Recent from talks
All channels
Be the first to start a discussion here.
Be the first to start a discussion here.
Be the first to start a discussion here.
Be the first to start a discussion here.
Welcome to the community hub built to collect knowledge and have discussions related to Calcium iodide.
Nothing was collected or created yet.
Calcium iodide
View on Wikipediafrom Wikipedia
 | |
| Names | |
|---|---|
| IUPAC name
calcium iodide
| |
| Identifiers | |
| |
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.030.238 |
| EC Number |
|
PubChem CID
|
|
| RTECS number |
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| CaI2 | |
| Molar mass | 293.887 g/mol (anhydrous) 365.95 g/mol (tetrahydrate) |
| Appearance | white solid |
| Density | 3.956 g/cm3 (anhydrous)[1] |
| Melting point | 779 °C (1,434 °F; 1,052 K) (anhydrous) [2] |
| Boiling point | 1,100 °C (2,010 °F; 1,370 K)[2] |
| 64.6 g/100 mL (0 °C) 66 g/100 mL (20 °C) 81 g/100 mL (100 °C) | |
| Solubility | soluble in acetone and alcohols |
| −109.0·10−6 cm3/mol | |
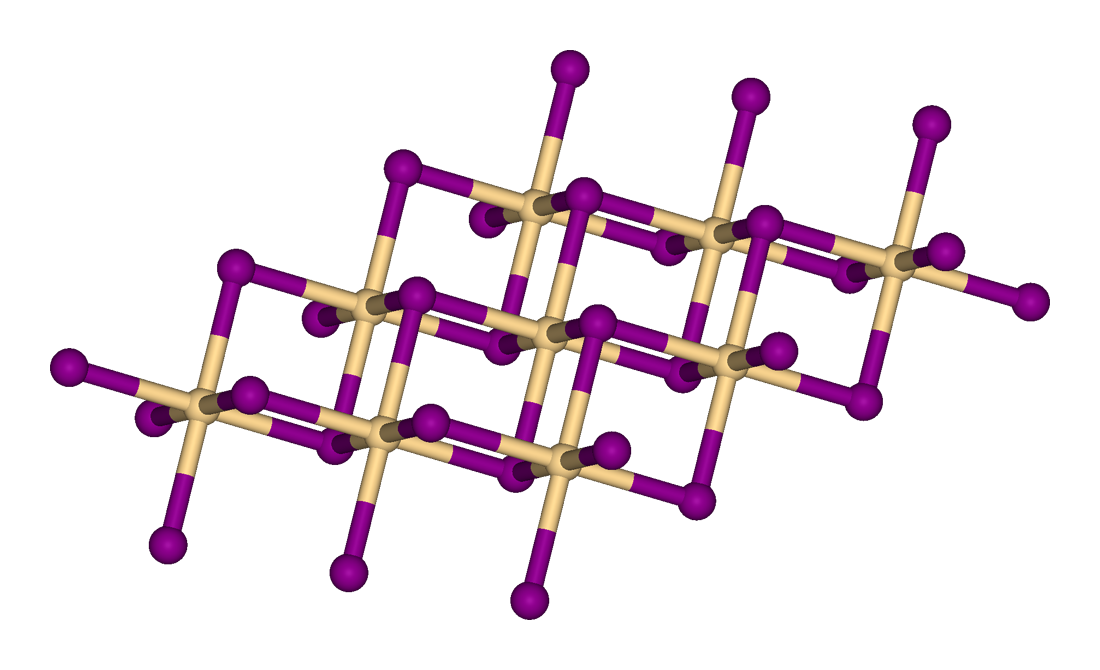
| Structure | |
| Rhombohedral, hP3 | |
| P-3m1, No. 164 | |
| octahedral | |
| Hazards | |
| NFPA 704 (fire diamond) | |
| Related compounds | |
Other anions
|
calcium fluoride calcium chloride calcium bromide |
Other cations
|
beryllium iodide magnesium iodide strontium iodide barium iodide |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Calcium iodide (chemical formula CaI2) is the ionic compound of calcium and iodine. This colourless deliquescent solid is a salt that is highly soluble in water. Its properties are similar to those for related salts, such as calcium chloride. It is used in photography.[1] It is also used in cat food as a source of iodine.
Reactions
[edit]Henri Moissan first isolated pure calcium in 1898 by reducing calcium iodide with pure sodium metal:[3]
Calcium iodide can be formed by treating calcium carbonate, calcium oxide, or calcium hydroxide with hydroiodic acid:[4]
Calcium iodide slowly reacts with oxygen and carbon dioxide in the air, liberating iodine, which is responsible for the faint yellow color of impure samples.[5]
- 2 CaI2 + 2 CO2 + O2 → 2 CaCO3 + 2 I2
References
[edit]- ^ a b Turner, Jr., Francis M., ed. (1920), The Condensed Chemical Dictionary (1st ed.), New York: Chemical Catalog Co., p. 127, retrieved 2007-12-08
- ^ a b R. J. Lewis (1993), Hawley's Condensed Chemical Dictionary 12th edition
- ^ Mellor, Joseph William (1912), Modern Inorganic Chemistry, New York: Longmans, Green, and Co, p. 334, 6909989325689, retrieved 2007-12-08
- ^ Gooch, Frank Austin; Walker, Claude Frederic (1905), Outlines of Inorganic Chemistry, New York: Macmillan, p. 340, retrieved 2007-12-08
- ^ Jones, Harry Clary (1906), Principles of Inorganic Chemistry, New York: Macmillan, p. 365, retrieved 2007-12-08
Calcium iodide
View on Grokipediafrom Grokipedia