Recent from talks
All channels
Be the first to start a discussion here.
Be the first to start a discussion here.
Be the first to start a discussion here.
Be the first to start a discussion here.
Welcome to the community hub built to collect knowledge and have discussions related to Ventricular flutter.
Nothing was collected or created yet.
Ventricular flutter
View on Wikipediafrom Wikipedia
| Ventricular flutter | |
|---|---|
 | |
| Specialty | Cardiology |
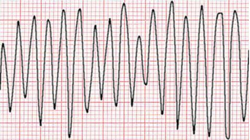
Ventricular flutter is an arrhythmia, more specifically a tachycardia affecting the ventricles with a rate over 250-350 beats/min, and one of the most indiscernible. It is characterized on the ECG by a sinusoidal waveform without clear definition of the QRS and T waves. It has been considered as a possible transition stage between ventricular tachycardia and fibrillation, and is a critically unstable arrhythmia that can result in sudden cardiac death.[citation needed][1]
It can occur in infancy,[2] youth,[3] or as an adult.
It can be induced by programmed electrical stimulation.[4][5]
References
[edit]- ^ "Heart Ventricle Flutter - an overview | ScienceDirect Topics". www.sciencedirect.com. Retrieved 2023-05-11.
- ^ Thies KC, Boos K, Müller-Deile K, Ohrdorf W, Beushausen T, Townsend P (January 2000). "Ventricular flutter in a neonate--severe electrolyte imbalance caused by urinary tract infection in the presence of urinary tract malformation". J Emerg Med. 18 (1): 47–50. doi:10.1016/S0736-4679(99)00161-4. PMID 10645837.
- ^ Hayashi M, Murata M, Satoh M, et al. (July 1985). "Sudden nocturnal death in young males from ventricular flutter". Jpn Heart J. 26 (4): 585–91. doi:10.1536/ihj.26.585. PMID 4057556.
- ^ Gurevitz O, Viskin S, Glikson M, et al. (April 2004). "Long-term prognosis of inducible ventricular flutter: not an innocent finding". Am. Heart J. 147 (4): 649–54. doi:10.1016/j.ahj.2003.11.012. PMID 15077080.
- ^ Viskin S, Ish-Shalom M, Koifman E, et al. (September 2003). "Ventricular flutter induced during electrophysiologic studies in patients with old myocardial infarction: clinical and electrophysiologic predictors, and prognostic significance". J. Cardiovasc. Electrophysiol. 14 (9): 913–9. doi:10.1046/j.1540-8167.2003.03082.x. PMID 12950532. S2CID 30924977.
External links
[edit]Ventricular flutter
View on Grokipediafrom Grokipedia
