Recent from talks
Nothing was collected or created yet.
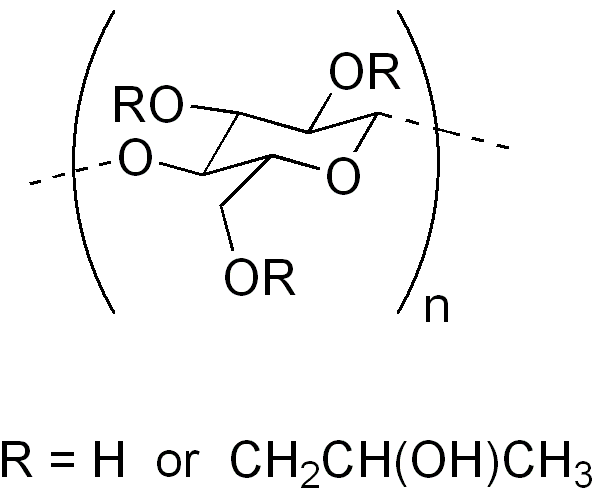
Hydroxypropyl cellulose
View on Wikipedia | |
| Names | |
|---|---|
| Other names
Cellulose, 2-hydroxypropyl ether; oxypropylated cellulose; E463; hyprolose
| |
| Identifiers | |
| ChEMBL | |
| ChemSpider |
|
| DrugBank | |
| ECHA InfoCard | 100.116.338 |
| E number | E463 (thickeners, ...) |
| UNII | |
CompTox Dashboard (EPA)
|
|
| Properties | |
| variable | |
| Molar mass | variable |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Hydroxypropyl cellulose (HPC) is a derivative of cellulose with both water solubility and organic solubility. It is used as an excipient; a topical ophthalmic protectant and lubricant; a thickener, emulsifier, and stabilizer in cosmetic formulations; a sieving matrix for DNA separations by capillary and microchip electrophoresis; a leather consolidant used in book preservation; and a wood consolidant.
Chemistry
[edit]HPC is an ether of cellulose in which some of the hydroxyl groups in the repeating glucose units have been hydroxypropylated forming -OCH2CH(OH)CH3 groups using propylene oxide. The average number of substituted hydroxyl groups per glucose unit is referred to as the degree of substitution (DS). Complete substitution would provide a DS of 3. Because the hydroxypropyl group added contains a hydroxyl group, this can also be etherified during preparation of HPC. When this occurs, the number of moles of hydroxypropyl groups per glucose ring, moles of substitution (MS), can be higher than 3.
Because cellulose is very crystalline, HPC must have an MS about 4 in order to reach a good solubility in water. HPC has a combination of hydrophobic and hydrophilic groups, so it has a lower critical solution temperature (LCST) at 45 °C. At temperatures below the LCST, HPC is readily soluble in water; above the LCST, HPC is not soluble.
At the right concentrations, HPC forms liquid crystals and many mesophases. Such mesophases include isotropic, anisotropic, nematic and cholesteric. The last one gives many colors such as violet, green and red.[1] These colors are structural colors by nature and are also mechanochromic, meaning the HPC mesophase changes color when stress is applied.[2]

Uses
[edit]HPC is well established in the medical, pharmaceutical, and food industries as a widely applicable non-toxic, and cost-effective raw material.[2] It is commonly used as a thickener, emulsifier, stabilizer, binder and anti-caking agent. It has E number E463.

In pharmaceuticals it is used as a binder[3] in tablets, and is used variously within cosmetic formulations such as shampoos, conditioners, and lotions.[4] Lacrisert, manufactured by Aton Pharma, is a formulation of HPC used for artificial tears. It is used to treat medical conditions characterized by insufficient tear production such as keratoconjunctivitis sicca, recurrent corneal erosions, decreased corneal sensitivity, exposure and neuroparalytic keratitis. HPC is also used as a lubricant for artificial eyes.[5][6][7]
HPC is used as a sieving matrix for DNA separations by capillary and microchip electrophoresis.[8]
HPC is the main ingredient in Cellugel, described as a "safe, penetrating consolidant for leather book covers affected by red rot" by Preservation Solutions, and used in book conservation.[9]
Due to its ability for structural color and mechanochromism at the right concentrations, it can also be utilised as an optical strain sensor,[10] or as a more environmentally responsible color display technology driven mechanically.[2]
HPC was used by conservators at the Firearms Museum at the Buffalo Bill Center of the West on the Forgotten Winchester, a Winchester Model 1873 lever-action centerfire rifle discovered in 2014 leaning against a tree in Great Basin National Park, Nevada.[11]
See also
[edit]Notes and references
[edit]- ^ a b Barty-King, Charles; Chan, Chun Lam Clement; Parker, Richard; Bay, Mélanie; Vadrucci, Roberto; De Volder, Michael; Vignolini, Silvia (29 July 2021). "Mechanochromic, Structurally Colored, and Edible Hydrogels Prepared from Hydroxypropyl Cellulose and Gelatin". Advanced Materials. 33 (37) 2102112. Bibcode:2021AdM....3302112B. doi:10.1002/adma.202102112. PMC 11468689. PMID 34323315. S2CID 236497081.
- ^ a b c d Barty-King, Charles H.; Burgonse, Maxime; Vignolini, Silvia; Baumberg, Jeremy; De Volder, Michael (2025). "Mechanochromic, Low-Cost, and Structurally Colored Displays Using Biodegradable Hydroxypropyl Cellulose". Advanced Materials. 37 (29) 2418880. doi:10.1002/adma.202418880. ISSN 1521-4095. PMC 12288781. PMID 40346773.
- ^ Weiner, Myra L.; Lois A. Kotkoskie (1999). Excipient Toxicity and Safety. Taylor & Francis. p. 8. ISBN 978-0-8247-8210-8.
- ^ "Hydroxypropyl Cellulose". kimachemical.com. Retrieved 7 March 2023.
- ^ Luchs J, Nelinson D, Macy J (December 2010). "Efficacy of hydroxypropyl cellulose inserts (LACRISERT®) in subsets of patients with dry eye syndrome (DES): Findings from a patient registry". Cornea. 29 (12): 1417–1427. doi:10.1097/ICO.0b013e3181e3f05b. PMID 20847657. S2CID 40120861.
- ^ McDonald M; D'Aversa G; Perry D; Wittpenn J; Nelinson D (Oct 2010). "Correlating patient-reported response to Hydroxypropyl cellulose ophthalmic insert (LACRISERT®) therapy with clinical outcomes: tools for predicting response". Curr Eye Res. 35 (10): 880–887. doi:10.3109/02713683.2010.495811. PMID 20858108. S2CID 207450381.
- ^ Koffler B, McDonald M, Nelinson D (May 2010). "Improvement in clinical signs, symptoms, and QoL associated with DES: Hydroxypropyl Cellulose Ophthalmic Insert Patient Registry". Eye & Contact Lens. 36 (3): 170–176. doi:10.1097/ICL.0b013e3181db352f. PMID 20351555. S2CID 1751728.
- ^ Sanders, Joshua C.; Breadmore, Michael C.; Kwok, Yien C.; Horsman, Katie M.; Landers, James P. (February 2003). "Hydroxypropyl Cellulose as an Adsorptive Coating Sieving Matrix for DNA Separations: Artificial Neural Network Optimization for Microchip Analysis". Analytical Chemistry. 75 (4): 986–994. doi:10.1021/ac020425z. PMID 12622396. INIST 14571070.
- ^ "Cellugel". Conservationresources.com. Archived from the original on 2022-06-25. Retrieved 2021-12-20.
- ^ Kamita, Gen; Frka-Petesic, Bruno; Allard, Antoine; Dargaud, Marielle; King, Katie; Dumanli, Ahu Gumrah; Vignolini, Silvia (2016). "Biocompatible and Sustainable Optical Strain Sensors for Large-Area Applications". Advanced Optical Materials. 4 (12): 1950–1954. doi:10.1002/adom.201600451. hdl:10044/1/53167. ISSN 2195-1071.
- ^ Forgotten Winchester visits the Center of the West's Cody Firearms Museum, Buffalo Bill Center of the West