomass and nutrient content in seedlings exposed to heavy metals.[49][50] Fungi such as Boletus edulis have also been associated with P. halepensis roots, aiding in micronutrient mobilization and overall plant vigor. The species relies primarily on wind for pollination, but its seeds interact with avian dispersers and predators, including birds like common crossbills (Loxia curvirostra), which specialize in extracting seeds from P. halepensis cones using their adapted bills. This interaction can facilitate limited dispersal through caching behavior, though crossbills more often act as predators in a coevolutionary dynamic that influences cone morphology across geographic ranges.[51][52] Major pests of P. halepensis include the pine bast scale (Marchalina hellenica), which infests bark and branches, sucking sap and weakening trees, leading to reduced growth and increased susceptibility to secondary infections in southeastern Europe.[53][54] The pine processionary moth (Thaumetopoea pityocampa) is another significant defoliator, with larvae causing extensive needle loss and tree decline during outbreaks.[53] Fungal pathogens, such as Sphaeropsis sapinea (syn. Diplodia sapinea), contribute to tip blight and dieback, particularly under stress conditions, and have been isolated from declining P. halepensis stands in Spain, where they exacerbate canopy thinning and mortality.[55][56] In fire ecology, P. halepensis exhibits serotinous cones that remain closed until heated by fire, releasing viable seeds to exploit post-fire environments for rapid regeneration and dominance in Mediterranean ecosystems.[18] This adaptation supports high post-fire seedling establishment, but the species' resin-rich foliage and retained dead branches increase flammability, intensifying wildfire spread and severity in dense stands.[57] Climate change poses severe threats through intensified drought stress, leading to widespread dieback in southern ranges since the early 2000s, as evidenced by growth declines during extreme events like the 2012 drought in Spain. As of 2025, continued dieback has been reported in southern ranges like western Algeria and Morocco, with studies indicating that increasing temperatures threaten post-fire auto-successional regeneration and overall resilience under projected warming scenarios of 2.5–3.5°C.[58][59][41][60][31][61]Conservation
Conservation status
Pinus halepensis is assessed as Least Concern on the IUCN Red List at the global level, with the evaluation conducted in 2013 and no major updates as of 2025.[4] The species has a wide distribution across the Mediterranean Basin, and its global population is considered stable due to its adaptability and extensive use in plantations.[4] However, the species faces local vulnerabilities in fragmented populations, such as those in the Algarve region of Portugal and the Costa Brava area of Spain on the Iberian Peninsula, where it is considered threatened primarily due to habitat destruction from urban development and agricultural expansion.[4] In its native range, population trends show declines in some natural stands attributable to urbanization, overgrazing, and habitat fragmentation, while populations in managed plantations remain stable or are increasing through active reforestation efforts.[4][62] Key protected areas supporting P. halepensis include Mount Carmel in Israel, which hosts one of the largest natural forests and serves as a genetic diversity hotspot for the eastern Mediterranean lineage.[63] Recent assessments from the 2020s highlight the species' resilience to drought but warn of climate-induced declines, particularly in North Africa; for instance, studies in Morocco's High Atlas Mountains document growth suppression and dieback linked to prolonged dry and hot periods at the southern range limit.[64]Management and restoration
Reforestation initiatives for Pinus halepensis in the Mediterranean region have been supported by EU-funded projects under the LIFE program since the 1990s, focusing on enhancing forest resilience and biodiversity through the use of native seeds.[65] The LIFE ADAPT-ALEPPO project, launched in 2020, exemplifies these efforts by developing adaptation tools for Iberian Aleppo pine forests, including assisted migration techniques that employ native seeds in restoration plots across approximately 110 hectares in three bioclimatic regions to promote post-fire regeneration and structural heterogeneity.[65] These initiatives emphasize silvicultural treatments to improve water availability and floristic diversity, with monitoring to ensure long-term conservation outcomes.[65] Fire management strategies for P. halepensis stands prioritize reducing fuel loads to mitigate wildfire risks, incorporating prescribed burns and thinning. Prescribed burning in Mediterranean ecosystems, including mixed P. halepensis stands, effectively decreases understory fuel and wildfire intensity while maintaining ecosystem functionality.[66] Thinning, particularly pre-commercial thinning in post-fire regenerations, accelerates cone and viable seed production, enhancing stand vitality and reducing competition among dense saplings.[67] Post-fire restoration often involves direct seeding techniques, such as applying seeds in patches or strips covered with wood chips from on-site debris, which improves germination rates and seedling establishment in semiarid conditions.[68][69] Pest control for P. halepensis relies on integrated pest management (IPM) approaches that integrate biological agents to target key insects like scales and moths while minimizing chemical use. For the pine processionary moth (Thaumetopoea pityocampa), a major defoliator of Aleppo pine, biological controls such as Bacillus thuringiensis formulations and natural predators like the Argentine ant (Linepithema humile) provide effective suppression of larval populations.[70][71] The Israeli pine bast scale (Matsucoccus josephi), which causes bud drying and tree decline in P. halepensis, is managed through IPM tactics including the promotion of parasitoids and predators as biological agents to disrupt scale life cycles.[72] Genetic conservation efforts for P. halepensis emphasize ex situ collections to preserve diversity for restoration, with seed banks storing lots from broad genepools (at least 30 trees per population across multiple sites) to support artificial regeneration after disturbances like fire.[73] These collections, including clonal archives and cold storage, target marginal populations at high altitudes or desert edges to safeguard drought-resistant provenances valuable for climate adaptation.[73] Restoration guidelines from the European Forest Genetic Resources Programme (EUFORGEN), in collaboration with FAO, recommend using locally adapted, drought-resistant provenances while avoiding inter-zone seed transfers to prevent genetic erosion and ensure ecological suitability.[73][74]Uses
Forestry and timber
The wood of Pinus halepensis is soft and highly resinous, making it suitable for pulp production, particleboard manufacturing, and utility poles, though it requires preservative treatment for outdoor structural uses due to moderate natural durability.[75] Its basic density ranges from 450 to 620 kg/m³, contributing to a lightweight yet workable material with compressive strength of 30–74 MPa and modulus of rupture in bending averaging 57 MPa, which is relatively low compared to other softwoods.[75] In Spain, it is commonly processed into pallets and furniture components.[75] Extensive plantations of P. halepensis were established in Spain and North Africa starting in the late 19th century, primarily to combat soil erosion on degraded lands and to support resin tapping for industrial applications such as varnishes and adhesives.[76] These efforts expanded in the 20th century, with the species valued for its rapid establishment on poor, calcareous soils in semiarid regions.[77] Resin tapping, historically prominent in Mediterranean countries including Spain and Portugal, involved V-shaped incisions on trunks to collect oleoresin, though production has declined since the mid-20th century due to synthetic alternatives.[14] In managed plantations, mean annual volume increments reaching up to 6.5 m³/ha in productive stands under moderate thinning regimes.[78] Site quality influences yields, with high-fertility areas achieving maximum annual growth of around 3.3 m³/ha at age 40, while overall stand volumes can exceed 200 m³/ha by rotation end in optimal conditions.[79] Rotation cycles for timber and poles typically span 70–90 years.[80] The wood is also used for fuelwood, particularly in rural Mediterranean areas for heating and cooking.[2] Additionally, P. halepensis supports pine honey production, a honeydew honey collected by bees from the excretions of the insect Marchalina hellenica on the trees, which is a significant product in Greece and Turkey. Sustainable forestry practices for P. halepensis face challenges from high fire risk due to the species' flammable resin and canopy structure, necessitating thinning and mixed-species planting to mitigate crown fires.[57] Forest Stewardship Council (FSC) certification is applied to some mixed plantations in Mediterranean Europe, promoting biodiversity through reduced-impact logging and pest monitoring.[81] Resin yields have declined in recent decades owing to insect pests like the pine processionary moth (Thaumetopoea pityocampa) and bark beetles, which weaken trees and reduce oleoresin flow by up to 50% in affected stands.[14]Ornamental and landscape
Pinus halepensis, commonly known as the Aleppo pine, is valued in ornamental landscaping for its picturesque, irregular crown that develops an umbrella-like shape with age, making it suitable for parks, avenues, and large open spaces.[7] Its fine-textured, pale green needles and attractive reddish-brown bark contribute to its aesthetic appeal in Mediterranean-style gardens.[14] The tree's high drought tolerance, once established, positions it as an ideal choice for xeriscaping in arid and semi-arid regions, requiring minimal irrigation after the initial years.[82] In landscape design, P. halepensis serves multiple functional roles, including as windbreaks and shelterbelts to protect against coastal winds and erosion.[83] It excels in soil stabilization, particularly on slopes and sandy soils, where its deep root system helps prevent erosion and bind loose substrates.[84] Notable examples include its use in California gardens, such as those in the low deserts and coastal areas like Sunnyvale, where it provides shade and screening in public landscapes.[85] In Australia, it is planted along coastal regions for similar protective purposes, enhancing stability in sandy, wind-exposed sites.[86] Cultivation of P. halepensis in ornamental settings requires full sun and well-drained soils, with spacing of 6–12 meters (20–40 feet) to accommodate its mature width and prevent crowding.[83] Pruning is recommended during winter dormancy to maintain shape, remove dead branches, and thin the foliage for better air circulation, though excessive pruning should be avoided to prevent stress.[87] For smaller spaces, compact varieties such as 'Compacta' offer a denser, more manageable form, reaching heights of 5–10 meters while retaining the species' ornamental qualities.[88] Despite its benefits, P. halepensis presents challenges in landscaping, including substantial litter from shedding needles and cones, which can form a thick carpet and require regular cleanup to maintain tidiness.[84] Additionally, its potential allelopathic effects, mediated by leaf leachates and volatile compounds, may inhibit understory plant growth, limiting biodiversity in mixed plantings.[89]Medicinal and pharmacological uses
The essential oils extracted from the needles of Pinus halepensis are rich in terpenes and exhibit various pharmacological properties. Key compounds include α-pinene (up to 17%) and β-pinene (up to 3.5%), which possess anti-inflammatory and antimicrobial activities; myrcene (up to 25%); β-caryophyllene (7-32%); as well as limonene, terpinolene, germacrene D, camphene, aromadendrene, and humulene.[90][91] These components contribute to antioxidant effects, with IC₅₀ values as low as 1.78 μg/mL in chemiluminescence assays, and demonstrate antibacterial activity against pathogens such as Escherichia coli and Staphylococcus aureus (MIC values 100-600 μg/mL).[92][91] Additionally, the oils show potential anti-inflammatory and antiviral properties, supporting traditional uses for respiratory ailments and immune system enhancement.[90][91] The needles of Pinus halepensis are not poisonous to humans and have been traditionally used in the Mediterranean region, including Algeria, to prepare a vitamin C-rich tea for immune support and treatment of colds, with no reliable reports of toxicity in these areas.[93][94][95] The needles also contain minerals such as iron, phosphorus, magnesium, and potassium, which contribute to general health support, though their quantities are not extremely high compared to vitamins.[96][97] Unlike certain global pine species such as Pinus ponderosa, which can pose risks particularly to pregnant individuals or in large amounts and cause toxicity in livestock, P. halepensis is safe and not present in Algeria.[98]Cultural and historical significance
In mythology and symbolism
In Mediterranean mythology, the Aleppo pine (Pinus halepensis) held symbolic significance tied to themes of death, resurrection, and eternal life, particularly through its association with the Phrygian god Attis, consort of Cybele. In the myth, Attis, driven mad, castrates himself and dies beneath a pine tree sacred to Cybele; an evergreen pine is annually felled and decorated with flowers and ribbons in his honor during spring festivals, symbolizing the cycle of vegetation and renewal, with the Aleppo pine's evergreen nature reinforcing this as a symbol of immortality and rebirth.[99] This practice is echoed in biblical critiques of pagan rituals.[99] During the Roman Saturnalia festival (December 17–23), honoring the god Saturn, Aleppo pines and other evergreens were incorporated into wreaths and decorations, symbolizing abundance and the return of light amid winter solstice observances; these traditions later influenced Christian Christmas customs, with the pine cone emerging as a fertility emblem linked to Roman gods like Dionysus.[99] Biblical texts possibly identify the Aleppo pine as the "oren" or "pine" in Isaiah 44:14, describing a tree planted for idols and sustained by rain, highlighting its utility in sacred contexts; its resilient, evergreen form also symbolizes endurance and divine provision in Jewish interpretive traditions.[99][100] In modern contexts, the Aleppo pine embodies Mediterranean cultural heritage, frequently appearing in environmental literature and art as an icon of ecological resilience and regional identity, from ancient Egyptian embalming resins to contemporary depictions of coastal landscapes.[99][101]Historical uses and modern references
In ancient Mediterranean societies, the resin of Pinus halepensis, commonly known as the Aleppo pine, was harvested for producing pitch and tar essential to shipbuilding, serving as a waterproofing agent for hulls and rigging to protect against seawater corrosion. This practice dates back to early maritime cultures, such as the Greeks, who relied on such resinous materials from native pines.[102] By the 19th century, turpentine distilled from P. halepensis found medicinal applications, particularly as an antiseptic and counterirritant applied topically to wounds, abrasions, and rheumatic conditions to promote healing and reduce inflammation, reflecting its longstanding role in folk and naval medicine.[103] The species appears in early botanical literature, with Theophrastus detailing its characteristics in Enquiry into Plants around 300 BCE, where his description of "pitys"—a resinous pine with serotinous cones—likely refers to P. halepensis, emphasizing its form, habitat, and utility in the Greek world. In modern novels set in Provence, such as Peter Mayle's A Year in Provence (1989), the pines of the region, including Aleppo pine, evoke the rugged, aromatic landscapes of the Mediterranean, symbolizing the region's enduring natural beauty and seasonal rhythms through vivid depictions of pine-shaded hills and forests.[105] Aleppo pines feature prominently in art, notably in Vincent van Gogh's Study of Pine Trees (1889), an oil painting from Saint-Rémy-de-Provence that captures the twisted forms and vibrant greens of the region's pines, likely including P. halepensis, against the Provençal terrain, influencing Impressionist portrayals of southern European scenery. Documentaries on Mediterranean wildfires, including From Devil's Breath (2022) about the 2017 Portuguese blazes, highlight fire-prone ecosystems in the region where P. halepensis often dominates, illustrating the species' role in recovery challenges. In 2020s climate discourse, P. halepensis serves as an indicator species in IPCC assessments of Mediterranean ecosystems, underscoring its susceptibility to drought-induced dieback and intensified wildfires under warming conditions. As of 2025, the species continues to feature in discussions on climate-resilient forestry, with ongoing projects in the Mediterranean focusing on its adaptation to increased wildfire frequency.[106][107][108]References
- https://www.[researchgate](/page/ResearchGate).net/publication/300020521_Therapeutic_Use_of_Aleppo_Pine_Pinus_halepensis_Mill











